Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature
Abstract
:1. Introduction
2. High-Grade Serous Carcinoma
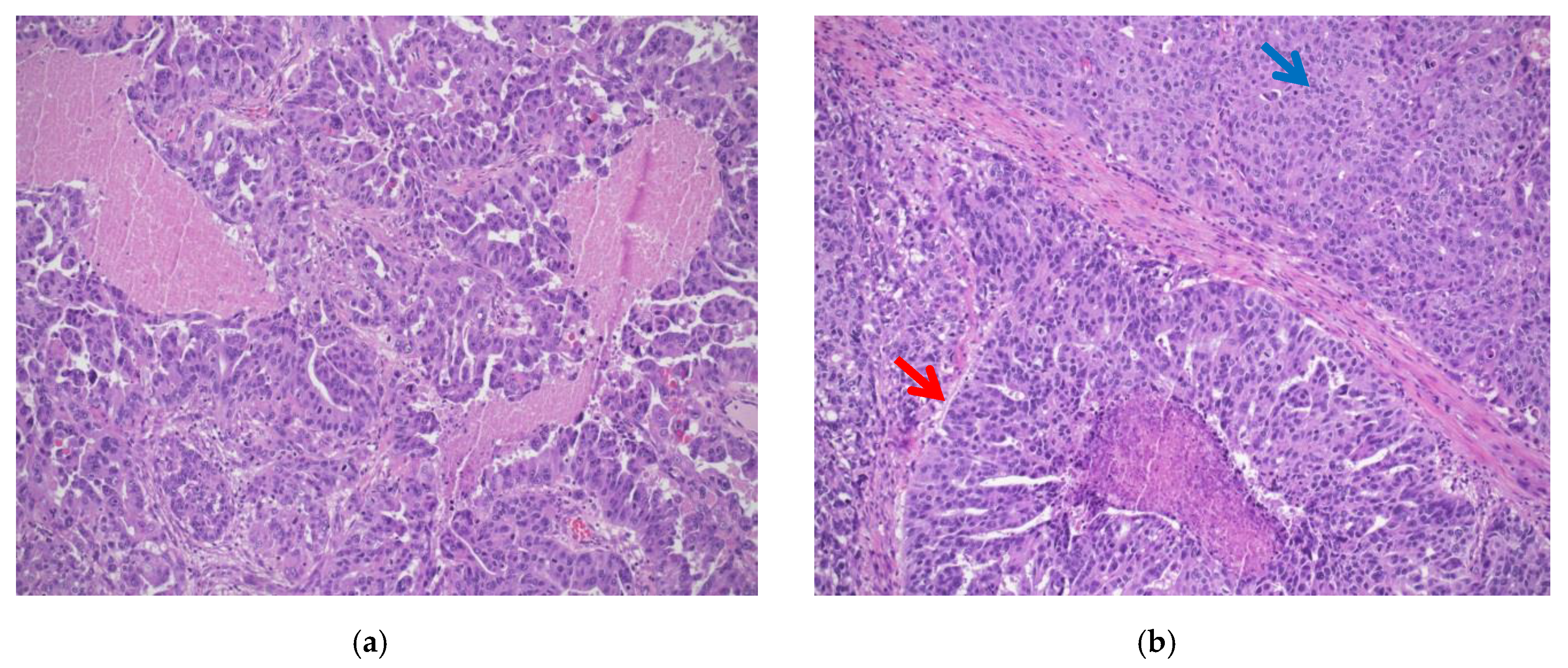
- Classic histotype: characterized by a papillary, micropapillary, and/or solid architecture, marked nuclear pleomorphism and high mitotic index (Figure 1a).
