Ultrasound Pitfalls in a Complex Fetal Cardiac Malformation—Case Report of a New Arteriovenous Central Communication
Abstract
:1. Introduction
2. Case Presentation Section
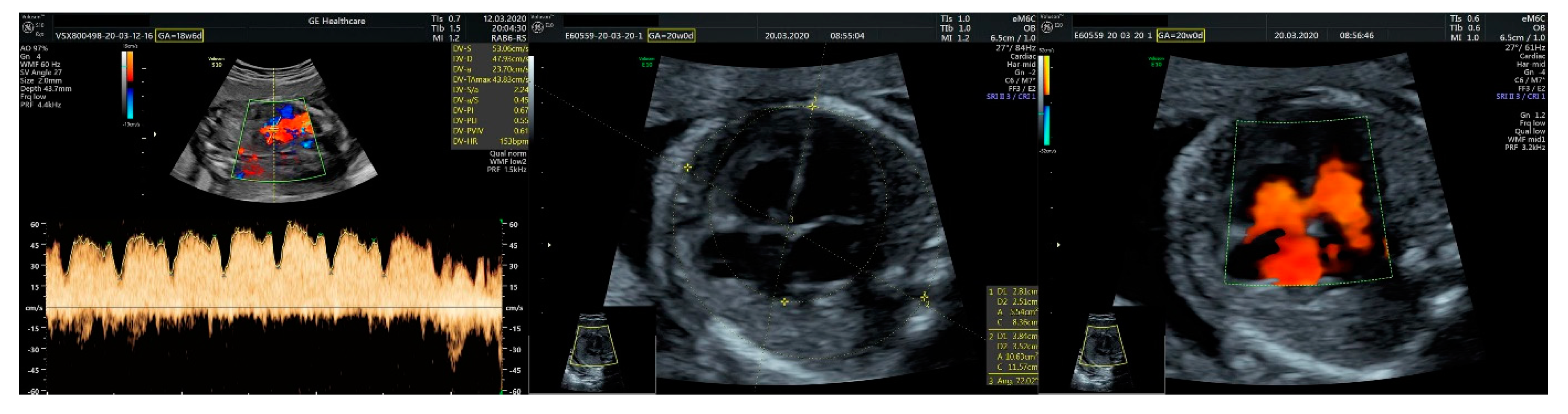
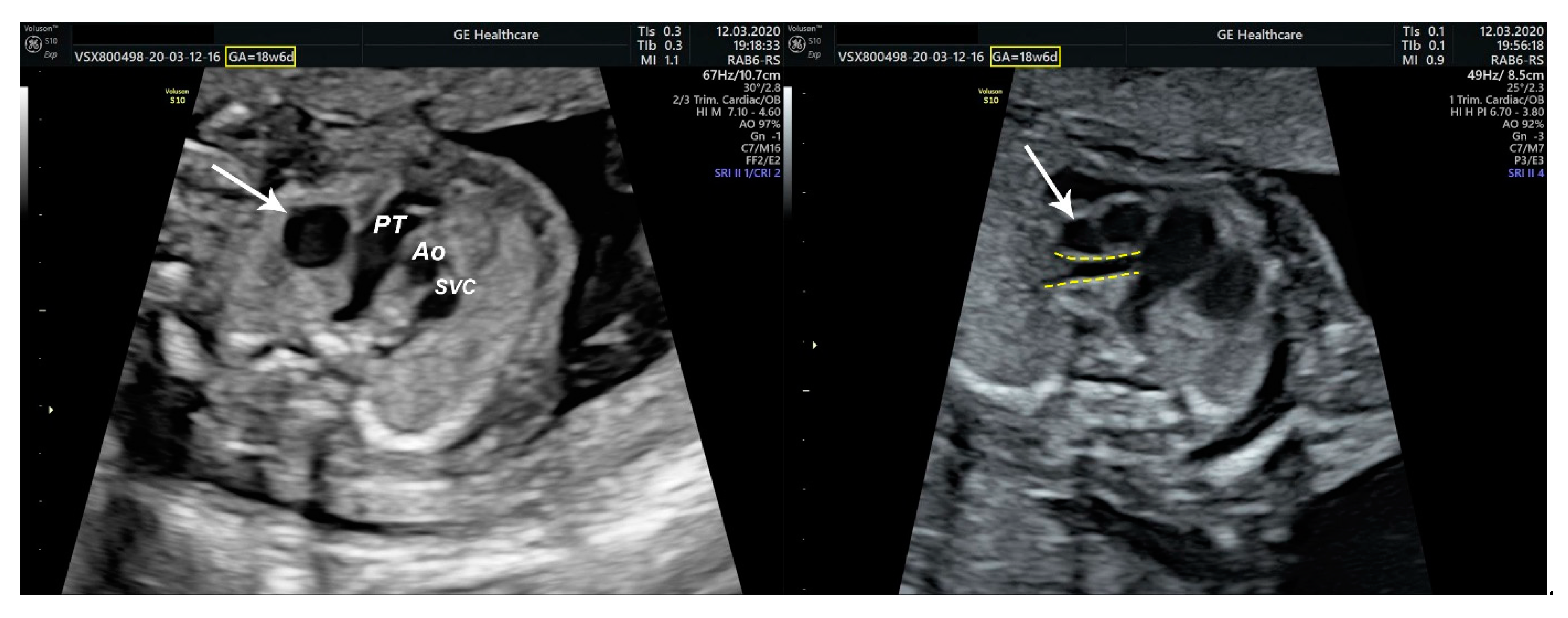
