Profiling of Myositis Specific Antibodies and Composite Scores as an Aid in the Differential Diagnosis of Autoimmune Myopathies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Autoantibody Testing
2.3. Statistical Methods
3. Results
3.1. Prevalence of Myositis Specific Antibodies in Myositis and Controls
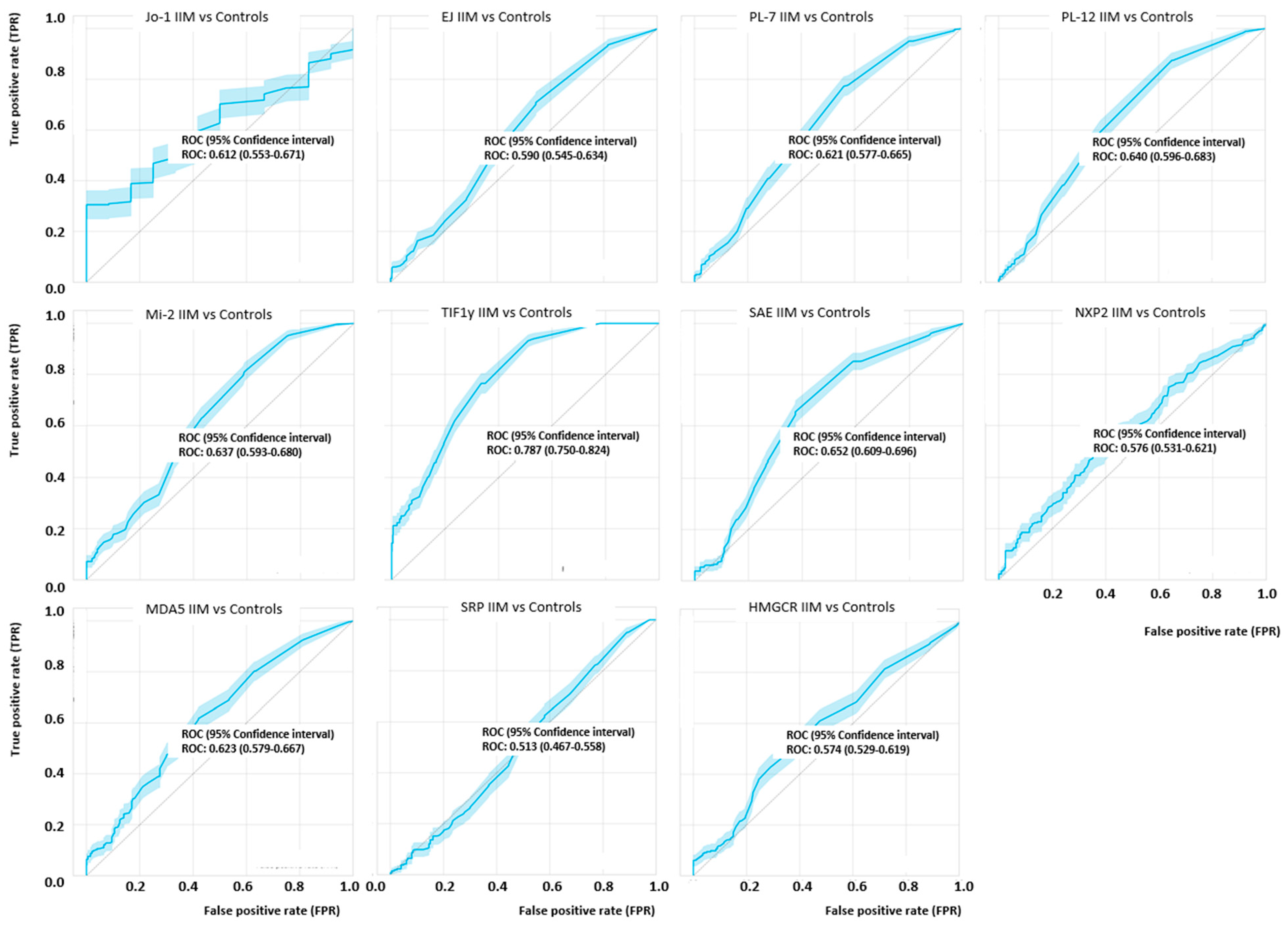
3.2. Quantitative Analysis of Myositis Specific Antibodies Studied
3.3. Prevalence of Myositis Specific Antibodies in Myositis Subsets
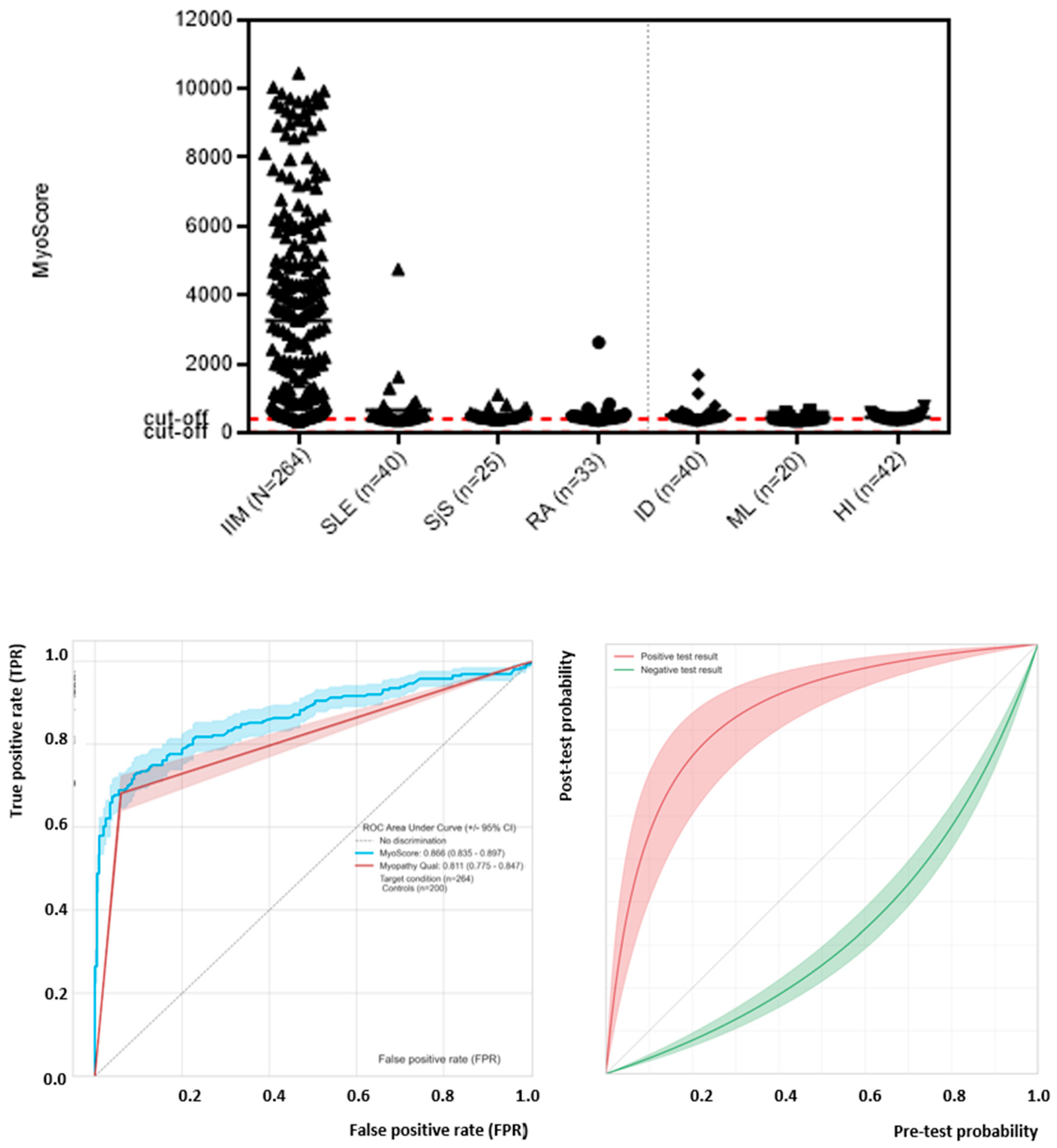
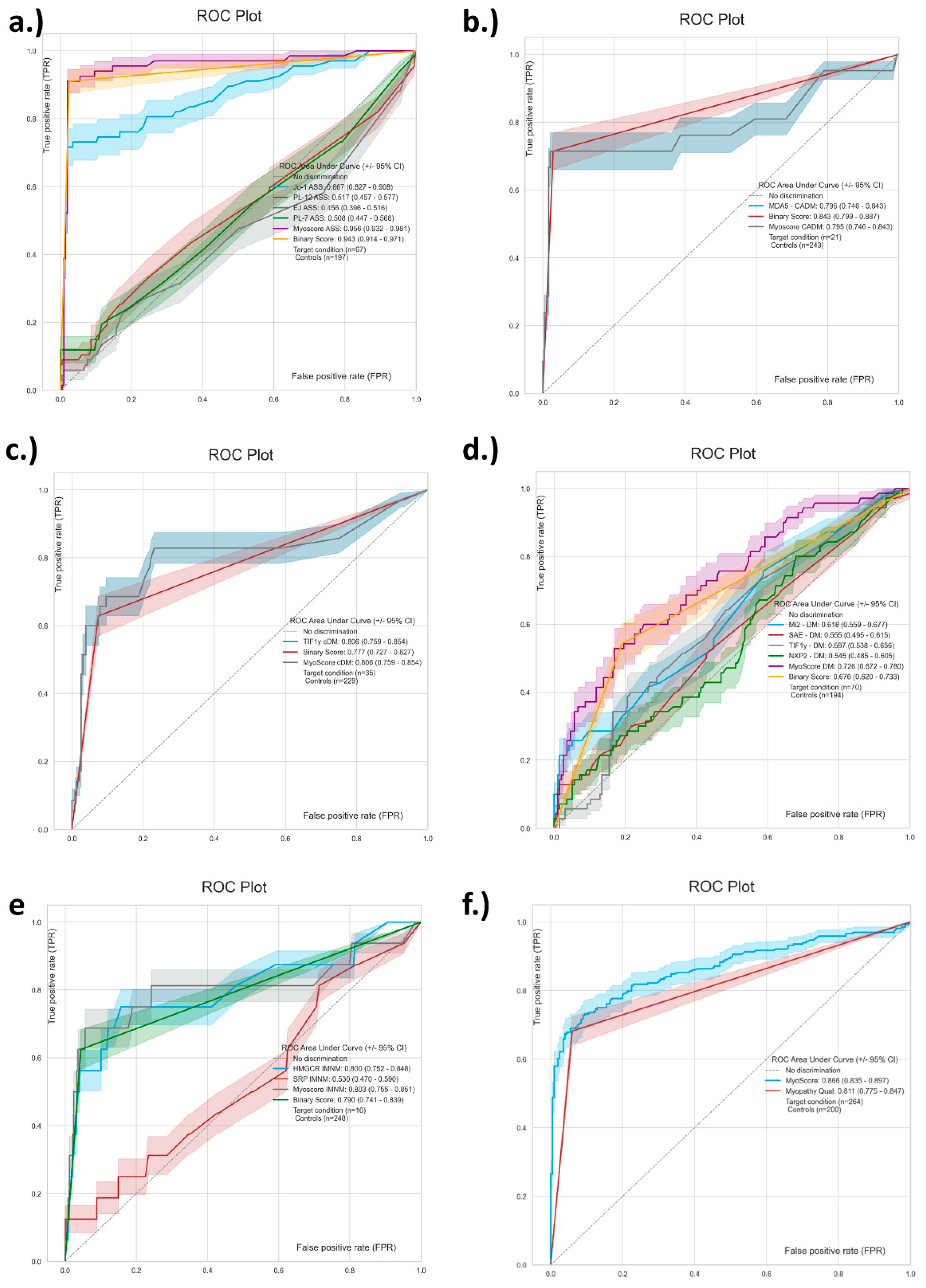
3.4. Generation and Performance Assessment of MyoScores for Idiopathic Inflammatory Myopathies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McHugh, N.J.; Tansley, S.L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 2018, 14, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Fritzler, M.J.; Choi, M.Y.; Mahler, M. The Antinuclear Antibody Test in the Diagnosis of Antisynthetase Syndrome and Other Autoimmune Myopathies. J. Rheumatol. 2018, 45, 444–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampoia, M.; Notarnicola, A.; Abbracciavento, L.; Fontana, A.; Giannini, M.; Humbel, R.L.; Iannone, F. A New Immunodot Assay for Multiplex Detection of Autoantibodies in a Cohort of Italian Patients with Idiopathic Inflammatory Myopathies. J. Clin. Lab. Anal. 2016, 30, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Damoiseaux, J.; Kallenberg, C.; Sack, U.; Witte, T.; Herold, M.; Bossuyt, X.; Musset, L.; Cervera, R.; Plaza-Lopez, A.; et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 2014, 73, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, R.; Dhillon, N.; Fertig, N.; Koontz, D.; Qi, Z.; Oddis, C.V. A Negative Antinuclear Antibody Does Not Indicate Autoantibody Negativity in Myositis: Role of Anticytoplasmic Antibody as a Screening Test for Antisynthetase Syndrome. J. Rheumatol. 