Clinical Usefulness of Red Cell Distribution Width/Albumin Ratio to Discriminate 28-Day Mortality in Critically Ill Patients with Pneumonia Receiving Invasive Mechanical Ventilation, Compared with Lacate/Albumin Ratio: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Study Outcome and Definition
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
3.2. RDW/Albumin Ratio and Factors Related to a 28-Day Mortality
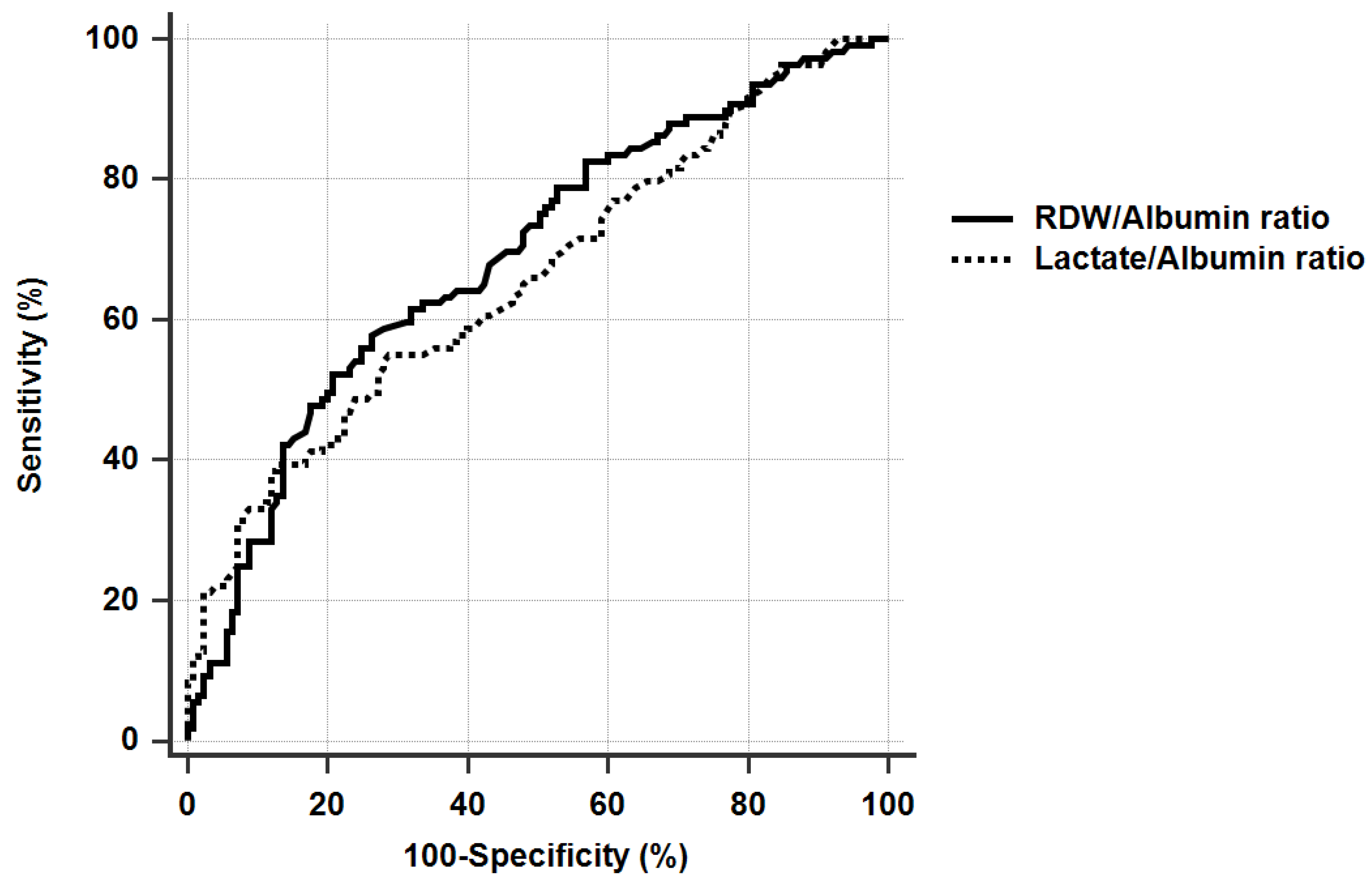
3.3. Determination of the Cut-Off Value for RDW/Albumin Ratio, and the Comparison with Lactate/Albumin Ratio for Discrimination of 28-Day Mortality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrado, R.E.; Lee, D.; Lucero, D.E.; Varma, J.K.; Vora, N.M. Burden of Adult Community-acquired, Health-care-Associated, Hospital-Acquired, and Ventilator-Associated Pneumonia: New York City, 2010 to 2014. Chest 2017, 152, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among US Adults. New Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, K.; Na, S.J.; Oh, D.K.; Park, S.; Choi, E.Y.; Kim, S.C.; Seong, G.M.; Heo, J.; Chang, Y.; Kwack, W.G.; et al. Characteristics, management and clinical outcomes of patients with sepsis: A multicenter cohort study in Korea. Acute Crit. Care 2019, 34, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Cilloniz, C.; Ferrer, M.; Liapikou, A.; Garcia-Vidal, C.; Gabarrus, A.; Ceccato, A.; De La Bellacasa, J.P.; Blasi, F.; Torres, A. Acute respiratory distress syndrome in mechanically ventilated patients with community-acquired pneumonia. Eur. Respir. J. 2018, 51, 1702215. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Blot, S.; Koulenti, D.; Dimopoulos, G.; Martin, C.; Komnos, A.; Krueger, W.A.; Spina, G.; Armaganidis, A.; Rello, J. Prevalence, Risk Factors, and Mortality for Ventilator-Associated Pneumonia in Middle-Aged, Old, and Very Old Critically Ill Patients*. Crit. Care Med. 2014, 42, 601–609. [Google Scholar] [CrossRef]
- Ferrer, M.; Travierso, C.; Cilloniz, C.; Gabarrus, A.; Ranzani, O.T.; Polverino, E.; Liapikou, A.; Blasi, F.; Torres, A. Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS ONE 2018, 13, e0191721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallazzi, R.; Furmanek, S.; Arnold, F.W.; Beavin, L.A.; Wunderink, R.G.; Niederman, M.S.; Ramirez, J.A. The Burden of Community-Acquired Pneumonia Requiring Admission to ICU in the United States. Chest 2020, 158, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Mizgerd, J.P. Respiratory Infection and the Impact of Pulmonary Immunity on Lung Health and Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Sibila, O.; Ferrer, M.; Mendendez, R.; Mensa, J.; Gabarrus, A.; Sellares, J.; Restrepo, M.; Anzueto, A.; Niederman, N.; et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. Acute Crit. Care 2015, 46, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Mizgerd, J.P. Inflammation and Pneumonia: Why Are Some More Susceptible than Others? Clin. Chest Med. 2018, 39, 669–676. [Google Scholar] [CrossRef]
