Distributions of Aβ42 and Aβ42/40 in the Cerebrospinal Fluid in View of the Probability Theory
Abstract
:1. Introduction
2. Theoretical Properties of the Variables
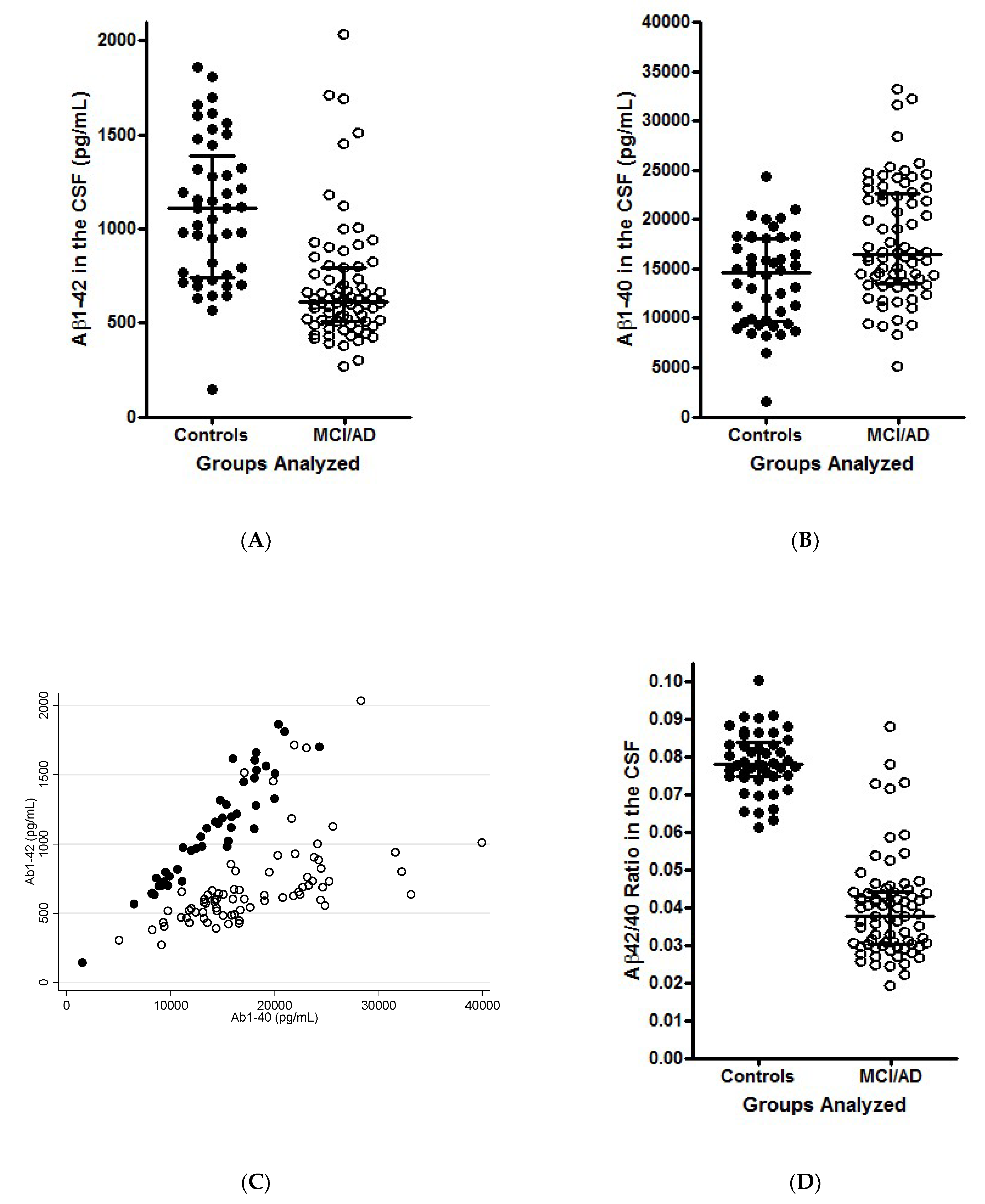
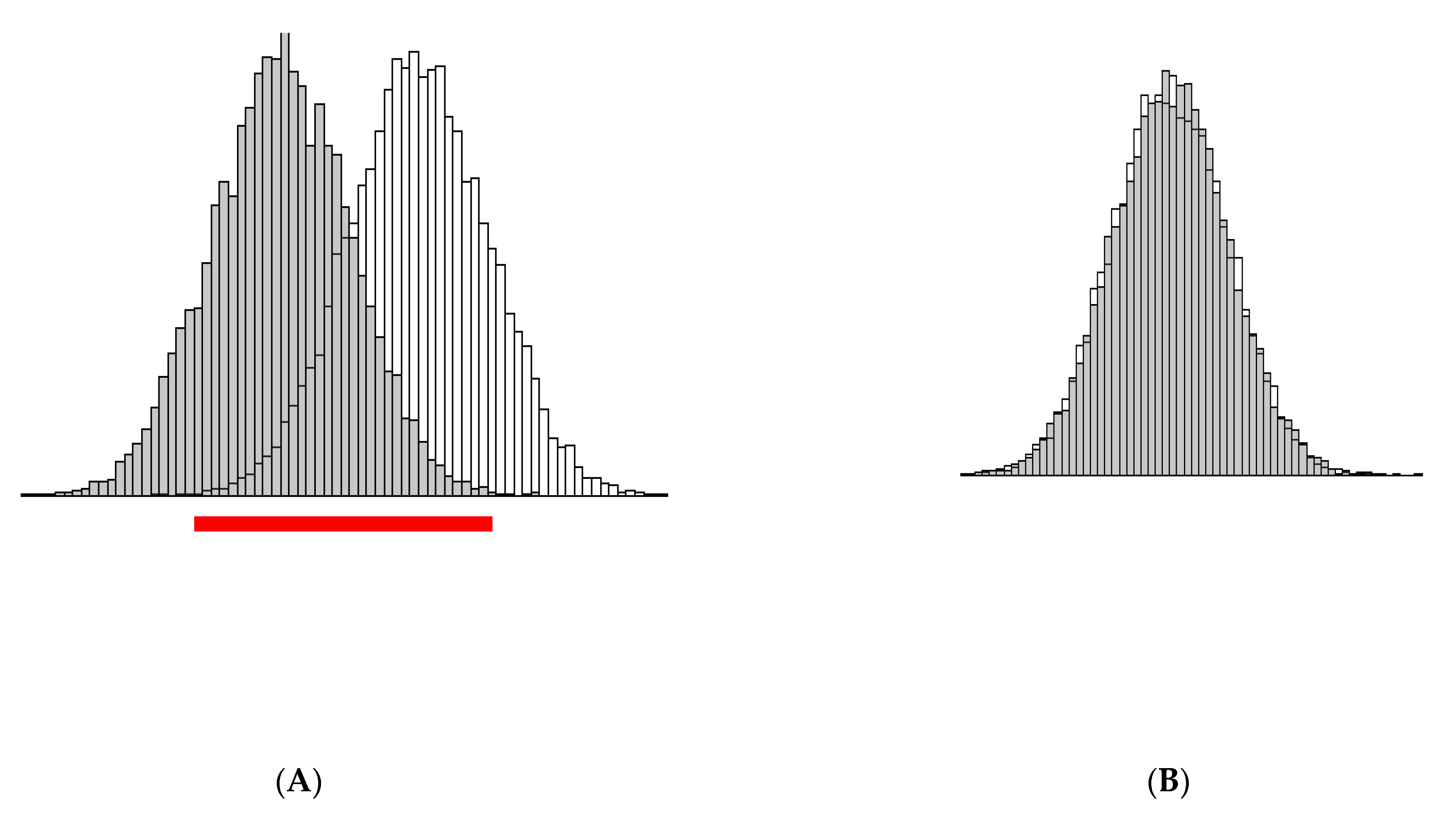
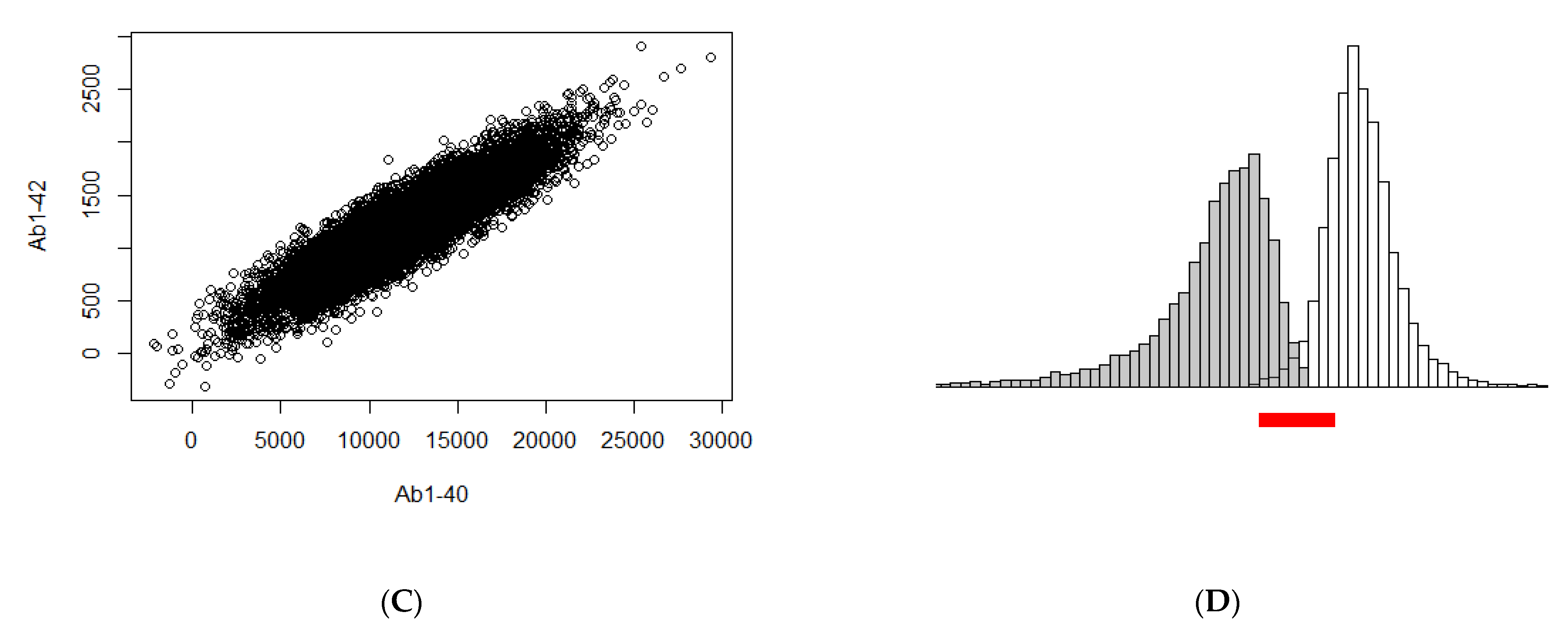
3. Illustration of the Theoretical Findings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bain, L.J.; Jedrziewski, K.; Morrison-Bogorad, M.; Albert, M.; Cotman, C.; Hendrie, H.; Trojanowski, J.Q. Healthy brain aging: A meeting report from the Sylvan M. Cohen Annual Retreat of the University of Pennsylvania Institute on Aging. Alzheimers Dement. 2008, 4, 443–446. [Google Scholar] [CrossRef] [Green Version]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- de Leon, M.J.; Golomb, J.; George, A.E.; Convit, A.; Tarshish, C.Y.; McRae, T.; De Santi, S.; Smith, G.; Ferris, S.H.; Noz, M.; et al. The radiologic prediction of Alzheimer disease: The atrophic hippocampal formation. AJNR Am. J. Neuroradiol. 1993, 14, 897–906. [Google Scholar] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Dekosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Petersen, R.C.; Aisen, P.S.; Beckett, L.A.; Donohue, M.C.; Gamst, A.C.; Harvey, D.J.; Jack, C.R., Jr.; Jagust, W.J.; Shaw, L.M.; Toga, A.W.; et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 2010, 74, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Hampel, H.; Burger, K.; Teipel, S.J.; Bokde, A.L.; Zetterberg, H.; Blennow, K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008, 4, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Zetterberg, H.; Buchhave, P.; Londos, E.; Blennow, K.; Minthon, L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006, 5, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Lemaire, H.G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.H.; Multhaup, G.; Beyreuther, K.; Muller-Hill, B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987, 325, 733–736. [Google Scholar] [CrossRef]
