Two Needle Passes Achieve Similar Diagnostic Yield Compared to Three Passes Regarding Diagnosis of Solid Pancreatic Lesions in Endoscopic Ultrasound-Guided Fine Needle Aspiration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participating Centers
2.2. Inclusion and Exclusion Criteria
2.3. EUS-FNA Procedure Performance
2.4. Cytologic Analysis
2.5. Study Definitions
2.6. Study Endpoints
2.7. Statistical Analyses
2.8. Ethical Approval
3. Results
3.1. Endpoints
3.1.1. Primary Endpoint
3.1.2. Secondary Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papanikolaou, I.S. Quality in pancreatic endoscopic ultrasound: What’s new in 2020? Ann. Gastroenterol. 2020, 33, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H.; Matsui, T.; Yabuuchi, Y.; Imai, K.; Tanaka, M.; Kakushima, N.; Sasaki, K.; Ono, H. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J. Gastroenterol. 2016, 22, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Wani, S.; Triantafyllou, K.; Tziatzios, G.; Cannizzaro, R.; Muscatiello, N.; Singh, S. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2019, 90, 893–903.e7. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Garcia, J.; Dominguez-Munoz, J.E.; Abdulkader, I.; Larino-Noia, J.; Eugenyeva, E.; Lozano-Leon, A.; Forteza-Vila, J. Influence of On-Site Cytopathology Evaluation on the Diagnostic Accuracy of Endoscopic Ultrasound-Guided Fine Needle Aspiration (EUS-FNA) of Solid Pancreatic Masses. Am. J. Gastroenterol. 2011, 106, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, M.; Jenssen, C.C.; Kaye, P.V.; Carrara, S.; Deprez, P.; Ginès, A.; Fernández-Esparrach, G.G.; Eisendrath, P.; Aithal, G.P.; Arcidiacono, P.P.; et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline – March 2017. Endoscopy 2017, 49, 989–1006. [Google Scholar] [CrossRef] [Green Version]
- Möller, K.; Papanikolaou, I.S.; Toermer, T.; Delicha, E.M.; Sarbia, M.; Schenck, U.; Koch, M.; Al-Abadi, H.; Meining, A.; Schmidt, H.; et al. EUS-guided FNA of solid pancreatic masses: High yield of 2 passes with combined histologic-cytologic analysis. Gastrointest. Endosc. 2009, 70, 60–69. [Google Scholar] [CrossRef]
- Suzuki, R.; Irisawa, A.; Bhutani, M.S.; Hikichi, T.; Takagi, T.; Sato, A.; Sato, M.; Ikeda, T.; Watanabe, K.; Nakamura, J.; et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig. Endosc. 2012, 24, 452–456. [Google Scholar] [CrossRef]