- Sungurlu, S.; Balk, R.A. The Role of Biomarkers in the Diagnosis and Management of Pneumonia. Clin. Chest Med. 2018, 39, 691–701. [Google Scholar] [CrossRef]
- Saleh, M.A.; van de Garde, E.M.; van Hasselt, J.C. Host-response biomarkers for the diagnosis of bacterial respiratory tract infections. Clin. Chem. Lab. Med. 2019, 57, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Y.; Chen, H.-L.; Ni, S.-S. Red cell distribution width in predicting 30-day mortality in patients with pulmonary embolism. J. Crit. Care 2017, 37, 197–201. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Ding, Y.; Pu, L.; Liu, J.; Xie, J.; Cabanero, M.; Li, J.; Xiang, R.; Xiong, S. Prognostic value of RDW in cancers: A systematic review and meta-analysis. Oncotarget 2016, 8, 16027–16035. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Park, J.T.; Kim, E.J.; Han, J.H.; Han, J.S.; Choi, J.Y.; Han, S.H.; Yoo, T.-H.; Kim, Y.S.; Kang, S.-W.; et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care 2013, 17, R282. [Google Scholar] [CrossRef] [Green Version]
- Nathan, S.D.; Brown, A.W.; Reffett, T.R.; Shlobin, O.A.; Ahmad, S.; Weir, N.; Sheridan, M.J. The red cell distribution width as a prognostic indicator in idiopathic pulmonary fibrosis. Chest 2012, 143, 1692–1698. [Google Scholar] [CrossRef]
- Bazick, H.S.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Christopher, K.B. Red cell distribution width and all-cause mortality in critically ill patients *. Crit. Care Med. 2011, 39, 1913–1921. [Google Scholar] [CrossRef] [Green Version]
- Bateman, R.M.; Sharpe, M.D.; Singer, M.; Ellis, C.G. The Effect of Sepsis on the Erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.S.; Lopez, M.F.; Fleming, M.; Rivera, A.; Martin, F.M.; Welsh, M.L.; Boyd, A.; Doctrow, S.R.; Burakoff, S.J. SOD2-deficiency anemia: Protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood 2004, 104, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef]
- Aman, J.; van der Heijden, M.; van Lingen, A.; Girbes, A.R.; van Nieuw Amerongen, G.P.; van Hinsbergh, V.W.; Groeneveld, A.J. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit. Care Med. 2011, 39, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.; Kim, K.; Jo, Y.H.; Rhee, J.; Kim, T.Y.; Na, S.H.; Hwang, S.S. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J. Crit. Care 2011, 26, 287–294. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Bao, J.; Shang, Y.; Yin, L.; Yu, Y.; Xie, Y.; Chen, L.; Zheng, Y.; Xu, Y.; Gao, Z. The prognostic value of serum albumin levels and respiratory rate for community-acquired pneumonia: A prospective, multi-center study. PLoS ONE 2021, 16, e0248002. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, R.; Kabata, D.; Shimizu, K.; Hisano, S.; Ogura, H.; Shintani, A.; Shimazu, T. Serum albumin as a risk factor for death in patients with prolonged sepsis: An observational study. J. Crit. Care 2019, 51, 139–144. [Google Scholar] [CrossRef]