2016, 44, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tansley, S.L.; Snowball, J.; Pauling, J.D.; Lissina, A.; Kuwana, M.; Rider, L.G.; Ronnelid, J.; McHugh, N.J. Assessment International Myositis, and Group Clinical Studies Group Myositis Autoantibody Scientific Interest. “The Promise, Perceptions, and Pitfalls of Immunoassays for Autoantibody Testing in Myositis”. Arthritis Res. Ther. 2020, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Piette, Y.; de Sloovere, M.; VandenDriessche, S.; Dehoorne, J.; de Bleecker, J.L.; van Praet, L.; Mijnsbrugge, A.-S.V.; de Schepper, S.; Jacques, P.; de Keyser, F.; et al. Pitfalls in the detection of myositis specific antibodies by lineblot in clinically suspected idiopathic inflammatory myopathy. Clin. Exp. Rheumatol. 2019, 38, 212–219. [Google Scholar]
- Mahler, M.; Fritzler, M.J. Detection of myositis-specific antibodies: Additional notes. Ann. Rheum. Dis. 2018, 78, e45. [Google Scholar] [CrossRef] [PubMed]
- Lackner, A.; Tiefenthaler, V.; Mirzayeva, J.; Posch, F.; Rossmann, C.; Kastrati, K.; Radner, H.; Demel, U.; Gretler, J.; Stotz, M.; et al. The use and diagnostic value of testing myositis-specific and myositis-associated autoantibodies by line immuno-assay: A retrospective study. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720–20975907. [Google Scholar] [CrossRef] [PubMed]
- Tansley, S.L.; Li, D.; Betteridge, Z.E.; McHugh, N.J. The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology 2020, 59, 2109–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahler, M.; Meroni, P.L.; Bossuyt, X.; Fritzler, M.J. Current Concepts and Future Directions for the Assessment of Autoantibodies to Cellular Antigens Referred to as Anti-Nuclear Antibodies. J. Immunol. Res. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mecoli, C.A.; Albayda, J.; Tiniakou, E.; Paik, J.J.; Zahid, U.; Danoff, S.K.; Casciola-Rosen, L.; Casal-Dominguez, M.; Pak, K.; Pinal-Fernandez, I.; et al. Myositis Autoantibodies: A Comparison of Results From the Oklahoma Medical Research Foundation Myositis Panel to the Euroimmun Research Line Blot. Arthritis Rheumatol. 2020, 72, 192–194. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol. 2017, 69, 2271–2282. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef]