- Carroll, C.M.; Li, Y.M. Physiological and pathological roles of the gamma-secretase complex. Brain Res. Bull. 2016, 126, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Wiltfang, J.; Esselmann, H.; Bibl, M.; Smirnov, A.; Otto, M.; Paul, S.; Schmidt, B.; Klafki, H.W.; Maler, M.; Dyrks, T.; et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Ab peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J. Neurochem. 2002, 81, 481–496. [Google Scholar] [CrossRef]
- Lewczuk, P.; Esselmann, H.; Meyer, M.; Wollscheid, V.; Neumann, M.; Otto, M.; Maler, J.M.; Rüther, E.; Kornhuber, J.; Wiltfang, J. The amyloid-b (Ab) peptide pattern in cerebrospinal fluid in Alzheimer’s disease: Evidence of a novel carboxyterminally elongated Ab peptide. Rapid Commun. Mass Spectrom. 2003, 17, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Mintun, M.A.; Mach, R.H.; Lee, S.Y.; Dence, C.S.; Shah, A.R.; LaRossa, G.N.; Spinner, M.L.; Klunk, W.E.; Mathis, C.A.; et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann. Neurol. 2006, 59, 512–519. [Google Scholar] [CrossRef]
- Lewczuk, P.; Matzen, A.; Blennow, K.; Parnetti, L.; Molinuevo, J.L.; Eusebi, P.; Kornhuber, J.; Morris, J.C.; Fagan, A.M. Cerebrospinal Fluid Abeta42/40 Corresponds Better than Abeta42 to Amyloid PET in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Sjögren, M.; Gisslen, M.; Vanmechelen, E.; Blennow, K. Low cerebrospinal fluid b-amyloid 42 in patients with acute bacterial meningitis and normalization after treatment. Neurosci. Lett. 2001, 314, 33–36. [Google Scholar] [CrossRef]
- Spies, P.E.; Verbeek, M.M.; van Groen, T.; Claassen, J.A. Reviewing reasons for the decreased CSF Abeta42 concentration in Alzheimer disease. Front. Biosci. 2012, 17, 2024–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mroczko, B.; Groblewska, M.; Litman-Zawadzka, A.; Kornhuber, J.; Lewczuk, P. Amyloid beta oligomers (AbetaOs) in Alzheimer’s disease. J. Neural Transm. 