- Yin, M.; Si, L.; Qin, W.; Li, C.; Zhang, J.; Yang, H.; Han, H.; Zhang, F.; Ding, S.; Zhou, M.; et al. Predictive Value of Serum Albumin Level for the Prognosis of Severe Sepsis Without Exogenous Human Albumin Administration: A Prospective Cohort Study. J. Intensive Care Med. 2016, 33, 687–694. [Google Scholar] [CrossRef]
- Shin, J.; Hwang, S.Y.; Jo, I.J.; Kim, W.Y.; Ryoo, S.M.; Kang, G.H.; Kim, K.; Jo, Y.H.; Chung, S.P.; Joo, Y.S.; et al. Prognostic Value of The Lactate/Albumin Ratio for Predicting 28-Day Mortality in Critically ILL Sepsis Patients. Shock 2018, 50, 545–550. [Google Scholar] [CrossRef]
- Gharipour, A.; Razavi, R.; Gharipour, M.; Mukasa, D. Lactate/albumin ratio: An early prognostic marker in critically ill patients. Am. J. Emerg. Med. 2020, 38, 2088–2095. [Google Scholar] [CrossRef]
- Wang, B.; Chen, G.; Cao, Y.; Xue, J.; Li, J.; Wu, Y. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J. Crit. Care 2015, 30, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, X.Y.; Zhu, C.Q. Prognostic value of albumin-red cell distribution width score in patients with severe community-acquired pneumonia. Ann. Palliat. Med. 2020, 9, 759–765. [Google Scholar] [CrossRef]
- Force, A.D.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Rhodes, A.A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Lee, J.H.; Chung, H.J.; Kim, K.; Jo, Y.H.; Rhee, J.E.; Kim, Y.J.; Kang, K.W. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am. J. Emerg. Med. 2013, 31, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, Y.; Ying, B.; Cheng, B. Relation between Red Cell Distribution Width and Mortality in Critically Ill Patients with Acute Respiratory Distress Syndrome. BioMed Res. Int. 2019, 2019, 1942078. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhou, H.; Tang, Q. Red Blood Cell Distribution Width: A Novel Predictive Indicator for Cardiovascular and Cerebrovascular Diseases. Dis. Markers 2017, 2017, 7089493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirahara, N.; Tajima, Y.; Fujii, Y.; Kaji, S.; Yamamoto, T.; Hyakudomi, R.; Taniura, T.; Kawabata, Y. Comprehensive Analysis of Red Blood Cell Distribution Width as a Preoperative Prognostic Predictor in Gastric Cancer. Anticancer Res. 2019, 39, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Finfer, S.; Bellomo, R.; Boyce, N.; French, J.; Myburgh, J.; Norton, R. A Comparison of Albumin and Saline for Fluid Resuscitation in the Intensive Care Unit. N. Engl. J. Med. 2004, 350, 2247–2256. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.-J.; E A Orellanajimenez, C.; Melot, C.; De Backer, D.; Berre, J.; Leeman, M.; Brimioulle, S.; Appoloni, O.; Creteur, J.; Vincent, J.-L. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study*. Crit. Care Med. 2006, 34, 2536–2540. [Google Scholar] [CrossRef]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef] [Green Version]
- Lichtenauer, M.; Wernly, B.; Ohnewein, B.; Franz, M.; Kabisch, B.; Muessig, J.; Masyuk, M.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. The Lactate/Albumin Ratio: A Valuable Tool for Risk Stratification in Septic Patients Admitted to ICU. Int. J. Mol. Sci. 2017, 18, 1893. [Google Scholar] [CrossRef] [Green Version]