- “SET” variant (solid-pseudoendometrioid and transitional): characterized by an admixture of solid, glandular/endometrioid-like, and transitional/malignant Brenner-like growth patterns), higher mitotic index compared to the classic histotype, and a high number of tumor-infiltrating lymphocytes (TILs) (Figure 1b). In 2012, a study from Soslow et al. demonstrated a statistical association between BRCA1/2 mutation and SET morphology [12]
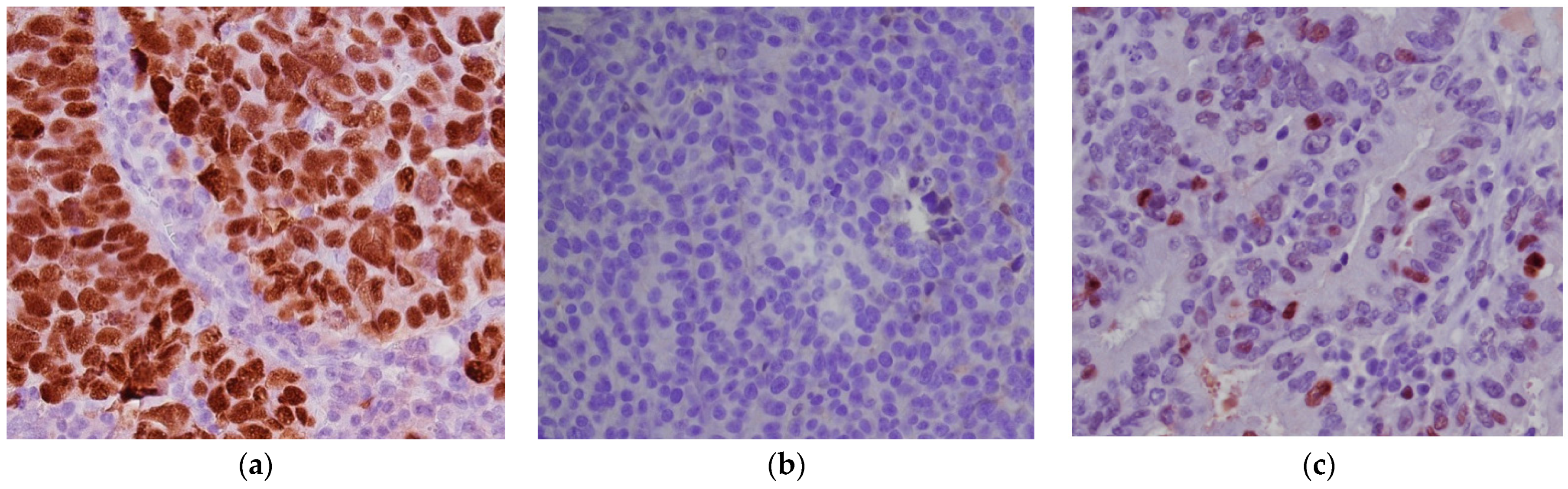
- p53 intense nuclear positivity in >80% tumor cells (overexpression pattern), complete absence of expression (null pattern) or cytoplasmic expression without nuclear staining were scored as p53 “abnormal” (p53abn) and correlated with TP53 mutation (Figure 2a,b).
- heterogeneous p53 expression was scored as “wild-type” (p53wt) and correlated with a TP53-wildtype gene (Figure 2c).
- Association with serous tubal intraepithelial carcinoma (STIC), low-grade serous-like areas, WT1 IHC positivity, mutation of CCNE1, BRCA1/2, and MDM2 amplification support the diagnosis of TP53-wildtype HGSC [17].
- Association with endometriosis, endometrioid cystadenofibroma, and borderline endometrioid tumor, as well as WT1 IHC negativity, support the diagnosis of p53abn grade 3 ENOC [18].
- identify patients with BRCA germline mutations eligible for PARPi therapy, prophylactic surgery, and genetic counseling;
- identify patients with BRCA somatic mutations potentially eligible for PARPi therapy;
- exclude unnecessary germline testing for somatic BRCA-negative tumors, in order to be cost-effective and reduce patients’ psychological distress.