2018, 125, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Esselmann, H.; Otto, M.; Maler, J.M.; Henkel, A.W.; Henkel, M.K.; Eikenberg, O.; Antz, C.; Krause, W.R.; Reulbach, U.; et al. Neurochemical diagnosis of Alzheimer’s dementia by CSF Ab42, Ab42/Ab40 ratio and total tau. Neurobiol. Aging 2004, 25, 273–281. [Google Scholar] [CrossRef]
- Klafki, H.W.; Wiltfang, J.; Staufenbiel, M. Electrophoretic separation of betaA4 peptides (1-40) and (1-42). Anal. Biochem. 1996, 237, 24–29. [Google Scholar] [CrossRef]
- Shoji, M.; Matsubara, E.; Kanai, M.; Watanabe, M.; Nakamura, T.; Tomidokoro, Y.; Shizuka, M.; Wakabayashi, K.; Igeta, Y.; Ikeda, Y.; et al. Combination assay of CSF tau, Ab1-40 and Ab1-42(43) as a biochemical marker of Alzheimer’s disease. J. Neurol. Sci. 1998, 158, 134–140. [Google Scholar] [CrossRef]
- Lewczuk, P.; Riederer, P.; O’Bryant, S.E.; Verbeek, M.M.; Dubois, B.; Visser, P.J.; Jellinger, K.A.; Engelborghs, S.; Ramirez, A.; Parnetti, L.; et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J. Biol. Psychiatry 2018, 19, 244–328. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid beta (Abeta) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res. Ther. 2019, 11, 34. [Google Scholar] [CrossRef]
- Fagan, A.M.; Mintun, M.A.; Shah, A.R.; Aldea, P.; Roe, C.M.; Mach, R.H.; Marcus, D.; Morris, J.C.; Holtzman, D.M. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol. Med. 2009, 1, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Zetterberg, H.; Blennow, K.; Vestberg, S.; Andreasson, U.; Brooks, D.J.; Owenius, R.; Hagerstrom, D.; Wollmer, P.; Minthon, L.; et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: A cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014, 71, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Dubois, B.; Fagan, A.M.; Lewczuk, P.; de Leon, M.J.; Hampel, H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement. 