- Malaviya, A.N. 2017 EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: Little emphasis on autoantibodies, why? Ann. Rheum. Dis. 2017, 77, e77. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Bottai, M.; Tjarnlund, A. Response To: ’Performance of the 2017 European League against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies in Clinical Practice’ by Hocevar Et Al. Ann. Rheum. Dis. 2018, 77, e91. [Google Scholar] [CrossRef] [Green Version]
- Mariampillai, K.; Granger, B.; Amelin, D.; Guiguet, M.; Hachulla, E.; Maurier, F.; Meyer, A.; Tohmé, A.; Charuel, J.-L.; Musset, L.; et al. Development of a New Classification System for Idiopathic Inflammatory Myopathies Based on Clinical Manifestations and Myositis-Specific Autoantibodies. JAMA Neurol. 2018, 75, 1528–1537. [Google Scholar] [CrossRef] [Green Version]
- Vulsteke, J.-B.; de Langhe, E.; Mahler, M. Autoantibodies at the Center of (sub)Classification-Issues of Detection. JAMA Neurol. 2019, 76, 867–868. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Labrador-Horrillo, M.; Solans-Laqué, R.; Simeón-Aznar, C.P.; Martínez-Gómez, X.; Vilardell-Tarrés, M. Myositis-specific and myositis-associated antibodies in a series of eighty-eight mediterranean patients with idiopathic inflammatory myopathy. Arthritis Rheum. 2006, 55, 791–798. [Google Scholar] [CrossRef]
- Richards, M.; LA Torre, I.G.-D.; González-Bello, Y.C.; Mercado, M.V.-D.; Andrade-Ortega, L.; Medrano-Ramírez, G.; Navarro-Zarza, J.E.; Maradiaga-Ceceña, M.; Loyo, E.; Rojo-Mejía, A.; et al. Autoantibodies to Mi-2 alpha and Mi-2 beta in patients with idiopathic inflammatory myopathy. Rheumatology 2019, 58, 1655–1661. [Google Scholar] [CrossRef]
- Mahler, M.; Betteridge, Z.; Bentow, C.; Richards, M.; Seaman, A.; Chinoy, H.; McHugh, N. Comparison of Three Immunoassays for the Detection of Myositis Specific Antibodies. Front. Immunol. 2019, 10, 848. [Google Scholar] [CrossRef] [Green Version]
- Cavazzana, I.; Richards, M.; Bentow, C.; Seaman, A.; Fredi, M.; Giudizi, M.; Palterer, B.; Pratesi, F.; Migliorini, P.; Franceschini, F.; et al. Evaluation of a novel particle-based assay for detection of autoantibodies in idiopathic inflammatory myopathies. J. Immunol. Methods 2019, 474, 112661. [Google Scholar] [CrossRef]
