Challenging Cases of Aortic Prosthesis Dysfunction, the Importance of Multimodality Imaging, a Case Series
Abstract
:1. Introduction
2. Case Series
2.1. Case 1
2.2. Case 2
2.3. Case 3
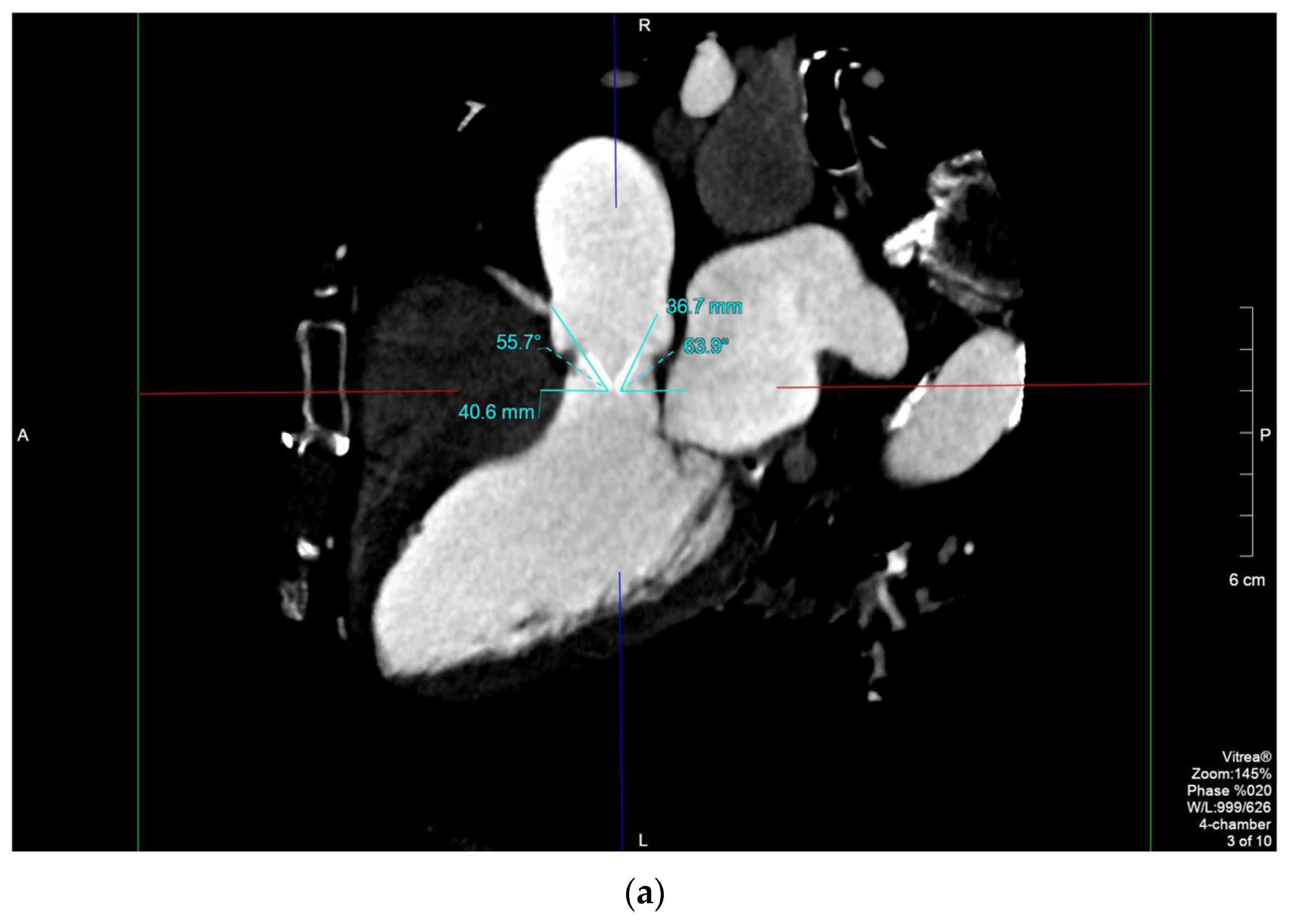
2.4. Case 4
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Manghat, N.E.; Rachapalli, V.; LINGEN, R.V.; Veitch, A.M.; Roobottom, C.A.; Morgan-Hughes, G.J. Imaging the heart valves using ECG-gated 64-detector row cardiac CT. Br. J. Radiol. 2008, 81, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Annabi, M.S.; Salaun, E.; Cosyns, B.; Lancellotti, P.; Pibarot, P. Multimodality Imaging Assessment of Prosthetic Aortic Valve. In Advances in Treatments for Aortic Valve and Root Diseases; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; Khandheria, B.K.; Levine, R.A.; Marx, G.R.; Miller, F.A.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 975–1014. [Google Scholar] [PubMed]
- Mahjoub, H.; Mathieu, P.; Larose, E.; Dahou, A.; Sénéchal, M.; Dumesnil, J.G.; Després, J.P.; Pibarot, P. Determinants of aortic bioprosthetic valve calcification assessed by multidetector CT. Heart 2015, 101, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Jilaihawi, H.; Asch, F.M.; Manasse, E.; Ruiz, C.E.; Jelnin, V.; Kashif, M.; Kawamori, H.; Maeno, Y.; Kazuno, Y.; Takahashi, N.; et al. Systematic CT Methodology for the Evaluation of Subclinical Leaflet Thrombosis. JACC Cardiovasc. Imaging 2017, 10, 461–470. [Google Scholar] [CrossRef]
- Makkar, R.R.; Chakravarty, T. Transcatheter aortic valve thrombosis; new problems, new insights. JACC Cardiovasc. Interv. 2017, 10, 698–700. [Google Scholar] [CrossRef]
- Tanis, W.; Habets, J.; Brink, R.B.A.V.D.; Symersky, P.; Budde, R.P.J.; Chamuleau, S.A.J. Differentiation of thrombus from pannus as the cause of acquired mechanical prosthetic heart valve obstruction by non-invasive imaging: A review of the literature. Eur. Heart J. Cardiovasc. Imaging 2013, 15, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Senapati, A.; Faza, N.N.; Mahmarian, J.; Chang, S.M. Cardiac Computed Tomography for Structural Heart Disease Assessment and Therapeutic Planning: Focus on Prosthetic Valve Dysfunction. Methodist Debakey Cardiovasc. J. 2020, 16, 86–96. [Google Scholar] [CrossRef]
- Habets, J.; Symersky, P.; van Herwerden, L.A.; de Mol, B.A.J.M.; Spijkerboer, A.M.; Mali, W.P.T.M.; Budde, R.P.J. Prosthetic heart valve assessment with multidetector-row CT: Imaging characteristics of 91 valves in 83 patients. Eur. Radiol. 2011, 21, 1390–1396. [Google Scholar] [CrossRef] [Green Version]
- Konen, E.; Goitein, O.; Feinberg, M.S.; Eshet, Y.; Raanani, E.; Rimon, U.; Di-Segni, E. The Role of ECG-Gated MDCT in the Evaluation of Aortic and Mitral Mechanical Valves: Initial Experience. Am. J. Roentgenol. 2008, 191, 26–31. [Google Scholar] [CrossRef]