2015, 11, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Leuzy, A.; Chiotis, K.; Hasselbalch, S.G.; Rinne, J.O.; de Mendonca, A.; Otto, M.; Lleo, A.; Castelo-Branco, M.; Santana, I.; Johansson, J.; et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-beta in a multicentre European memory clinic study. Brain 2016, 139, 2540–2553. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, N.; Insel, P.S.; Donohue, M.; Landau, S.; Jagust, W.J.; Shaw, L.M.; Trojanowski, J.Q.; Zetterberg, H.; Blennow, K.; Weiner, M.W.; et al. Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer’s disease. Brain 2015, 138, 772–783. [Google Scholar] [CrossRef] [Green Version]
- Janelidze, S.; Zetterberg, H.; Mattsson, N.; Palmqvist, S.; Vanderstichele, H.; Lindberg, O.; van Westen, D.; Stomrud, E.; Minthon, L.; Blennow, K.; et al. CSF Abeta42/Abeta40 and Abeta42/Abeta38 ratios: Better diagnostic markers of Alzheimer disease. Ann. Clin. Transl. Neurol. 2016, 3, 154–165. [Google Scholar] [CrossRef] [Green Version]
- Engelborghs, S. Clinical indications for analysis of Alzheimer’s disease CSF biomarkers. Rev. Neurol. 2013, 169, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Slaets, S.; Le Bastard, N.; Martin, J.J.; Sleegers, K.; Van Broeckhoven, C.; De Deyn, P.P.; Engelborghs, S. Cerebrospinal fluid Abeta1-40 improves differential dementia diagnosis in patients with intermediate P-tau181P levels. J. Alzheimers Dis. 2013, 36, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Renard, D.; Wacongne, A.; Ayrignac, X.; Charif, M.; Fourcade, G.; Azakri, S.; Le Floch, A.; Bouly, S.; Marelli, C.; Arquizan, C.; et al. Cerebrospinal Fluid Alzheimer’s Disease Biomarkers in Cerebral Amyloid Angiopathy-Related Inflammation. J. Alzheimers Dis. 2016, 50, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Bibl, M.; Mollenhauer, B.; Lewczuk, P.; Esselmann, H.; Wolf, S.; Otto, M.; Kornhuber, J.; Ruther, E.; Wiltfang, J. Cerebrospinal fluid tau, p-tau 181 and amyloid-beta38/40/42 in frontotemporal dementias and primary progressive aphasias. Dement. Geriatr. Cogn. Disord. 