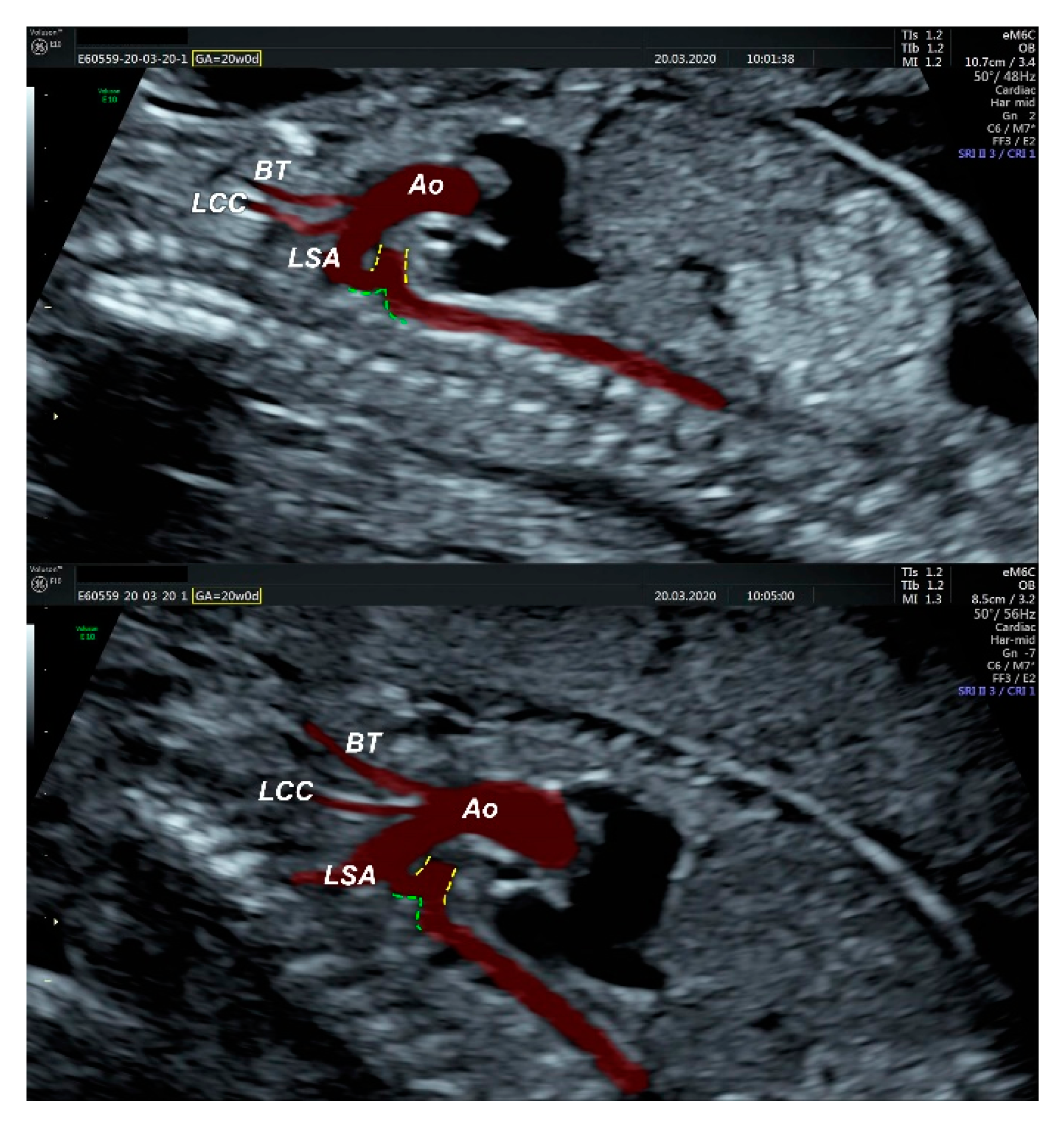
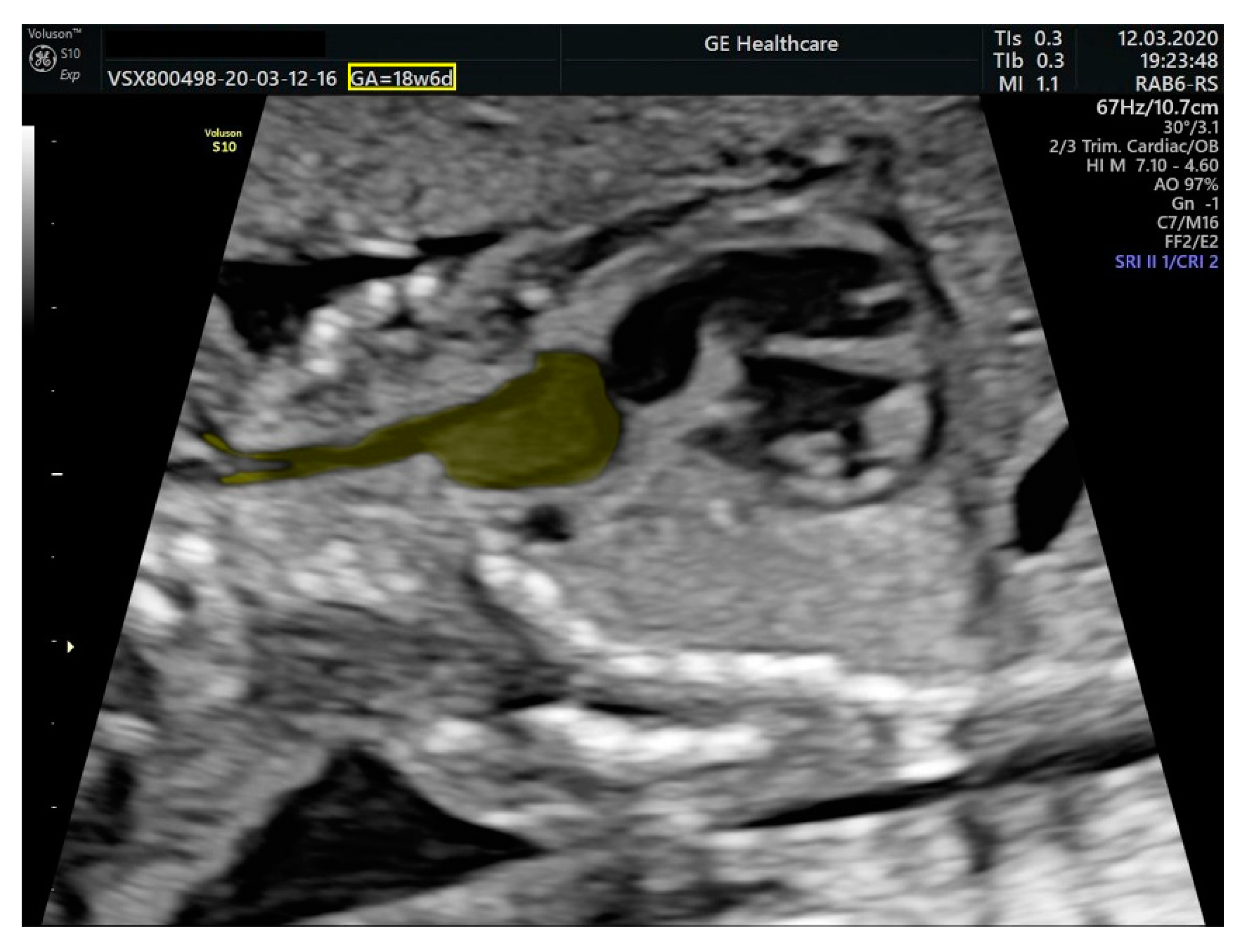
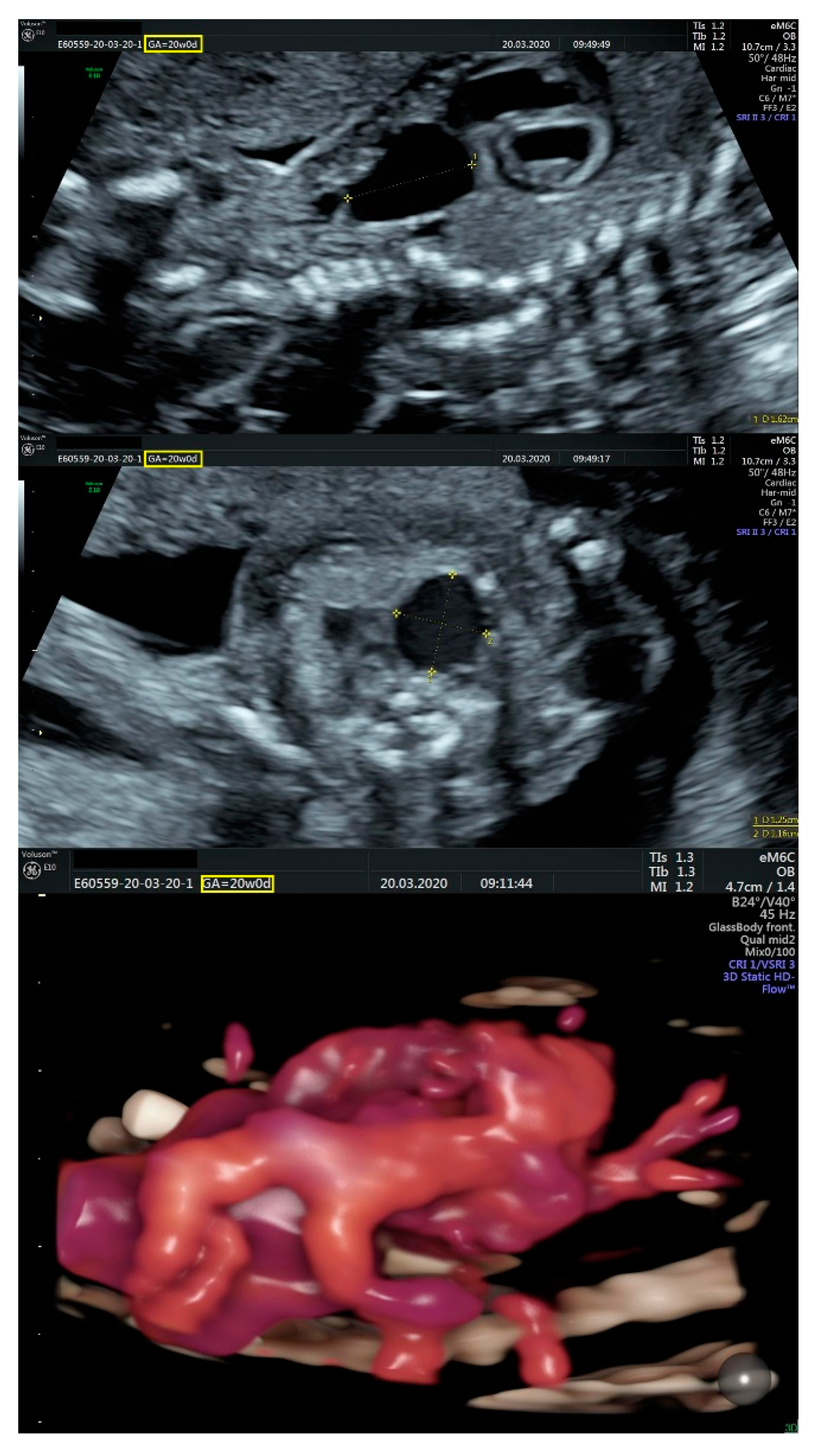
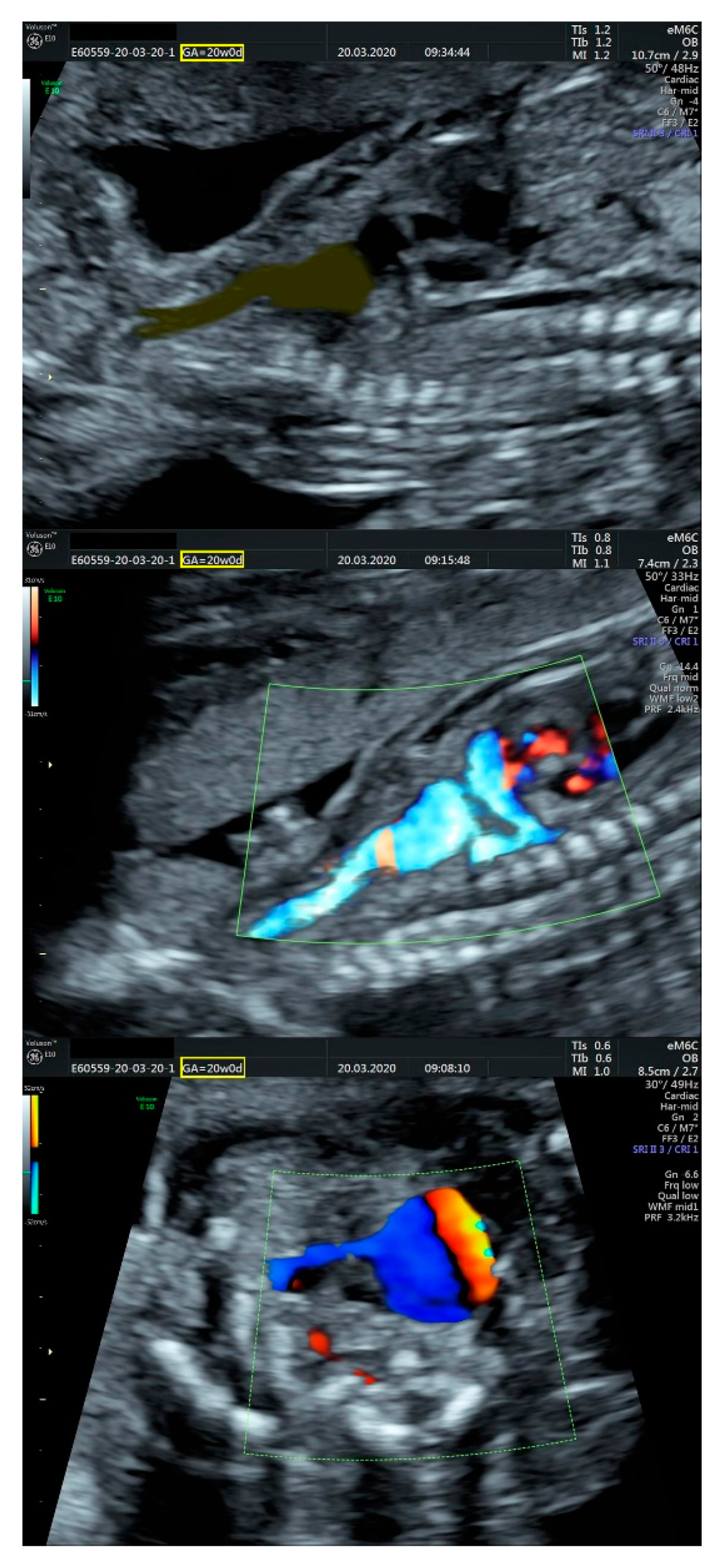
2.1. Ultrasound Findings
- an isolated aortic arch anomaly (supposedly aneurysmal dilation from which the left common carotid artery emerges) and coarctation of the aorta with the anterograde flow;
- ventricular septal defect, coarctation of the aorta, and a vascular formation located superior from the aortic arch with the appearance of an arteriovenous fistula;
- aneurysmal dilation located above the pulmonary trunk bifurcation and a dilated left common carotid artery with a retrograde flow;
- minor ventricular septal defect with a normal ductus venosus triphasic flow.