3. Low-Grade Serous Carcinoma
3.1. Ovarian Endometrioid Carcinoma
3.2. Clear Cell Carcinoma
3.3. Mucinous Carcinoma
3.4. Other Rare Primary Ovarian Tumors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Female Genital Tumours. In WHO Classification of Tumours, 5th ed.; WHO Classification of Tumours Editorial Board: Geneva, Switzerland, 2020. [Google Scholar]
- Prat, J.; D’Angelo, E.; Espinosa, I. Ovarian carcinomas: At least five different diseases with distinct histological features and molecular genetics. Hum. Pathol. 2018, 80, 11–27. [Google Scholar] [CrossRef]
- The Global Cancer Observatory. Source: Globocan 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Facts & Figures 2019; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Weren, R.D.A.; Mensenkamp, A.R.; Simons, M.; Eijkelenboom, A.; Sie, A.S.; Ouchene, H.; van Asseldonk, M.; Gomez-Garcia, E.B.; Blok, M.J.; de Hullu, J.A.; et al. Novel BRCA1 and BRCA2 Tumor Test as Basis for Treatment Decisions and Referral for Genetic Counselling of Patients with Ovarian Carcinomas. Hum. Mutat. 2017, 38, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Miller, R.E. Targeted therapies in gynaecological cancers. Histopathology 2020, 76, 157–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurman, R.J.; Shih, I.-M. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurman, R.J.; Shih, I.M. The dualistic model of ovarian carcinogenesis revisited, revised, and expanded. Am. J. Pathol. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; Disaia, P.; Gabra, H.; Glenn, P.; et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Soslow, R.A.; Han, G.; Park, K.J.; Garg, K.; Olvera, N.; Spriggs, D.R.; Kauff, N.D.; Levine, D.A. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod. Pathol. 2012, 25, 625–636. [Google Scholar] [CrossRef]
- Köbel, M.; Piskorz, A.M.; Lee, S.; Lui, S.; LePage, C.; Marass, F.; Rosenfeld, N.; Mes Masson, A.-M.; Brenton, J.D. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J. Pathol. Clin. Res. 2016, 2, 247–258. [Google Scholar] [CrossRef]
- Wing-Cheuk Wong, R.; Palicelli, A.; Hoang, L.; Singh, N. Interpretation of p16, p53 and mismatch repair protein immunohistochemistry in gynaecological neoplasia. Diagn. Histopathol. 2020, 26, 257–277. [Google Scholar] [CrossRef]
- Casey, L.; Singh, N. Metastases to the ovary arising from endometrial, cervical and fallopian tube cancer: Recent advances. Histopathology 2020, 76, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Krämer, P.; Talhouk, A.; Brett, M.A.; Chiu, D.S.; Cairns, E.S.; Scheunhage, D.A.; Hammond, R.F.L.; Farnell, D.; Nazeran, T.M.; Grube, M.; et al. Endometrial Cancer Molecular Risk Stratification is Equally Prognostic for Endometrioid Ovarian Carcinoma. Clin. Cancer Res. 2020, 26, 5400–5410. [Google Scholar] [CrossRef] [PubMed]
- Chui, M.H.; Momeni Boroujeni, A.; Mandelker, D.; Ladanyi, M.; Soslow, R.A. Characterization of TP53-wildtype tubo-ovarian high-grade serous carcinomas: Rare exceptions to the binary classification of ovarian serous carcinoma. Mod. Pathol. 2021, 34, 490–501. [Google Scholar] [CrossRef]
- McCluggage, W.G. Endometriosis-related pathology: A discussion of selected uncommon benign, premalignant and malignant lesions. Histopathology 2020, 76, 76–92. [Google Scholar] [CrossRef]
- Nosé, V. Protocol for Examination of Specimens From Patients with Primary Pituitary Tumors. In Diagnostic Pathology: Endocrine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 606–609. [Google Scholar]