- Budoff, M.J.; Achenbach, S.S.; Hecht, H.S.; Narula, J. Atlas of Cardiovascular Computed Tomography, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Rajiah, P.; Moore, A.; Saboo, S.; Goerne, H.; Ranganath, P.; MacNamara, J.; Joshi, P.; Abbara, S. Multimodality Imaging of Complications of Cardiac Valve Surgeries. Radio Graph. 2019, 39, 932–956. [Google Scholar] [CrossRef]
- Aladmawi, M.A.; Pragliola, C.; Vriz, O.; Galzerano, D. Use of multidetector-row computed tomography scan to detect pannus formation in prosthetic mechanical aortic valves. J. Thorac. Dis. 2017, 9, S343–S348. [Google Scholar] [CrossRef] [Green Version]
- Moss, A.J.; Dweck, M.R.; Dreisbach, J.G.; Williams, M.C.; Mak, S.M.; Cartlidge, T.; Nicol, E.D.; Morgan-Hughes, G.J. Complementary role of cardiac CT in the assessment of aortic valve replacement dysfunction. Open Heart 2016, 3, e000494. [Google Scholar] [CrossRef]
- Qanadli, S.D.; Jouannic, A.-M.; Dehmeshki, J.; Lu, T.-L. CT Attenuation Values of Blood and Myocardium: Rationale for Accurate Coronary Artery Calcifications Detection with Multi-Detector CT. PLoS ONE 2015, 10, e0124175. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Ha, H.; Kang, J.-W.; Kim, J.A.; Song, J.-K.; Kim, H.J.; Lim, T.-H.; Yang, D.H. Impact of pannus formation on hemodynamic dysfunction of prosthetic aortic valve: Pannus extent and its relationship to prosthetic valve motion and degree of stenosis. Clin. Res. Cardiol. 2018, 107, 554–564. [Google Scholar] [CrossRef]
- Tsuneyoshi, H.; Komiya, T.; Shimamoto, T. Accuracy of Aortic Annulus Diameter Measurement: Comparison of Multi-Detector CT, Two- and Three-Dimensional Echocardiography. J. Card. Surg. 2015, 31, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Schoenhagen, P. The role of computed tomography in pre-procedural planning of cardiovascular surgery and intervention. Insights Imaging 2013, 4, 671–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchá, D.; Chamuleau, S.A.J.; Symersky, P.; Meijs, M.F.L.; Brink, R.B.A.V.D.; De Mol, B.A.J.M.; Mali, W.P.T.M.; Habets, J.; Van Herwerden, L.A.; Budde, R.P.J. Baseline MDCT findings after prosthetic heart valve implantation provide important complementary information to echocardiography for follow-up purposes. Eur. Radiol. 2015, 26, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seng Low, S.C.; Attili, A.; Bach, D.; Agarwal, P.P. CT and MRI features of pseudoaneurysms of the mitral-aortic intervalvular fibrosa. Clin. Imaging 2018, 47, 74–79. [Google Scholar]