- Agresti, A. On logit confidence intervals for the odds ratio with small samples. Biometrics 1999, 55, 597–602. [Google Scholar] [CrossRef]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Vulsteke, J.-B.; Bossuyt, X.; de Langhe, E.; Satoh, M. Standardisation of myositis-specific antibodies: Where are we today? Ann. Rheum. Dis. 2021, 80, e132. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, I.E.; de Visser, M.; Werth, V.P. Classification of myositis. Nat. Rev. Rheumatol. 2018, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Yoo, I.S.; Kim, J.; Kang, S.W.; Kwon, M.; Joung, C.-I.; Choi, I.A.; Chang, S.H.; Kang, M.I.; Hong, S.-J.; et al. Comparison of the 2017 EULAR/ACR Criteria with Clinicoserologic Criteria for the Classification of Idiopathic Inflammatory Myopathies in Korean Patients. Yonsei Med. J. 2021, 62, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Vulsteke, J.-B.; de Langhe, E.; Claeys, K.; Dillaerts, D.; Poesen, K.; Lenaerts, J.; Westhovens, R.; van Damme, P.; Blockmans, D.; de Haes, P.; et al. Detection of myositis-specific antibodies. Ann. Rheum. Dis. 2018, 78, e7. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, A.; Milán, M.; Baucells, A.; Martínez, M.A.; Guillen, A.G.; Trallero-Araguás, E.; Alvarado-Cardenas, M.; Martínez-Martínez, L.; Alserawan, L.; Franco-Leyva, T.; et al. Anti-Tif-1γ Antibody Detection Using a Commercial Kit Vs in-House Immunoblot: Usefulness in Clinical Practice. Front. Immunol. 2021, 11, 625896. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Malyavantham, K.; Fritzler, M.J.; Satoh, M. Comment on: The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology 2021, 60, e35–e37. [Google Scholar] [CrossRef]
- To, F.; Ventín-Rodríguez, C.; Elkhalifa, S.; Lilleker, J.B.; Chinoy, H. Line blot immunoassays in idiopathic inflammatory myopathies: Retrospective review of diagnostic accuracy and factors predicting true positive results. BMC Rheumatol. 2020, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Ghirardello, A.; Rampudda, M.; Ekholm, L.; Bassi, N.; Tarricone, E.; Zampieri, S.; Zen, M.; Vattemi, G.; Lundberg, I.; Doria, A. Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology 2010, 49, 2370–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampeli, E.; Cinoku, I.I.; Mavragani, C.P.; Skopouli, F.N.; Moutsopoulos, H.M. Clinical Significance of Higher Cutoffs for Myositis Autoantibody Positivity Using the Euroimmun Research Line Blot: Comment on the Article by Mecoli et al. Arthritis Rheumatol. 2020, 72, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Lilleker, J.B.; Vencovsky, J.; Wang, G.; Wedderburn, L.; Diederichsen, L.P.; Schmidt, J.; Oakley, P.; Benveniste, O.; Danieli, M.G.; Danko, K.; et al. The EuroMyositis registry: An international collaborative tool to facilitate myositis research. Ann. Rheum. Dis. 2018, 77, 30–39. [Google Scholar] [CrossRef]
- Sheldon, J.; Dellavance, A. Strategies for Building Reference Standards for Autoantibodies. Front. Immunol. 2015, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.F.; Bossuyt, X. Standardization and harmonization of autoimmune diagnostics. Clin. Chem. Lab. Med. 2018, 56, 1563–1567. [Google Scholar] [CrossRef]
- Betteridge, Z.; Tansley, S.; Shaddick, G.; Chinoy, H.; Cooper, R.; New, R.; Lilleker, J.; Vencovsky, J.; Chazarain, L.; Danko, K.; et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J. Autoimmun. 2019, 101, 48–55. [Google Scholar] [CrossRef]
- Picard, C.; Vincent, T.; Lega, J.-C.; Hue, S.; Fortenfant, F.; Lakomy, D.; Humbel, R.-L.; Goetz, J.; Molinari, N.; Bardin, N.; et al. Heterogeneous clinical spectrum of anti-SRP myositis and importance of the methods of detection of anti-SRP autoantibodies: A multicentric study. Immunol. Res. 2016, 64, 677–686. [Google Scholar] [CrossRef]
- Aguila, L.A.; Lopes, M.R.U.; Pretti, F.Z.; Sampaio-Barros, P.D.; de Souza, F.H.C.; Borba, E.F.; Shinjo, S.K. Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin. Rheumatol. 2014, 33, 1093–1098. [Google Scholar] [CrossRef]