- Uehara, H.; Sueyoshi, H.; Takada, R.; Fukutake, N.; Katayama, K.; Ashida, R.; Ioka, T.; Takenaka, A.; Nagata, S.; Tomita, Y. Optimal number of needle passes in endoscopic ultrasound-guided fine needle aspiration for pancreatic lesions. Pancreatol. 2015, 15, 392–396. [Google Scholar] [CrossRef]
- Cherian, P.T.; Mohan, P.; Douiri, A.; Taniere, P.; Hejmadi, R.K.; Mahon, B.S. Role of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic and peripancreatic lesions: Is onsite cytopathology necessary? HPB 2010, 12, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, R.A.; Sayage-Rabie, L.; Beissner, R. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest. Endosc. 2000, 51, 184–190. [Google Scholar] [CrossRef]
- Ge, P.S.; Wani, S.; Watson, R.R.; Sedarat, A.; Kim, S.; Marshall, C.; Wilson, R.H.; Makker, J.; Mohamadnejad, M.; Komanduri, S.; et al. Per-Pass Performance Characteristics of Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Malignant Solid Pancreatic Masses in a Large Multicenter Cohort. Pancreas 2018, 47, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Muthusamy, V.R.; Komanduri, S. EUS-guided tissue acquisition: An evidence-based approach (with videos). Gastrointest. Endosc. 2014, 80, 939–959.e7. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; Mullady, D.; Early, D.S.; Collins, B.; Marshall, C.; Sams, S.; Yen, R.; Rizeq, M.; Romanas, M.; Nawaz, S.; et al. Increasing Number of Passes Beyond 4 Does Not Increase Sensitivity of Detection of Pancreatic Malignancy by Endoscopic Ultrasound–Guided Fine-Needle Aspiration. Clin. Gastroenterol. Hepatol. 2017, 15, 1071–1078.e2. [Google Scholar] [CrossRef] [PubMed]
- Eloubeidi, M.A.; Tamhane, A. EUS-guided FNA of solid pancreatic masses: A learning curve with 300 consecutive procedures. Gastrointest. Endosc. 2005, 61, 700–708. [Google Scholar] [CrossRef]
- Wani, S.; Coté, G.A.; Keswani, R.; Mullady, D.; Azar, R.; Murad, F.; Edmundowicz, S.; Komanduri, S.; McHenry, L.; Al-Haddad, M.A.; et al. Learning curves for EUS by using cumulative sum analysis: Implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest. Endosc. 2013, 77, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.P.; Maple, J.T.; Zhang, Q.; Ylagan, L.R.; Zhai, J.; Kohlmeier, C.; Jonnalagadda, S.; Early, D.S.; Edmundowicz, S.A.; Azar, R.R. Reliability of gross visual assessment of specimen adequacy during EUS-guided FNA of pancreatic masses. Gastrointest. Endosc. 2009, 69, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Harewood, G.C.; Wiersema, L.M.; Halling, A.C.; Keeney, G.L.; Salamao, D.R.; Wiersema, M.J. Influence of EUS training and pathology interpretation on accuracy of EUS-guided fine needle aspiration of pancreatic masses. Gastrointest. Endosc. 