- Crum, C.P.; Drapkin, R.; Kindelberger, D.; Medeiros, F.; Miron, A.; Lee, Y. Lessons from BRCA: The Tubal Fimbria Emerges as an Origin for Pelvic Serous Cancer. Clin. Med. Res. 2007, 5, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial Carcinoma of the Fimbria and Pelvic Serous Carcinoma: Evidence for a Causal Relationship. Am. J. Surg. Pathol. 2007, 31, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.A.; Rouzbahman, M.; Pizer, E.S.; Pintilie, M.; Begley, H. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod. Pathol. 2009, 22, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Vang, R.; Visvanathan, K.; Gross, A.; Maambo, E.; Gupta, M.; Kuhn, E.; Li, R.F.; Ronnett, B.M.; Seidman, J.D.; Yemelyanova, A.; et al. Validation of an Algorithm for the Diagnosis of Serous Tubal Intraepithelial Carcinoma. Int. J. Gynecol. Pathol. 2012, 31, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.A.; Clarke, B.; George, S.H.L. Precursors of High-Grade Serous Carcinoma. In Precancerous Lesions of the Gynecologic Tract; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–22. ISBN 9783319225098. [Google Scholar]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2012, 12, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015, 137, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaak, R.G.W.; Tamayo, P.; Yang, J.-Y.; Hubbard, D.; Zhang, H.; Creighton, C.J.; Fereday, S.; Lawrence, M.; Carter, S.L.; Mermel, C.H.; et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J. Clin. Investig. 2012. [Google Scholar] [CrossRef] [PubMed]
- Le Page, C.; Rahimi, K.; Köbel, M.; Tonin, P.N.; Meunier, L.; Portelance, L.; Bernard, M.; Nelson, B.H.; Bernardini, M.Q.; Bartlett, J.M.S.; et al. Characteristics and outcome of the COEUR Canadian validation cohort for ovarian cancer biomarkers. BMC Cancer 2018, 18, 347. [Google Scholar] [CrossRef]
- Girolimetti, G.; Perrone, A.M.; Santini, D.; Barbieri, E.; Guerra, F.; Ferrari, S.; Zamagni, C.; De Iaco, P.; Gasparre, G.; Turchetti, D. BRCA-associated ovarian cancer: From molecular genetics to risk management. Biomed Res. Int. 2014. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin No. 103: Hereditary Breast and Ovarian Cancer Syndrome. Obstet. Gynecol. 2009, 113, 957–966. [CrossRef]
- Meisel, J.L.; Hyman, D.M.; Garg, K.; Zhou, Q.; Dao, F.; Bisogna, M.; Gao, J.; Schultz, N.D.; Grisham, R.N.; Phillips, M.; et al. The performance of BRCA1 immunohistochemistry for detecting germline, somatic, and epigenetic BRCA1 loss in high-grade serous ovarian cancer. Ann. Oncol. 2014, 25, 2372–2378. [Google Scholar] [CrossRef]
- Teixeira, L.A.; Candido dos Reis, F.J. Immunohistochemistry for the detection of BRCA1 and BRCA2 proteins in patients with ovarian cancer: A systematic review. J. Clin. Pathol. 2020, 73, 191–196. [Google Scholar] [CrossRef]
- Naipal, K.A.T.; Verkaik, N.S.; Ameziane, N.; van Deurzen, C.H.M.; ter Brugge, P.; Meijers, M.; Sieuwerts, A.M.; Martens, J.W.; O’Connor, M.J.; Vrieling, H.; et al. Functional Ex Vivo Assay to Select Homologous Recombination–Deficient Breast Tumors for PARP Inhibitor Treatment. Clin. Cancer Res. 2014, 20, 4816–4826. [Google Scholar] [CrossRef] [Green Version]
- Cruz, C.; Castroviejo-Bermejo, M.; Gutiérrez-Enríquez, S.; Llop-Guevara, A.; Ibrahim, Y.H.; Gris-Oliver, A.; Bonache, S.; Morancho, B.; Bruna, A.; Rueda, O.M.; et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann. Oncol. 2018, 29, 1203–1210. [Google Scholar] [CrossRef]
- de Jonge, M.M.; Auguste, A.; van Wijk, L.M.; Schouten, P.C.; Meijers, M.; ter Haar, N.T.; Smit, V.T.H.B.M.; Nout, R.A.; Glaire, M.A.; Church, D.N.; et al. Frequent Homologous Recombination Deficiency in High-grade Endometrial Carcinomas. Clin. Cancer Res. 2019, 25, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, B.T.J.; Timms, K.M.; Carey, M.S.; Gutin, A.; Meyer, L.A.; Flake, D.D.; Abkevich, V.; Potter, J.; Pruss, D.; Glenn, P.; et al. Somatic Mutations in BRCA1 and BRCA2 Could Expand the Number of Patients That Benefit From Poly (ADP Ribose) Polymerase Inhibitors in Ovarian Cancer. J. Clin. Oncol. 2010, 28, 3570–3576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Kaye, S. PARP Inhibitors in BRCA Gene-Mutated Ovarian Cancer and Beyond. Curr. Oncol. Rep. 2011, 13, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [Green Version]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian Cancer Immunotherapy: Turning up the Heat. Int. J. Mol. Sci. 2019, 20, 2927. [Google Scholar] [CrossRef] [Green Version]