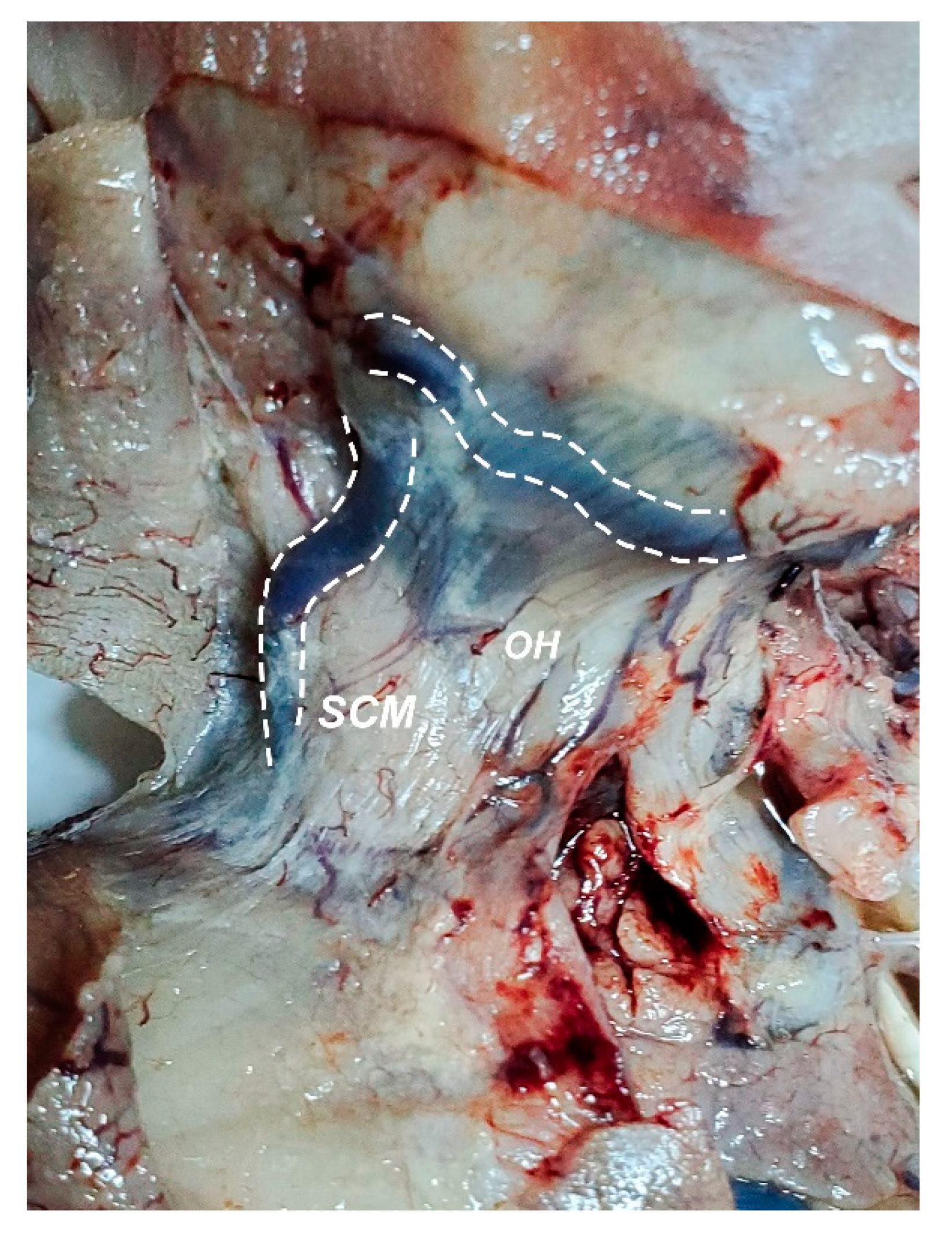
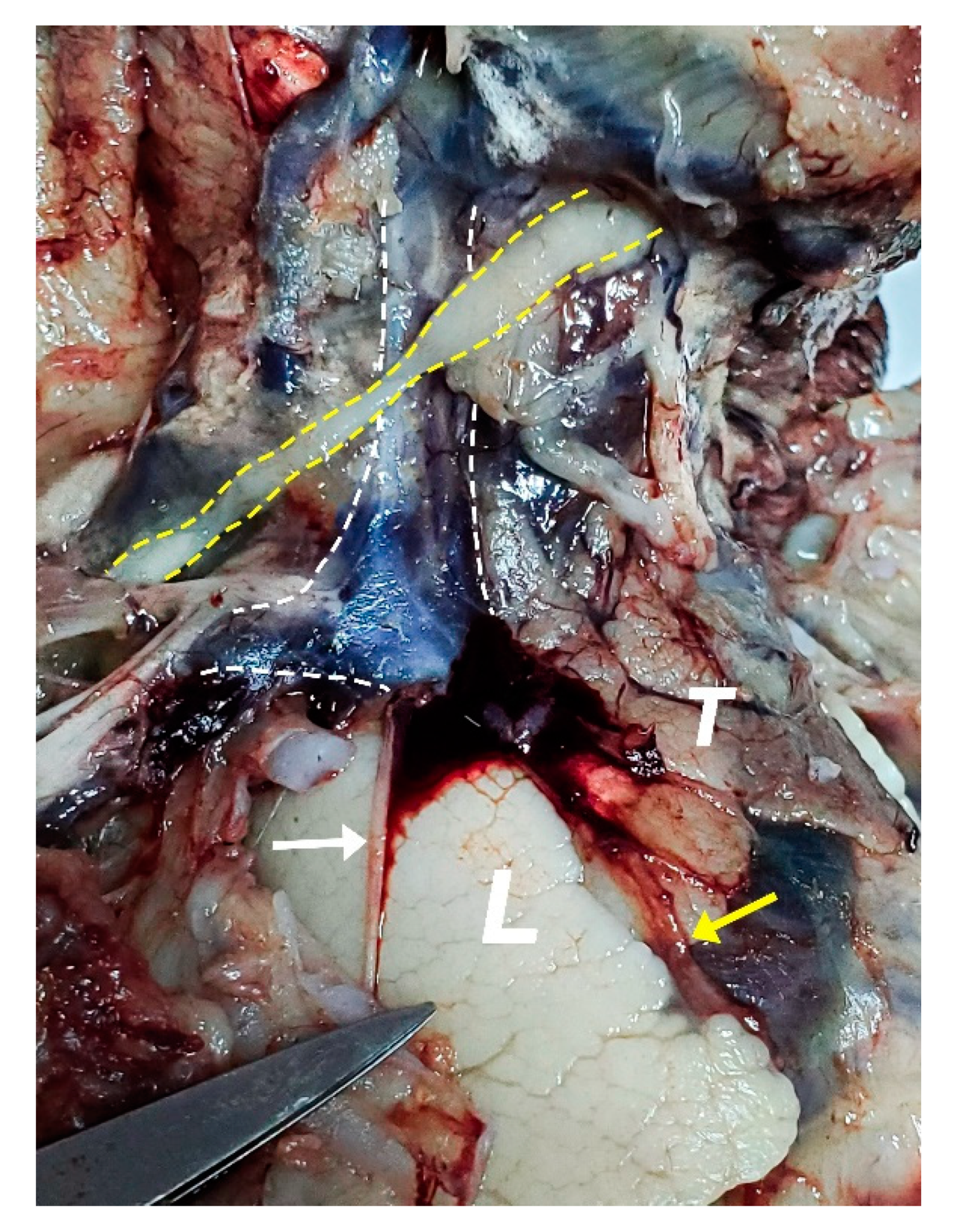
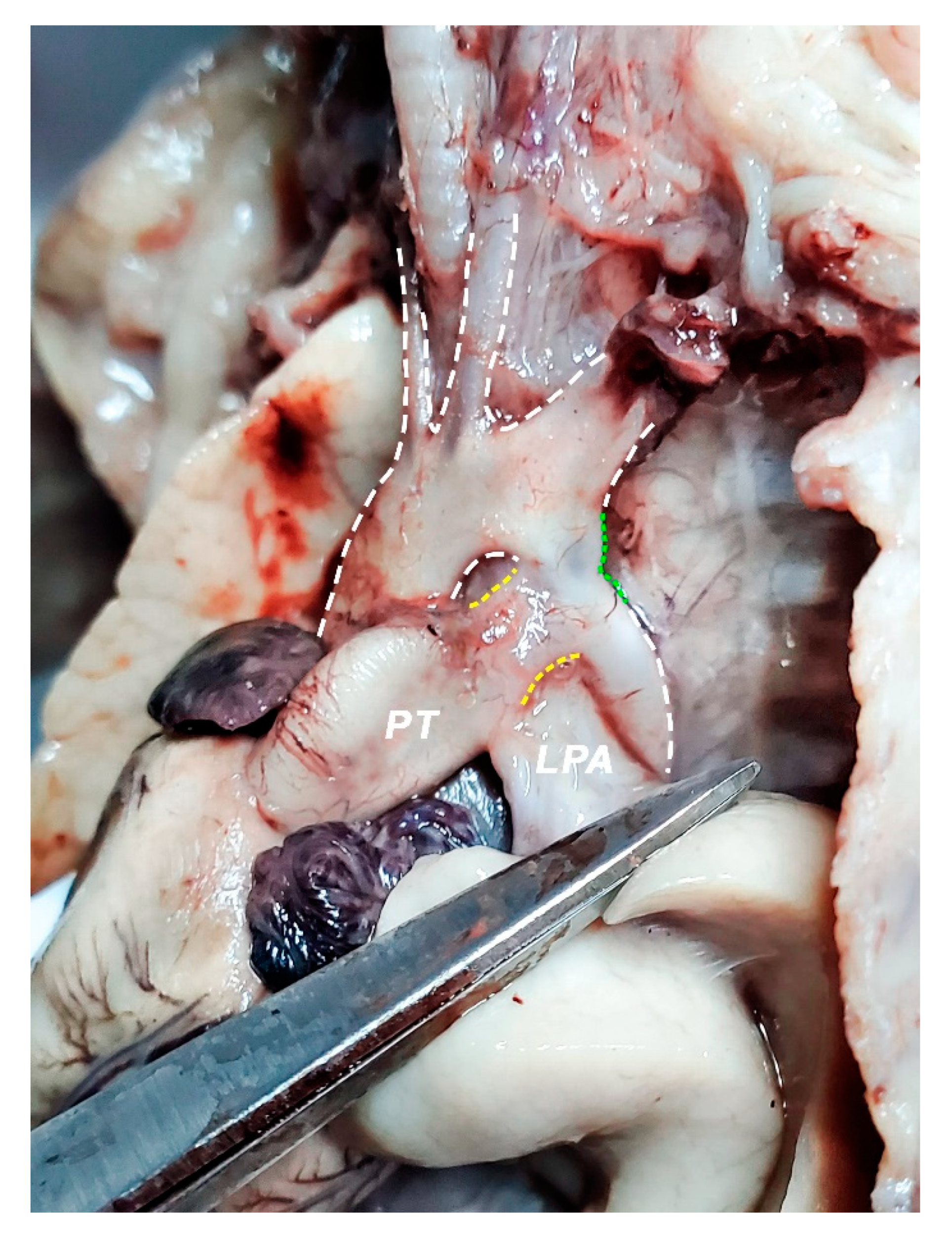
2.2. Dissection
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacy, T.A.; Silverman, N.H. Systemic venous abnormalities: Embryologic and echocardiographic considerations. Echocardiography 2001, 18, 401–413. [Google Scholar] [CrossRef]
- Fasouliotis, S.J.; Achiron, R.; Kivilevitch, Z.; Yagel, S. The Human Fetal Venous System. J. Ultrasound Med. 2002, 21, 1145–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecher, K.; Campbell, S. Characteristics of fetal venous blood flow under normal circumstances and during fetal disease. Ultrasound Obstet. Gynecol. 1996, 7, 68–83. [Google Scholar] [CrossRef]
- Huisman, T.W.A.; Stewart, P.A.; Wladimiroff, J.W. Doppler assessment of the normal early fetal circulation. Ultrasound Obstet. Gynecol. 1992, 2, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Anteby, E.Y.; Shimonovitz, S.; Yagel, S. Fetal echocardiography: The identification of two of the pulmonary veins from the four-chamber view during the second trimester of pregnancy. Ultrasound Obstet. Gynecol. 1994, 4, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Yagel, S.; Kivilevitch, Z.; Cohen, S.M.; Valsky, D.V.; Messing, B.; Shen, O.; Achiron, R. The fetal venous system, Part I: Normal embryology, anatomy, hemodynamics, ultrasound evaluation and Doppler investigation. Ultrasound Obstet. Gynecol. 2010, 35, 741–750. [Google Scholar] [CrossRef]
- Magro, E.; Sénécail, B.; Gentric, J.C.; Alavi, Z.; Palombi, O.; Seizeur, R. Contribution of embryology in the understanding of cervical venous system anatomy within and around the transverse foramen: A review of the classical literature. Surg. Radiol. Anat. 2014, 36, 411–418. [Google Scholar] [CrossRef]