2002, 55, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Hupp, M.M.; Khan, S.; Dincer, H.E.; Mallery, J.S.; Shyne, M.T.; Mettler, T.; Stewart, J.; Amin, K. Evaluation and Comparison of Performance Parameters and Impact of Telepathology and Operator Experience on Endobronchial and Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Am. J. Clin. Pathol. 2021, 155, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Weston, B.R.; Bhutani, M.S. Optimizing Diagnostic Yield for EUS-Guided Sampling of Solid Pancreatic Lesions: A Technical Review. Gastroenterol. Hepatol. 2013, 9, 352–363. [Google Scholar]
- Irisawa, A.; Yamabe, A.; Bhutani, M.S.; Shibukawa, G.; Fujisawa, M.; Sato, A.; Yoshida, Y.; Arakawa, N.; Ikeda, T.; Igarashi, R.; et al. Efforts to improve the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic tumors. Endosc. Ultrasound 2016, 5, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwashita, T.; Yasuda, I.; Mukai, T.; Doi, S.; Nakashima, M.; Uemura, S.; Mabuchi, M.; Shimizu, M.; Hatano, Y.; Hara, A.; et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study). Gastrointest. Endosc. 2015, 81, 177–185. [Google Scholar] [CrossRef]
- Park, C.; Kim, H.J.; Kim, S.Y.; Lee, S.S.; Byun, J.H.; Kim, S.C.; Kim, M.-H. Growth rate of serous pancreatic neoplasms in vivo: A retrospective, observational study. Acta Radiol. 2019, 60, 433–440. [Google Scholar] [CrossRef]
- Sugiura, R.; Kuwatani, M.; Hirata, K.; Sano, I.; Kato, S.; Kawakubo, K.; Sakamoto, N. Effect of Pancreatic Mass Size on Clinical Outcomes of Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Dig. Dis. Sci. 2019, 64, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Larghi, A.; Bernardoni, L.; Parisi, A.; Frulloni, L.; Gabbrielli, A.; Parcesepe, P.; Scarpa, A.; Manfrin, E. Touch imprint cytology on endoscopic ultrasound fine-needle biopsy provides comparable sample quality and diagnostic yield to standard endoscopic ultrasound fine-needle aspiration specimens in the evaluation of solid pancreatic lesions. Cytopathology 2019, 30, 179–186. [Google Scholar] [CrossRef]