- Van Tienhoven, A.J.; van Beers, C.A.J.; Siegert, C.E.H. Agreement between arterial and peripheral venous lactate levels in the ED: A systematic review. Am. J. Emerg. Med. 2019, 37, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Shadvar, K.; Sanaie, S.; Golzari, S.E.; Parthvi, R.; Hamishehkar, H.; Nader, N.D. Arterial vs venous lactate: Correlation and predictive value of mortality of patients with sepsis during early resuscitation phase. J. Crit. Care 2020, 58, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Theerawit, P.; Na Petvicharn, C.; Tangsujaritvijit, V.; Sutherasan, Y. The Correlation Between Arterial Lactate and Venous Lactate in Patients With Sepsis and Septic Shock. J. Intensive Care Med. 2018, 33, 116–120. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Ju, S.; Lee, S.J.; Cho, Y.J.; Lee, J.D.; Kim, H.C. Red cell distribution width/albumin ratio is associated with 60-day mortality in patients with acute respiratory distress syndrome. Infect. Dis. 2020, 52, 266–270. [Google Scholar] [CrossRef]

| Variables | Total | Survivor | Non-Survivors | p-Value |
|---|---|---|---|---|
| N = 234 | N = 125 | N = 109 | ||
| Age, years old | 76 (64.8–81) | 76 (61.5–80) | 76 (65.5–81) | 0.323 |
| Gender, male | 171 (73.1) | 83 (66.4) | 88 (80.7) | 0.014 |
| BMI, (kg/m2) | 20.9 (18.5–23.3) | 21.3 (18.3–23.7) | 20.8 (18.6–23) | 0.684 |
| Diabetes mellitus | 95 (40.6) | 47 (37.6) | 48 (44) | 0.317 |
| Chronic kidney disease | 31 (12.5) | 16 (12.8) | 15 (13.8) | 0.829 |
| Chronic heart failure | 25 (10.7) | 13 (10.4) | 12 (11) | 0.880 |
| Chronic liver disease | 28 (12) | 9 (7.2) | 19 (17.4) | 0.016 |
| Cerebrovascular disease | 49 (20.9) | 28 (22.4) | 21 (19.3) | 0.557 |
| COPD | 43 (18.4) | 27 (21.6) | 16 (14.7) | 0.173 |
| ILD | 12 (5.1) | 5 (4) | 7 (6.4) | 0.402 |
| APACHE II | 24 (19–28) | 22 (17–25) | 27 (22–33) | <0.001 |
| SOFA | 11 (8–13) | 9 (7–11) | 13 (10–15) | <0.001 |
| Types of pneumonia | ||||
| CAP | 117 (50) | 65 (52) | 52 (47.7) | 0.512 |
| Nosocomial pneumonia | 117 (50) | 60 (48.1) | 57 (52.3) | |
| Septic shock | 178 (76.1) | 85 (68) | 93 (85.3) | 0.002 |
| ARDS | 131 (56) | 58 (46.4) | 73 (67) | 0.002 |
| AKI | 117 (50) | 46 (36.8) | 71 (65.1) | <0.001 |
| HFNC before IMV | 77 (32.9) | 28 (22.4) | 49 (45) | <0.001 |
| RRT | 54 (23.1) | 14 (11.2) | 40 (36.7) | <0.001 |
| Prone position | 15 (6.4) | 6 (4.8) | 9 (8.3) | 0.282 |
| ECMO | 10 (4.3) | 5 (4) | 5 (4.6) | 0.825 |
| Variables | Total | Survivors | Non-Survivors | p-Value |
|---|---|---|---|---|
| N = 234 | N = 125 | N = 109 | ||
| WBC, · 103/mm3 | 13.6 (6.6–20.5) | 13.2 (8–19.1) | 14.1 (5.5–21.9) | 0.994 |
| Hb, g/dL | 10.9 (9.4–12.2) | 11.2 (9.9–12.5) | 10.5 (8.9–11.9) | 0.007 |
| RDW, % | 14.4 (13.4–15.6) | 14 (13.2–15.2) | 14.9 (13.6–16.2) | 0.008 |
| Platelet, · 103/mm3 | 181 (106–248) | 190 (143–264) | 146 (83.5–225.5) | 0.001 |