- Sinkovskaya, E.; Klassen, A.; Abuhamad, A. A novel systematic approach to the evaluation of the fetal venous system. Semin. Fetal Neonatal Med. 2013, 18, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Yagel, S.; Kivilevitch, Z.; Cohen, S.M.; Valsky, D.V.; Messing, B.; Shen, O.; Achiron, R. The fetal venous system, Part II: Ultrasound evaluation of the fetus with congenital venous system malformation or developing circulatory compromise. Ultrasound Obstet. Gynecol. 2010, 36, 93–111. [Google Scholar] [CrossRef]
- Chaoui, R.; Heling, K.S.; Karl, K. Ultrasound of the fetal veins part 1: The intrahepatic venous system. Ultraschall Der Med.-Eur. J. Ultrasound 2014, 35, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, R.; Heling, K.S.; Karl, K. Ultrasound of the fetal veins part 2: Veins at the cardiac level. Ultraschall Der Med.-Eur. J. Ultrasound 2014, 35, 302–321. [Google Scholar] [CrossRef]
- Yagel, S.; Cohen, S.M.; Valsky, D.V.; Shen, O.; Lipschuetz, M.; Messing, B. Systematic examination of the fetal abdominal precordial veins: A cohort study. Ultrasound Obstet. Gynecol. 2015, 45, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Viora, E.; Sciarrone, A.; Bastonero, S.; Errante, G.; Mortara, G.; Chiappa, E.; Campogrande, M. Anomalies of the fetal venous system: A report of 26 cases and review of the literature. Fetal Diagn. Ther. 2004, 19, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.D.; Martins, I. Congenital systemic venous return anomalies to the right atrium review. Insights Imaging 2019, 10. [Google Scholar] [CrossRef]
- Sinkovskaya, E.; Abuhamad, A.; Horton, S.; Chaoui, R.; Karl, K. Fetal left brachiocephalic vein in normal and abnormal conditions. Ultrasound Obstet. Gynecol. 2012, 40, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Newman, B.; Alkhori, N. Congenital central pulmonary artery anomalies: Part 1. Pediatr. Radiol. 2020, 50, 1022–1029. [Google Scholar] [CrossRef]
- Newman, B.; Alkhori, N. Congenital central pulmonary artery anomalies: Part 2. Pediatr. Radiol. 2020, 50, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Suzui, I.; Masuyama, H.; Hirano, Y.; Nishida, T.; Hayata, K.; Hiramatsu, Y. Prenatal diagnosis of umbilical arteriovenous malformation. J. Matern. Fetal. Neonatal Med. 2017, 30, 85–87. [Google Scholar] [CrossRef]