- Pouliakis, A.; Karakitsou, E.; Margari, N.; Bountris, P.; Haritou, M.; Panayiotides, J.; Koutsouris, D.; Karakitsos, P. Artificial Neural Networks as Decision Support Tools in Cytopathology: Past, Present, and Future. Biomed. Eng. Comput. Biol. 2016, 7, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouw, R.E.; Barret, M.; Biermann, K.; Bisschops, R.; Czakó, L.; Gecse, K.B.; de Hertogh, G.; Hucl, T.; Iacucci, M.; Jansen, M.; et al. Endoscopic tissue sampling – Part 1: Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endosc. 2021, 53, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Wallace, M.B.; Cohen, J.; Pike, I.M.; Adler, D.G.; Kochman, M.L.; Lieb, J.G.; Park, W.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for EUS. Gastrointest. Endosc. 2015, 81, 67–80. [Google Scholar] [CrossRef]
- McQuaid, K.R.; Laine, L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest. Endosc. 2008, 67, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Erickson, R.A.; Sayage-Rabie, L.; Avots-Avotins, A. Clinical utility of endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 1997, 41, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- A Erickson, R.; Tretjak, Z. Clinical Utility of Endoscopic Ultrasound and Endoscopic Ultrasound-Guided Fine Needle Aspiration in Retroperitoneal Neoplasms. Am. J. Gastroenterol. 2000, 95, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef] [PubMed]

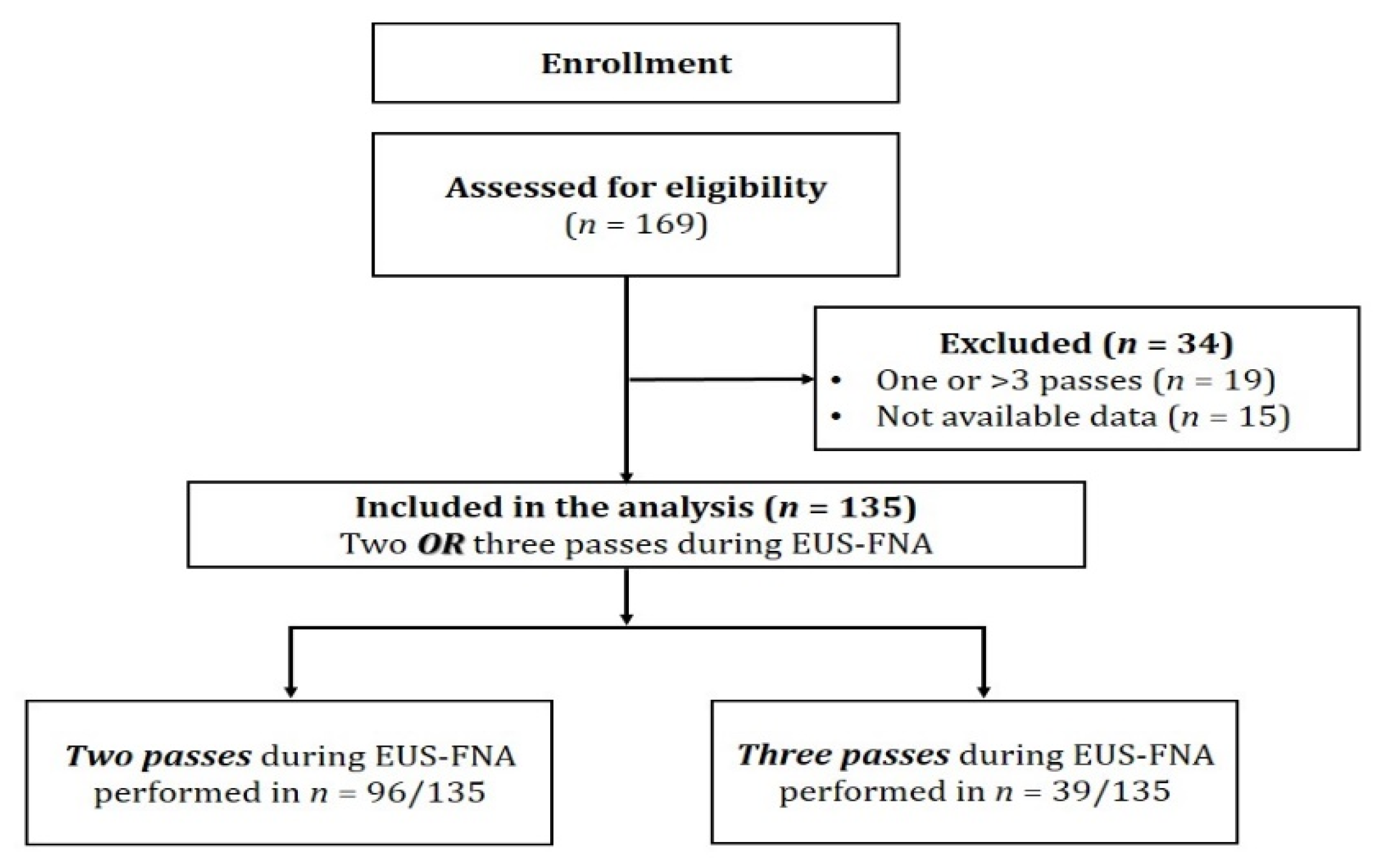
| All Patients (n = 135) | 2 Passes (n = 96) | 3 Passes (n = 39) | p * | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Gender (males) | 83 (61.5%) | 55 (57.3%) | 28 (71.8%) | 0.125 |
| Age (years) # | 66.9 ± 12.8 | 67.6 ± 13.3 | 65.2 ± 11.2 | 0.332 |
| Lesion location (head/body/tail) | 101 (74.8%)/24 (17.8%)/10 (7.4%) | 71 (74%)/19 (19.8%)/6 (6.3%) | 30 (76.9%)/5 (12.8%)/4 (10.3%) | 0.480 |
| Size of lesion (mm) # | 28.8 ± 11.9 | 27.9 ± 12.7 | 31.2 ± 9.7 | 0.115 |
| Procedural characteristics | ||||
| Needle type (22G) | 120 (88.9%) | 85 (88.5%) | 35 (89.7%) | 1.000 |
| Procedure time, mean (SD), minutes | 23.2 (8.7) | 22.2 (9.2) | 24.1 (8.1) | 0.19 |
| Propofol dose (mg) | 204.7 ± 88.7 | 188.7 ± 75.4 | 223.0 ± 100.7 | 0.210 |
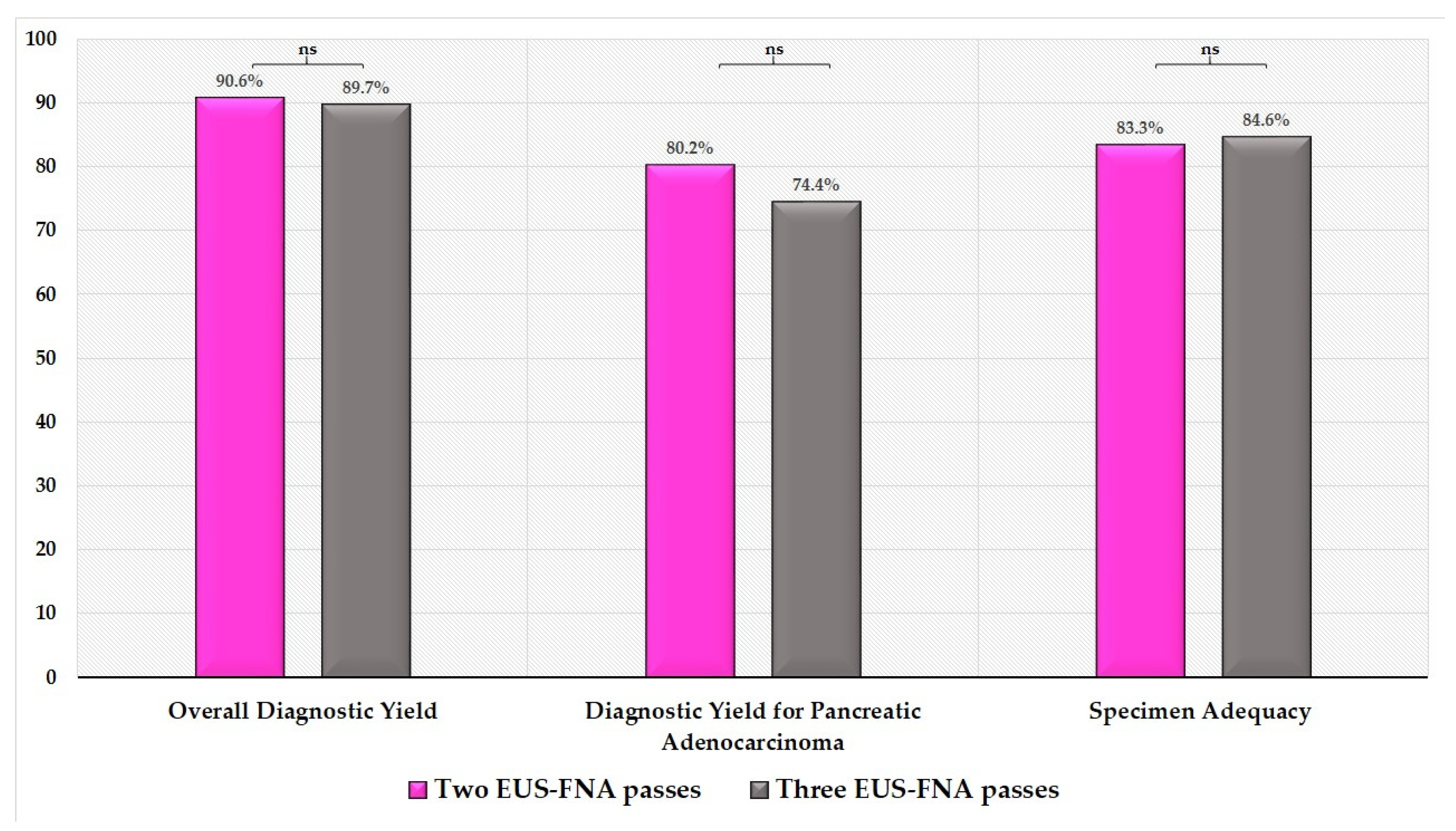
| Overall diagnostic yield | 122 (90.4%) | |||
| Diagnostic yield for adenocarcinoma | 106 (78.5%) | |||
| Specimen adequacy | 113 (83.7%) | |||
| Parameters | Overall Diagnostic Yield (OR, 95%CI) | p Value | Diagnostic Yield for Adenocarcinoma (OR, 95%CI) | p Value | Specimen Adequacy (OR, 95%CI) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 1.043 (0.999–1.089) | 0.063 | 1.033 (0.998–1.069) | 0.092 | 1.031 (0.994–1.069) | 0.056 |
| Gender (Female vs. Male) | 0.958 (0.275–3.342) | 0.844 | 1.117 (0.440–2.833) | 0.374 | 1.463 (0.521–4.108) | 0.687 |
| Lesion size | 1.010 (0.954–1.069) | 0.569 | 0.994 (0.954–1.035) | 0.591 | 1.008 (0.964–1.055) | 0.918 |