| D-dimer, ug/mL (n = 175) | 3.6 (1.7–7.3) | 3.4 (1.6–6.4) [90/125] | 3.7 (1.9–8.4) [85/109] | 0.195 |
| Urea, mg/dL | 26.9 (17.9–42.5) | 23.6 (15.9–39) | 30.7 (21.4–45) | 0.03 |
| Albumin, g/dL | 2.8 (2.3–3.2) | 3 (2.6–3.5) | 2.6 (2.2–3) | <0.001 |
| CRP, mg/dL | 15.9 (9.1–25.9) | 14.4 (7.9–25.9) | 18.8 (11–26) | 0.141 |
| Lactate, | 3 (1.8–5.2) | 2.6 (1.6–4.1) | 3.6 (2–6.5) | 0.002 |
| P/F ratio | 140 (102–198.4) | 161.7 (125.7–226.9) | 122.9 (93.2–171.7) | <0.001 |
| CURB-65 score | 4 (4–5) | 4 (3–5) | 5 (4–5) | <0.001 |
| ≥4 | 188 (80.3) | 90 (72) | 98 (89.9) | 0.001 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Age | 1.018 | 0.997–1.039 | 0.086 | 1.046 | 1.017–1.076 | 0.002 |
| Male gender | 2.120 | 1.160–3.878 | 0.015 | 2.015 | 0.955–4.248 | 0.066 |
| CLD | 2.721 | 1.175–6.300 | 0.019 | - | - | - |
| APACHEII | 1.150 | 1.098–1.205 | <0.001 | - | - | - |
| SOFA | 1.340 | 1.220–1.471 | <0.001 | 1.280 | 1.151–1.424 | <0.001 |
| ARDS | 2.342 | 1.376–3.987 | 0.002 | 1.931 | 0.981–3.798 | 0.057 |
| Hb | 0.848 | 0.746–0.964 | 0.012 | 0.939 | 0.813–1.085 | 0.394 |
| Platelet | 0.996 | 0.994–0.999 | 0.002 | - | - | - |
| Lactate | 1.187 | 1.084–1.299 | <0.001 | - | - | - |
| P/F ratio | 0.991 | 0.987–0.995 | <0.001 | 0.993 | 0.988–0.999 | 0.03 |
| RDW/Albumin ratio | 1.545 | 1.282–1.862 | <0.001 | 1.379 | 1.103–1.723 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.H.; Heo, M.; Lee, S.J.; Jeong, Y.Y.; Lee, J.D.; Yoo, J.-W. Clinical Usefulness of Red Cell Distribution Width/Albumin Ratio to Discriminate 28-Day Mortality in Critically Ill Patients with Pneumonia Receiving Invasive Mechanical Ventilation, Compared with Lacate/Albumin Ratio: A Retrospective Cohort Study. Diagnostics 2021, 11, 2344. https://doi.org/10.3390/diagnostics11122344
Jeong JH, Heo M, Lee SJ, Jeong YY, Lee JD, Yoo J-W. Clinical Usefulness of Red Cell Distribution Width/Albumin Ratio to Discriminate 28-Day Mortality in Critically Ill Patients with Pneumonia Receiving Invasive Mechanical Ventilation, Compared with Lacate/Albumin Ratio: A Retrospective Cohort Study. Diagnostics. 2021; 11(12):2344. https://doi.org/10.3390/diagnostics11122344
Chicago/Turabian StyleJeong, Jong Hwan, Manbong Heo, Seung Jun Lee, Yi Yeong Jeong, Jong Deog Lee, and Jung-Wan Yoo. 2021. "Clinical Usefulness of Red Cell Distribution Width/Albumin Ratio to Discriminate 28-Day Mortality in Critically Ill Patients with Pneumonia Receiving Invasive Mechanical Ventilation, Compared with Lacate/Albumin Ratio: A Retrospective Cohort Study" Diagnostics 11, no. 12: 2344. https://doi.org/10.3390/diagnostics11122344
APA StyleJeong, J. H., Heo, M., Lee, S. J., Jeong, Y. Y., Lee, J. D., & Yoo, J.-W. (2021). Clinical Usefulness of Red Cell Distribution Width/Albumin Ratio to Discriminate 28-Day Mortality in Critically Ill Patients with Pneumonia Receiving Invasive Mechanical Ventilation, Compared with Lacate/Albumin Ratio: A Retrospective Cohort Study. Diagnostics, 11(12), 2344. https://doi.org/10.3390/diagnostics11122344