- Strickland, K.C.; Howitt, B.E.; Shukla, S.A.; Rodig, S.; Ritterhouse, L.L.; Liu, J.F.; Garber, J.E.; Chowdhury, D.; Wu, C.J.; D’Andrea, A.D.; et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 2016, 7, 13587–13598. [Google Scholar] [CrossRef] [Green Version]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, J.R.; Milne, K.; Kroeger, D.R.; Nelson, B.H. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 2016, 141, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin de la Fuente, L.; Westbom-Fremer, S.; Arildsen, N.S.; Hartman, L.; Malander, S.; Kannisto, P.; Måsbäck, A.; Hedenfalk, I. PD-1/PD-L1 expression and tumor-infiltrating lymphocytes are prognostically favorable in advanced high-grade serous ovarian carcinoma. Virchows Arch. 2020, 477, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Molberg, K.; Strickland, A.L.; Castrillon, D.H.; Carrick, K.; Jiang, Q.; Niu, S.; Rivera-Colon, G.; Gwin, K.; Hinson, S.; et al. PD-L1 Expression and CD8+ Tumor-infiltrating Lymphocytes in Different Types of Tubo-ovarian Carcinoma and Their Prognostic Value in High-grade Serous Carcinoma. Am. J. Surg. Pathol. 2020, 44, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. Pathology of borderline and invasive cancers. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Oldt, R.; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih, I.-M. Mutations in BRAF and KRAS Characterize the Development of Low-Grade Ovarian Serous Carcinoma. JNCI J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehari, R.; Kurman, R.J.; Logani, S.; Shih, I.-M. The Development of High-grade Serous Carcinoma From Atypical Proliferative (Borderline) Serous Tumors and Low-grade Micropapillary Serous Carcinoma. Am. J. Surg. Pathol. 2007, 31, 1007–1012. [Google Scholar] [CrossRef]
- Hunter, S.M.; Anglesio, M.S.; Ryland, G.L.; Sharma, R.; Chiew, Y.-E.; Rowley, S.M.; Doyle, M.A.; Li, J.; Gilks, C.B.; Moss, P.; et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget 2015, 6, 37663–37677. [Google Scholar] [CrossRef] [Green Version]
- Tsang, Y.T.; Deavers, M.T.; Sun, C.C.; Kwan, S.-Y.; Kuo, E.; Malpica, A.; Mok, S.C.; Gershenson, D.M.; Wong, K.-K. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J. Pathol. 2013, 231, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Zarei, S.; Wang, Y.; Jenkins, S.M.; Voss, J.S.; Kerr, S.E.; Bell, D.A. Clinicopathologic, immunohistochemical, and molecular characteristics of ovarian serous carcinoma with mixed morphologic features of high-grade and low-grade serous carcinoma. Am. J. Surg. Pathol. 2020, 44, 316–328. [Google Scholar] [CrossRef]
- Coleman, R.L.; Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian Cancer Version 1.2020—March 11, 2020; NCCN: Plymouth Meeting, PA, USA, 2020; Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 29 January 2021).
- Combe, P.; Chauvenet, L.; Lefrère-Belda, M.-A.; Blons, H.; Rousseau, C.; Oudard, S.; Pujade-Lauraine, E. Sustained response to vemurafenib in a low grade serous ovarian cancer with a BRAF V600E mutation. Investig. New Drugs 2015, 33, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Moujaber, T.; Etemadmoghadam, D.; Kennedy, C.J.; Chiew, Y.-E.; Balleine, R.L.; Saunders, C.; Wain, G.V.; Gao, B.; Hogg, R.; Srirangan, S.; et al. BRAF Mutations in Low-Grade Serous Ovarian Cancer and Response to BRAF Inhibition. JCO Precis. Oncol. 2018, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Turashvili, G.; Grisham, R.N.; Chiang, S.; DeLair, D.F.; Park, K.J.; Soslow, R.A.; Murali, R. BRAF V 600E mutations and immunohistochemical expression of VE1 protein in low-grade serous neoplasms of the ovary. Histopathology 2018, 73, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. JNCI J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Meng, B.; Hoang, L.N.; McIntyre, J.B.; Duggan, M.A.; Nelson, G.S.; Lee, C.-H.; Köbel, M. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol. Oncol. 2014, 134, 15–19. [Google Scholar] [CrossRef]