- Milam, E.C.; Futran, J.; Franks, A.G., Jr. Anti-MDA5 Antibody Dermatomyositis Overlap with Systemic Lupus Erythematosus: A Case Report and Review of the Literature. Open Rheumatol. J. 2016, 10, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Toquet, S.; Granger, B.; Uzunhan, Y.; Mariampillai, K.; Nunes, H.; Benveniste, O.; Allenbach, Y. The seasonality of Dermatomyositis associated with anti-MDA5 antibody: An argument for a respiratory viral trigger. Autoimmun. Rev. 2021, 20, 102788. [Google Scholar] [CrossRef]
- Berger, J.; Volc, S. Autoantibodies in Covid19-a Model for Viral Induced Autoimmunity. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e571–e573. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, M.; Lu, Z.; Li, C.; Zeng, Y.; Fan, J.; Yu, K. Multiple Neurological Manifestations in a Patient with Systemic Lupus Erythematosus and Anti-Nxp2-Positive Myositis: A Case Report. Medicine 2021, 100, e25063. [Google Scholar] [CrossRef] [PubMed]
- Pinal-Fernandez, I.; Pak, K.; Casal-Dominguez, M.; Hosono, Y.; Mecoli, C.; Christopher-Stine, L.; Mammen, A.L. Validation of anti-Mi2 autoantibody testing by line blot. Autoimmun. Rev. 2019, 19, 102425. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, F.; Babačić, H.; Eichhorn, P.; Schoser, B. Evaluating the diagnostic utility of new line immunoassays for myositis antibodies in clinical practice: A retrospective study. J. Neurol. 2019, 266, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Platteel, A.C.; Wevers, B.A.; Lim, J.; Bakker, J.A.; Bontkes, H.J.; Curvers, J.; Damoiseaux, J.; Heron, M.; de Kort, G.; Limper, M.; et al. Frequencies and clinical associations of myositis-related antibodies in The Netherlands: A one-year survey of all Dutch patients. J. Transl. Autoimmun. 2019, 2, 100013. [Google Scholar] [CrossRef]
- Bossuyt, X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmun. Rev. 2009, 8, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Rossin, B.; Kubassova, O. Augmented versus artificial intelligence for stratification of patients with myositis. Ann. Rheum. Dis. 2020, 79, e162. [Google Scholar] [CrossRef] [Green Version]