2011, 31, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Spies, P.E.; Slats, D.; Sjogren, J.M.; Kremer, B.P.; Verhey, F.R.; Rikkert, M.G.; Verbeek, M.M. The cerebrospinal fluid amyloid beta42/40 ratio in the differentiation of Alzheimer’s disease from non-Alzheimer’s dementia. Curr. Alzheimer Res. 2010, 7, 470–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewczuk, P.; Lelental, N.; Spitzer, P.; Maler, J.M.; Kornhuber, J. Amyloid β 42/40 CSF concentration ratio in the diagnostics of Alzheimer’s Disease: Validation of two novel assays. J. Alzheimers Dis. 2015, 43, 183–191. [Google Scholar] [CrossRef]
- Koop, J.C. On an Identity for the Variances of a Ratio of Two Random Variables. J. R. Stat. Soc. Ser. B 1964, 26, 484–486. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewczuk, P.; Wiltfang, J.; Kornhuber, J.; Verhasselt, A. Distributions of Aβ42 and Aβ42/40 in the Cerebrospinal Fluid in View of the Probability Theory. Diagnostics 2021, 11, 2372. https://doi.org/10.3390/diagnostics11122372
Lewczuk P, Wiltfang J, Kornhuber J, Verhasselt A. Distributions of Aβ42 and Aβ42/40 in the Cerebrospinal Fluid in View of the Probability Theory. Diagnostics. 2021; 11(12):2372. https://doi.org/10.3390/diagnostics11122372
Chicago/Turabian StyleLewczuk, Piotr, Jens Wiltfang, Johannes Kornhuber, and Anneleen Verhasselt. 2021. "Distributions of Aβ42 and Aβ42/40 in the Cerebrospinal Fluid in View of the Probability Theory" Diagnostics 11, no. 12: 2372. https://doi.org/10.3390/diagnostics11122372
APA StyleLewczuk, P., Wiltfang, J., Kornhuber, J., & Verhasselt, A. (2021). Distributions of Aβ42 and Aβ42/40 in the Cerebrospinal Fluid in View of the Probability Theory. Diagnostics, 11(12), 2372. https://doi.org/10.3390/diagnostics11122372