- Church, D.N.; Stelloo, E.; Nout, R.A.; Valtcheva, N.; Depreeuw, J.; ter Haar, N.; Noske, A.; Amant, F.; Tomlinson, I.P.M.; Wild, P.J.; et al. Prognostic Significance of POLE Proofreading Mutations in Endometrial Cancer. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Billingsley, C.C.; Cohn, D.E.; Mutch, D.G.; Hade, E.M.; Goodfellow, P.J. Prognostic Significance of POLE Exonuclease Domain Mutations in High-Grade Endometrioid Endometrial Cancer on Survival and Recurrence: A Subanalysis. Int. J. Gynecol. Cancer 2016, 26, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Pawlik, T.M.; Raut, C.P.; Rodriguez-Bigas, M.A. Colorectal Carcinogenesis: MSI-H Versus MSI-L. Dis. Markers 2004, 20, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Yamamoto, H. Carcinogenesis and microsatellite instability: The interrelationship between genetics and epigenetics. Carcinogenesis 2008, 29, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Latham, A.; Srinivasan, P.; Kemel, Y.; Shia, J.; Bandlamudi, C.; Mandelker, D.; Middha, S.; Hechtman, J.; Zehir, A.; Dubard-Gault, M.; et al. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J. Clin. Oncol. 2019, 37, 286–295. [Google Scholar] [CrossRef]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis. Int. J. Gynecol. Pathol. 2019, 38, S64–S74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra-Herran, C.; Lerner-Ellis, J.; Xu, B.; Khalouei, S.; Bassiouny, D.; Cesari, M.; Ismiil, N.; Nofech-Mozes, S. Molecular-based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod. Pathol. 2017, 30, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, I.C.; Ubachs, J.E.H.; Stelloo, E.; de Kroon, C.D.; Goeman, J.J.; Smit, V.T.H.B.M.; Creutzberg, C.L.; Bosse, T. Blinded histopathological characterisation of POLE exonuclease domain—Mutant endometrial cancers: Sheep in wolf’s clothing. Histopathology 2018, 72, 248–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, L.N.; McConechy, M.K.; Köbel, M.; Anglesio, M.; Senz, J.; Maassen, M.; Kommoss, S.; Meng, B.; Postovit, L.; Kelemen, L.E.; et al. Polymerase Epsilon Exonuclease Domain Mutations in Ovarian Endometrioid Carcinoma. Int. J. Gynecol. Cancer 2015, 25, 1187–1193. [Google Scholar] [CrossRef]
- Leskela, S.; Romero, I.; Cristobal, E.; Pérez-Mies, B.; Rosa-Rosa, J.M.; Gutierrez-Pecharroman, A.; Caniego-Casas, T.; Santón, A.; Ojeda, B.; López-Reig, R.; et al. Mismatch Repair Deficiency in Ovarian Carcinoma. Am. J. Surg. Pathol. 2020, 44, 649–656. [Google Scholar] [CrossRef]
- Hicks, S.C.; Ward, R.L.; Hawkins, N.J. Immunohistochemistry for PMS2 and MSH6 alone can replace a four antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma. Pathology 2011, 43, 84–85. [Google Scholar] [CrossRef]
- O’Regan, T.; Chau, K.; Tatton, T.; Smith, T.; Parry, S.; Bissett, I. Immunochemistry screening for Lynch syndrome in colorectal adenocarcinoma using an initial two antibody panel can replace a four antibody panel. N. Z. Med. J. 2013, 126, 70–77. [Google Scholar]
- Wernicke, A. Immunohistochemistry for detecting colorectal (CRC) and endometrial cancer (EC) patients at risk for Lynch Syndrome (LS). Utility of a 2-antibody panel. Virchows Arch. 2018, 473, S72. [Google Scholar]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.-Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [Green Version]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase e–Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319. [Google Scholar] [CrossRef]