- Iramina, H.; Hamaguchi, T.; Nakamura, M.; Mizowaki, T.; Kanno, I. Metal artifact reduction by filter-based dual-energy cone-beam computed tomography on a bench-top micro-CBCT system: Concept and demonstration. J. Radiat. Res. 2018, 59, 511–520. [Google Scholar] [CrossRef]
- Stolzmann, P.; Scheffel, H.; Bettex, D.; Karlo, C.; Frauenfelder, T.; Prêtre, R.; Marincek, B.; Alkadhi, H. Subvalvular aortic stenosis: Comprehensive cardiac evaluation with dual-source computed tomography. J. Thorac. Cardiovasc. Surg. 2007, 134, 240–241.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podberesky, D.J.; Angel, E.; Yoshizumi, T.T.; Toncheva, G.; Salisbury, S.R.; Alsip, C.; Barelli, A.; Egelhoff, J.C.; Anderson-Evans, C.; Nguyen, G.B.; et al. Radiation Dose Estimation for Prospective and Retrospective ECG-Gated Cardiac CT Angiography in Infants and Small Children Using a 320-MDCT Volume Scanner. Am. J. Roentgenol. 2012, 199, 1129–1135. [Google Scholar] [CrossRef] [PubMed]







| 2D and 3D TEE | Cardiotriggered MDCT |
|---|---|
| Worse spatial resolution | Better spatial resolution |
| Better temporal resolution | Worse temporal resolution |
| Harmful for patients (esophageal tear, bleeding) | Harmful for patients (radio exposition, contrast nephropathy, allergic reactions) |
| Able to measure gradients | Unable to measure gradients |
| Unbale to evaluate extracardiac structures | Able to evaluate extracardiac structures (large FOV) |
| Bed side technique | Radiology department facility |
| Operator-dependent technique | More accurate measurement [15] |
| Invasive technique | Noninvasive technique |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergola, V.; Cabrelle, G.; De Conti, G.; Barbiero, G.; Mele, D.; Motta, R. Challenging Cases of Aortic Prosthesis Dysfunction, the Importance of Multimodality Imaging, a Case Series. Diagnostics 2021, 11, 2305. https://doi.org/10.3390/diagnostics11122305
Pergola V, Cabrelle G, De Conti G, Barbiero G, Mele D, Motta R. Challenging Cases of Aortic Prosthesis Dysfunction, the Importance of Multimodality Imaging, a Case Series. Diagnostics. 2021; 11(12):2305. https://doi.org/10.3390/diagnostics11122305
Chicago/Turabian StylePergola, Valeria, Giulio Cabrelle, Giorgio De Conti, Giulio Barbiero, Donato Mele, and Raffaella Motta. 2021. "Challenging Cases of Aortic Prosthesis Dysfunction, the Importance of Multimodality Imaging, a Case Series" Diagnostics 11, no. 12: 2305. https://doi.org/10.3390/diagnostics11122305
APA StylePergola, V., Cabrelle, G., De Conti, G., Barbiero, G., Mele, D., & Motta, R. (2021). Challenging Cases of Aortic Prosthesis Dysfunction, the Importance of Multimodality Imaging, a Case Series. Diagnostics, 11(12), 2305. https://doi.org/10.3390/diagnostics11122305