- Bakhru, S.; Jamalpuri, V.; Koneti, N.R. Diagnosis and management of congenital umbilical arteriovenous malformation. Cardiol. Young 2021, 31, 493–495. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy E-Book: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; ISBN 9780702068515. [Google Scholar]
- Vaida, M.A.; Niculescu, V.; Motoc, A.; Bolintineanu, S.; Sargan, I.; Niculescu, M.C. Correlations between anomalies of jugular veins and areas of vascular drainage of head and neck. Rom. J. Morphol. Embryol. 2006, 47, 287–290. [Google Scholar]
- Ghosh, S.; Mandal, L.; Roy, S.; Bandyopadhyay, M. Two Rare Anatomical Variations of External Jugular Vein: An Embryological Overview. Int. J. Morphol. 2012, 30, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Tudorache, S.; Florea, M.; Dragusin, R.; Zorila, L.; Patru, C.L. Learning Curve for Ultrasound Assessment of the Fetal Heart at Nuchal Scan. JBR J. Clin. Diagn. Res. 2017, 5, 133. [Google Scholar] [CrossRef] [Green Version]
- Maiz, N.; Kagan, K.O.; Milovanovic, Z.; Celik, E.; Nicolaides, K.H. Learning curve for Doppler assessment of ductus venosus flow at 11 + 0 to 13 + 6 weeks’ gestation. Ultrasound Obstet. Gynecol. 2008, 31, 503–506. [Google Scholar] [CrossRef]
- Iliescu, D.; Tudorache, S.; Comanescu, A.; Antsaklis, P.; Cotarcea, S.; Novac, L.; Cernea, N.; Antsaklis, A. Improved detection rate of structural abnormalities in the first trimester using an extended examination protocol. Ultrasound Obstet. Gynecol. 2013, 42, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Hofstaetter, C.; Plath, H.; Hansmann, M. Prenatal diagnosis of abnormalities of the fetal venous system. Ultrasound Obstet. Gynecol. 2000, 15, 231–241. [Google Scholar] [CrossRef]
- Achiron, R.; Hegesh, J.; Yagel, S.; Lipitz, S.; Cohen, S.B.; Rotstein, Z. Abnormalities of the fetal central veins and umbilico-portal system: Prenatal ultrasonographic diagnosis and proposed classification. Ultrasound Obstet. Gynecol. 2000, 16, 539–548. [Google Scholar] [CrossRef]