| Size (<20 mm vs. ≥20 mm) | 2.010 (0.254–11.069) | 0.419 | 2.214 (0.204–12.031) | 0.119 | 1.408 (0.104–11.05) | 0.180 |
| Lesion location (Body vs. Head) | 2.730 (0.346–23.599) | 0.195 | 3.156 (0.667–14.945) | 0.163 | 5.610 (0.682–46.126) | 0.179 |
| Lesion location (Tail vs. Head) | 0.275 (0.056–1.355) | 0.302 | 0.293 (0.072–1.189) | 0.275 | 0.328 (0.076–1.408) | 0.174 |
| Number of passes (3 vs. 2) | 1.614 (0.178–14.601) | 0.736 | 1.029 (0.389–2.726) | 0.827 | 1.423 (0.473–4.285) | 0.767 |
| Needle type (25G vs. 22G) | 2.243 (0.260–19.341) | 0.463 | 4.075 (0.495–3.531) | 0.205 | 3.126 (0.371–26.322) | 0.134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukoulioti, E.; Tziatzios, G.; Tadic, M.; Dimitriadis, S.; Gkolfakis, P.; Politi, E.; Stoos-Veic, T.; Turcic, P.; Chatzidakis, A.; Lazaridis, L.-D.; et al. Two Needle Passes Achieve Similar Diagnostic Yield Compared to Three Passes Regarding Diagnosis of Solid Pancreatic Lesions in Endoscopic Ultrasound-Guided Fine Needle Aspiration. Diagnostics 2021, 11, 2272. https://doi.org/10.3390/diagnostics11122272
Koukoulioti E, Tziatzios G, Tadic M, Dimitriadis S, Gkolfakis P, Politi E, Stoos-Veic T, Turcic P, Chatzidakis A, Lazaridis L-D, et al. Two Needle Passes Achieve Similar Diagnostic Yield Compared to Three Passes Regarding Diagnosis of Solid Pancreatic Lesions in Endoscopic Ultrasound-Guided Fine Needle Aspiration. Diagnostics. 2021; 11(12):2272. https://doi.org/10.3390/diagnostics11122272
Chicago/Turabian StyleKoukoulioti, Eleni, Georgios Tziatzios, Mario Tadic, Stavros Dimitriadis, Paraskevas Gkolfakis, Ekaterini Politi, Tajana Stoos-Veic, Petra Turcic, Alexandros Chatzidakis, Lazaros-Dimitrios Lazaridis, and et al. 2021. "Two Needle Passes Achieve Similar Diagnostic Yield Compared to Three Passes Regarding Diagnosis of Solid Pancreatic Lesions in Endoscopic Ultrasound-Guided Fine Needle Aspiration" Diagnostics 11, no. 12: 2272. https://doi.org/10.3390/diagnostics11122272
APA StyleKoukoulioti, E., Tziatzios, G., Tadic, M., Dimitriadis, S., Gkolfakis, P., Politi, E., Stoos-Veic, T., Turcic, P., Chatzidakis, A., Lazaridis, L.-D., Farmaki, M., Vezakis, A., Triantafyllou, K., Polydorou, A., & Papanikolaou, I. S. (2021). Two Needle Passes Achieve Similar Diagnostic Yield Compared to Three Passes Regarding Diagnosis of Solid Pancreatic Lesions in Endoscopic Ultrasound-Guided Fine Needle Aspiration. Diagnostics, 11(12), 2272. https://doi.org/10.3390/diagnostics11122272