- Mahler, M.; Miller, F.; Fritzler, M.J. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: A comprehensive review. Autoimmun. Rev. 2014, 13, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Muro, Y.; Yamano, Y.; Yoshida, K.; Oto, Y.; Nakajima, K.; Mitsuma, T.; Kikuchi, S.; Matsumae, A.; Ogawa-Momohara, M.; Takeichi, T.; et al. Immune Recognition of Lysyl-Trna Synthetase and Isoleucyl-Trna Synthetase by Anti-Oj Antibody-Positive Sera. J. Autoimmun. 2021, 122, 102680. [Google Scholar] [CrossRef]
- Vulsteke, J.-B.; Satoh, M.; Malyavantham, K.; Bossuyt, X.; de Langhe, E.; Mahler, M. Anti-OJ autoantibodies: Rare or underdetected? Autoimmun. Rev. 2019, 18, 658–664. [Google Scholar] [CrossRef] [PubMed]
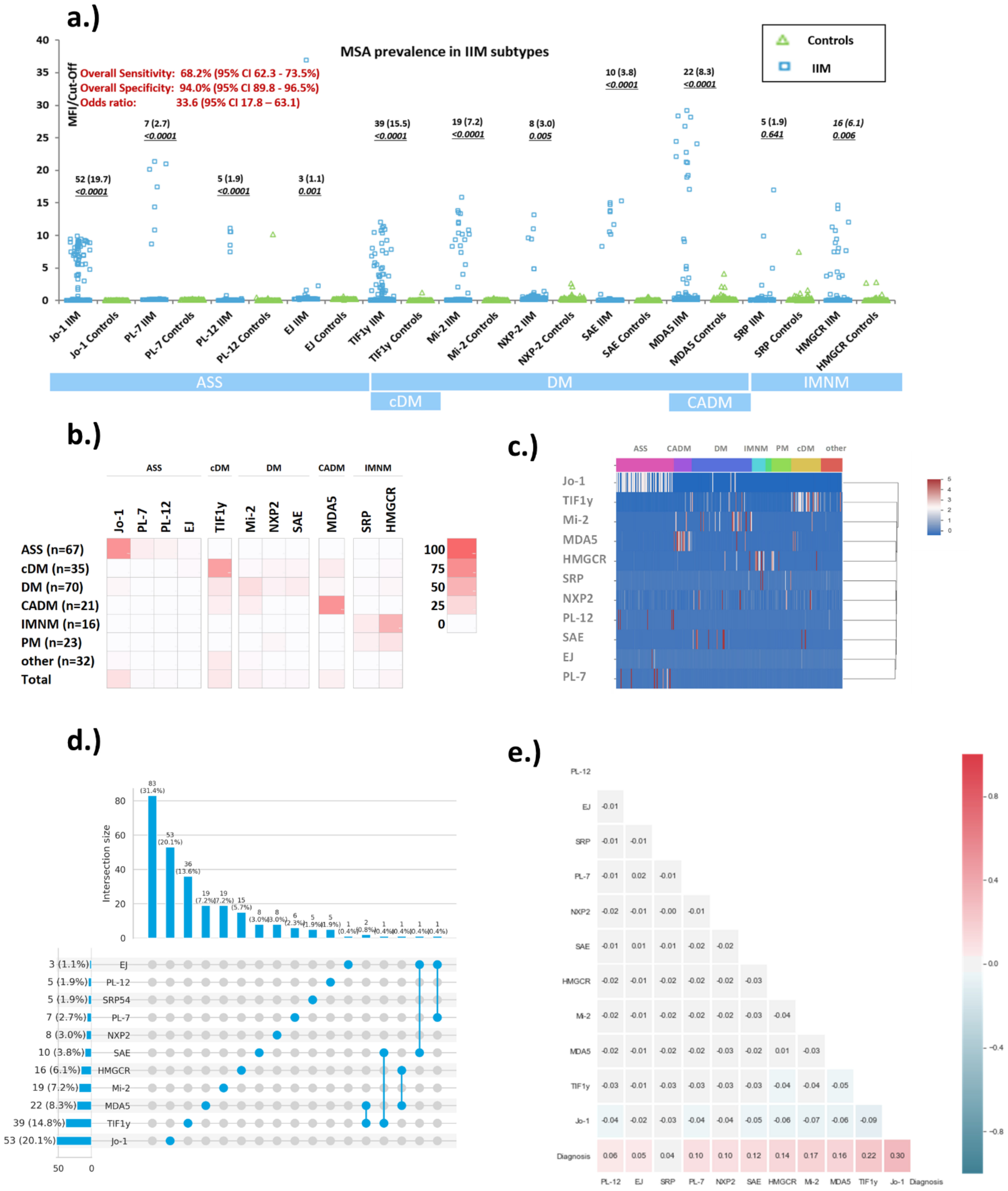
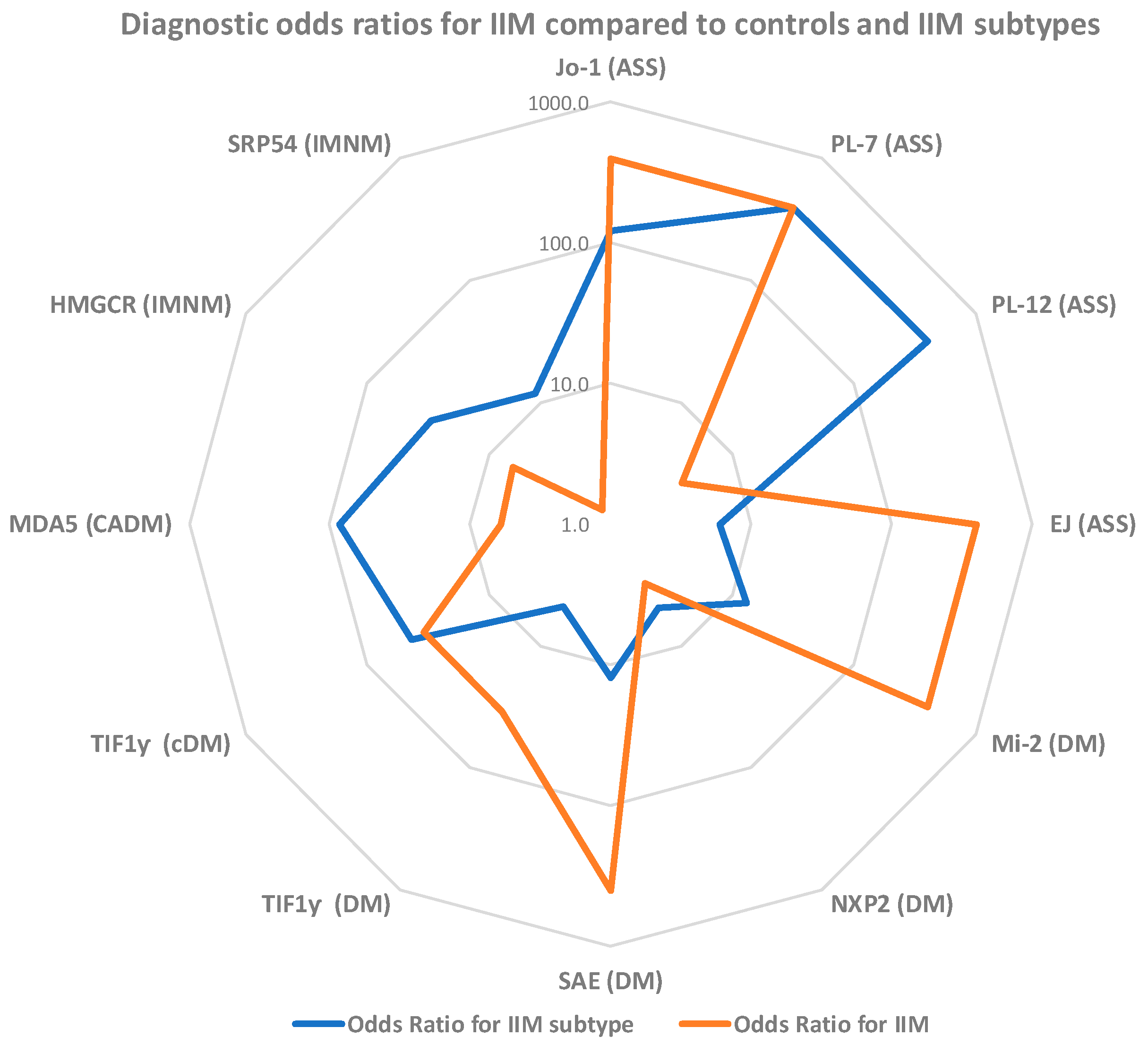
| IIM MSA | Sensitivity n pos, % pos (95% CI) | Specificity n pos, % pos (95% CI | Odds Ratio (95% CI) |
|---|---|---|---|
| Jo-1 | 52/264 19.7 (15.3–24.9) | 0/200 100.0 (98.1–100.0) | 98.1 (6.0–1600.3) |
| PL-7 | 7/264 2.7 (1.3–5.4) | 0/200 100.0 (98.1–100.0) | 27.2 (0.3–2329.2) |
| PL-12 | 5/264 1.9 (0.8–4.4) | 1/200 99.5 (97.2–99.9) | 3.8 (0.6–25.0) |
| EJ | 3/264 1.1 (0.4–3.3) | 0/200 100.0 (98.1–100.0) | 11.5 (0.1–1066.4) |
| Mi-2 | 19/264 7.2 (4.7–11.0) | 0/200 100.0 (98.1–100.0) | 77.6 (0.9–6377.9) |
| NXP2 | 8/264 3.0 (1.5–5.9) | 2/200 99.0 (96.4–99.7) | 3.1 (0.7–13.0) |
| SAE | 10/264 3.8 (2.1–6.8) | 0/200 100.0 (98.1–100.0) | 39.4 (0.5–3304.9) |
| TIF1γ | 39/264 15.5 (11.0–19.6) | 1/200 99.5 (97.2–99.9) | 34.5 (5.9–200.6) |
| MDA5 | 22/264 8.3 (5.6–12.3) | 3/200 98.5 (95.7–99.5) | 6.0 (1.9–19.0) |
| HMGCR | 16/264 6.1 (1.6–23.5) | 2/200 99.0 (96.4–99.7) | 6.4 (1.6–25.2) |
| SRP | 5/264 1.9 (0.8–4.4) | 3/200 98.5 (95.7–99.5) | 1.3 (0.3–4.9) |
| MSA IIM Subtype | Sensitivity IIM Subtype n pos, % ( 95% CI) | Specificity IIM Subtype n pos, % (95% CI) | Odds Ratio (95% CI) |
|---|---|---|---|
| Jo-1 (ASS) | 48/67 71.6 (59.9–81.0) | 4/197 98.0 (94.9–99.2) | 121.9 (40.8–360.0) |
| PL-7 (ASS) | 7/67 10.4 (5.2–20.0) | 0/197 100.0 (98.1–100.0) | 45.9 (2.6–821.9) |
| PL-12 (ASS) | 5/67 7.5 (3.2–16.3) | 0/197 100.0, 98.1–100.0 | 79.4 (0.9–6999.1) |
| EJ (ASS) | 2/67 3.0 (0.8–10.2) | 1/197 99.5 (97.2–99.9) | 6.0 (0.7–46.8) |