- Seravalli, V.; Miller, J.L.; Block-Abraham, D.; Baschat, A.A. Ductus venosus Doppler in the assessment of fetal cardiovascular health: An updated practical approach. Acta Obstet. Gynecol. Scand. 2016, 95, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Gembruch, U.; Meise, C.; Germer, U.; Berg, C.; Geipel, A. Venous Doppler ultrasound in 146 fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2003, 22, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.H.; Chong, Y.F.; Lu, J.H.; Hung, C.Y.S. Ductus venosus blood flow resistance and congenital heart defects in the second trimester. J. Clin. Ultrasound 2008, 36, 72–78. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohîlțea, R.E.; Dumitru, A.; Vlădăreanu, R.; Pleș, L.; Georgescu, T.A.; Petrescu, I.-A.; Munteanu, O. Ultrasound Pitfalls in a Complex Fetal Cardiac Malformation—Case Report of a New Arteriovenous Central Communication. Diagnostics 2021, 11, 2398. https://doi.org/10.3390/diagnostics11122398
Bohîlțea RE, Dumitru A, Vlădăreanu R, Pleș L, Georgescu TA, Petrescu I-A, Munteanu O. Ultrasound Pitfalls in a Complex Fetal Cardiac Malformation—Case Report of a New Arteriovenous Central Communication. Diagnostics. 2021; 11(12):2398. https://doi.org/10.3390/diagnostics11122398
Chicago/Turabian StyleBohîlțea, Roxana Elena, Adrian Dumitru, Radu Vlădăreanu, Liana Pleș, Tiberiu Augustin Georgescu, Ioan-Andrei Petrescu, and Octavian Munteanu. 2021. "Ultrasound Pitfalls in a Complex Fetal Cardiac Malformation—Case Report of a New Arteriovenous Central Communication" Diagnostics 11, no. 12: 2398. https://doi.org/10.3390/diagnostics11122398
APA StyleBohîlțea, R. E., Dumitru, A., Vlădăreanu, R., Pleș, L., Georgescu, T. A., Petrescu, I. -A., & Munteanu, O. (2021). Ultrasound Pitfalls in a Complex Fetal Cardiac Malformation—Case Report of a New Arteriovenous Central Communication. Diagnostics, 11(12), 2398. https://doi.org/10.3390/diagnostics11122398