| Mi-2 (DM) | 15/70 21.4 (13.4–32.4) | 4/194 97.9 (94.8–99.2) | 13.0 (4.3–38.7) |
| NXP2 (DM) | 5/70 7.1 (3.1–15.7) | 3/194 98.5 (95.6–99.5) | 4.9 (1.3–19.1) |
| SAE (DM) | 8/70 11.4 (5.9–21.0) | 2/194 99.0 (96.3–99.7) | 12.4 (2.9–52.9) |
| TIF1γ (DM) | 11/70 15.7 (9.0- 26.0) | 28/194 85.6 (79.9–89.8) | 4.8 (1.7–13.0) |
| TIF1γ (cDM ) | 22/35 62.9 (46.3–76.8) | 17/229 92.6 (88.4–95.3) | 43.2 (15.1–122.8) |
| MDA5 (CADM) | 15/21 71.4 (50.0–86.2) | 7/243 97.1 (94.2–98.6) | 84.3 (25.7–277.5) |
| HMGCR (IMNM) | 8/16 50.0 (28.0–72.0) | 8/248 96.8 (93.8–98.4) | 30.0 (9.2–98.2) |
| SRP (IMNM) | 2/16 12.5 (35.0–36.0) | 3/248 98.8 (96.5–99.6) | 11.7 (2.15–64.41) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahler, M.; Malyavantham, K.; Seaman, A.; Bentow, C.; Anunciacion-Llunell, A.; Sanz-Martínez, M.T.; Viñas-Gimenez, L.; Selva-O’Callaghan, A. Profiling of Myositis Specific Antibodies and Composite Scores as an Aid in the Differential Diagnosis of Autoimmune Myopathies. Diagnostics 2021, 11, 2246. https://doi.org/10.3390/diagnostics11122246
Mahler M, Malyavantham K, Seaman A, Bentow C, Anunciacion-Llunell A, Sanz-Martínez MT, Viñas-Gimenez L, Selva-O’Callaghan A. Profiling of Myositis Specific Antibodies and Composite Scores as an Aid in the Differential Diagnosis of Autoimmune Myopathies. Diagnostics. 2021; 11(12):2246. https://doi.org/10.3390/diagnostics11122246
Chicago/Turabian StyleMahler, Michael, Kishore Malyavantham, Andrea Seaman, Chelsea Bentow, Ariadna Anunciacion-Llunell, María Teresa Sanz-Martínez, Laura Viñas-Gimenez, and Albert Selva-O’Callaghan. 2021. "Profiling of Myositis Specific Antibodies and Composite Scores as an Aid in the Differential Diagnosis of Autoimmune Myopathies" Diagnostics 11, no. 12: 2246. https://doi.org/10.3390/diagnostics11122246
APA StyleMahler, M., Malyavantham, K., Seaman, A., Bentow, C., Anunciacion-Llunell, A., Sanz-Martínez, M. T., Viñas-Gimenez, L., & Selva-O’Callaghan, A. (2021). Profiling of Myositis Specific Antibodies and Composite Scores as an Aid in the Differential Diagnosis of Autoimmune Myopathies. Diagnostics, 11(12), 2246. https://doi.org/10.3390/diagnostics11122246