- Gargiulo, P.; Della Pepa, C.; Berardi, S.; Califano, D.; Scala, S.; Buonaguro, L.; Ciliberto, G.; Brauchli, P.; Pignata, S. Tumor genotype and immune microenvironment in POLE-ultramutated and MSI-hypermutated Endometrial Cancers: New candidates for checkpoint blockade immunotherapy? Cancer Treat. Rev. 2016, 48, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; DeFazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Rambau, P.; Kelemen, L.; Steed, H.; Quan, M.; Ghatage, P.; Köbel, M. Association of Hormone Receptor Expression with Survival in Ovarian Endometrioid Carcinoma: Biological Validation and Clinical Implications. Int. J. Mol. Sci. 2017, 18, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Roock, W.; De Vriendt, V.; Normanno, N.; Ciardiello, F.; Tejpar, S. KRAS, BRAF, PIK3CA, and PTEN mutations: Implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011, 12, 594–603. [Google Scholar] [CrossRef]
- Bosse, T.; Ter Haar, N.T.; Seeber, L.M.; Diest, P.J.v.; Hes, F.J.; Vasen, H.F.; Nout, R.A.; Creutzberg, C.L.; Morreau, H.; Smit, V.T. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod. Pathol. 2013, 26, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Janku, F.; Hong, D.S.; Fu, S.; Piha-Paul, S.A.; Naing, A.; Falchook, G.S.; Tsimberidou, A.M.; Stepanek, V.M.; Moulder, S.L.; Lee, J.J.; et al. Assessing PIK3CA and PTEN in Early-Phase Trials with PI3K/AKT/mTOR Inhibitors. Cell Rep. 2014, 6, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Rambau, P.F.; Kelemen, L.E.; Anglesio, M.S.; Leung, S.; Talhouk, A.; Köbel, M. Nuclear β-catenin and CDX2 expression in ovarian endometrioid carcinoma identify patients with favourable outcome. Histopathology 2019, 74, 452–462. [Google Scholar] [CrossRef]
- Zyla, R.E.; Olkhov-Mitsel, E.; Amemiya, Y.; Bassiouny, D.; Seth, A.; Djordjevic, B.; Nofech-Mozes, S.; Parra-Herran, C. CTNNB1 Mutations and Aberrant β-Catenin Expression in Ovarian Endometrioid Carcinoma. Am. J. Surg. Pathol. 2021, 45, 68–76. [Google Scholar] [CrossRef]
- Rambau, P.F.; Vierkant, R.A.; Intermaggio, M.P.; Kelemen, L.E.; Goodman, M.T.; Herpel, E.; Pharoah, P.D.; Kommoss, S.; Jimenez-Linan, M.; Karlan, B.Y.; et al. Association of p16 expression with prognosis varies across ovarian carcinoma histotypes: An Ovarian Tumor Tissue Analysis consortium study. J. Pathol. Clin. Res. 2018, 4, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.A.; Dong, F.; Young, R.H.; Oliva, E. Clear cell carcinoma of the ovary: Evaluation of prognostic parameters based on a clinicopathological analysis of 100 cases. Histopathology 2015, 66, 808–815. [Google Scholar] [CrossRef]
- Sangoi, A.R.; Soslow, R.A.; Teng, N.N.; Longacre, T.A. Ovarian Clear Cell Carcinoma With Papillary Features: A Potential Mimic of Serous Tumor of Low Malignant Potential. Am. J. Surg. Pathol. 2008, 32, 269–274. [Google Scholar] [CrossRef] [PubMed]
- DeLair, D.; Oliva, E.; Köbel, M.; Macias, A.; Gilks, C.B.; Soslow, R.A. Morphologic Spectrum of Immunohistochemically Characterized Clear Cell Carcinoma of the Ovary. Am. J. Surg. Pathol. 2011, 35, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Young, R.H.; Scully, R.E. Oxyphilic Clear Cell Carcinoma of the Ovary A Report of Nine Cases. Am. J. Surg. Pathol. 1987, 11, 661–662. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Young, R.H. Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumors. Semin. Diagn. Pathol. 2005, 22, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Magno, E.; Zhang, R. SWI/SNF Complexes in Ovarian Cancer: Mechanistic Insights and Therapeutic Implications. Mol. Cancer Res. 2018, 16, 1819–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, I.G.; Russell, S.E.; Choong, D.Y.H.; Montgomery, K.G.; Ciavarella, M.L.; Hooi, C.S.F.; Cristiano, B.E.; Pearson, R.B.; Phillips, W.A. Mutation of the PIK3CA Gene in Ovarian and Breast Cancer. Cancer Res. 2004, 64, 7678–7681. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.-C.; Ayhan, A.; Maeda, D.; Kim, K.-R.; Clarke, B.A.; Shaw, P.; Chui, M.H.; Rosen, B.; Shih, I.-M.; Wang, T.-L. Frequent somatic mutations of the telomerase reverse transcriptase promoter in ovarian clear cell carcinoma but not in other major types of gynaecological malignancy. J. Pathol. 2014, 232, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Li, Y.; Pan, L. The target therapy of ovarian clear cell carcinoma. Onco Targets Ther. 2014, 2014, 1647–1652. [Google Scholar] [CrossRef] [Green Version]
- Chui, M.H.; Gilks, C.B.; Cooper, K.; Clarke, B.A. Identifying Lynch Syndrome in Patients With Ovarian Carcinoma. Adv. Anat. Pathol. 2013, 20, 378–386. [Google Scholar] [CrossRef]
- Gadducci, A.; Guerrieri, M.E. Immune Checkpoint Inhibitors in Gynecological Cancers: Update of Literature and Perspectives of Clinical Research. Anticancer Res. 2017, 37. [Google Scholar] [CrossRef] [Green Version]
- Morice, P.; Gouy, S.; Leary, A. Mucinous Ovarian Carcinoma. N. Engl. J. Med. 2019, 380, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Scully, R.E. Mucinous Tumors of the Ovary. Am. J. Surg. Pathol. 2000, 24, 1447–1464. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P. Morphologic, Immunophenotypic, and Molecular Features of Epithelial Ovarian Cancer. Oncology 2016, 30, 166–176. [Google Scholar] [PubMed]
- Kelemen, L.E.; Köbel, M. Mucinous carcinomas of the ovary and colorectum: Different organ, same dilemma. Lancet Oncol. 2011, 12, 1071–1080. [Google Scholar] [CrossRef]
- Moh, M.; Krings, G.; Ates, D.; Aysal, A.; Kim, G.E.; Rabban, J.T. SATB2 Expression Distinguishes Ovarian Metastases of Colorectal and Appendiceal Origin From Primary Ovarian Tumors of Mucinous or Endometrioid Type. Am. J. Surg. Pathol. 2016, 40, 419–432. [Google Scholar] [CrossRef]
- Cheasley, D.; Wakefield, M.J.; Ryland, G.L.; Allan, P.E.; Alsop, K.; Amarasinghe, K.C.; Ananda, S.; Anglesio, M.S.; Au-Yeung, G.; Böhm, M.; et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat. Commun. 2019, 10, 3935. [Google Scholar] [CrossRef] [Green Version]
- McAlpine, J.N.; Wiegand, K.C.; Vang, R.; Ronnett, B.M.; Adamiak, A.; Köbel, M.; Kalloger, S.E.; Swenerton, K.D.; Huntsman, D.G.; Gilks, C.B.; et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer 2009, 9, 433. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.; McCluggage, W.G. Ovarian Seromucinous Carcinoma. Am. J. Surg. Pathol. 2015, 39, 983–992. [Google Scholar] [CrossRef]
- Rambau, P.F.; McIntyre, J.B.; Taylor, J.; Lee, S.; Ogilvie, T.; Sienko, A.; Morris, D.; Duggan, M.A.; McCluggage, W.G.; Köbel, M. Morphologic Reproducibility, Genotyping, and Immunohistochemical Profiling Do Not Support a Category of Seromucinous Carcinoma of the Ovary. Am. J. Surg. Pathol. 2017, 41, 685–695. [Google Scholar] [CrossRef]
- Pors, J.; Cheng, A.; Leo, J.M.; Kinloch, M.A.; Gilks, B.; Hoang, L. A Comparison of GATA3, TTF1, CD10, and Calretinin in Identifying Mesonephric and Mesonephric-like Carcinomas of the Gynecologic Tract. Am. J. Surg. Pathol. 2018, 42, 1596–1606. [Google Scholar] [CrossRef]
- Mirkovic, J.; McFarland, M.; Garcia, E.; Sholl, L.M.; Lindeman, N.; MacConaill, L.; Dong, F.; Hirsch, M.; Nucci, M.R.; Quick, C.M.; et al. Targeted Genomic Profiling Reveals Recurrent KRAS Mutations in Mesonephric-like Adenocarcinomas of the Female Genital Tract. Am. J. Surg. Pathol. 2018, 42, 227–233. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Vosmikova, H.; Laco, J. Ovarian Combined Low-grade Serous and Mesonephric-like Adenocarcinoma. Int. J. Gynecol. Pathol. 2020, 39, 84–92. [Google Scholar] [CrossRef]
- Espinosa, I.; De Leo, A.; D’Angelo, E.; Rosa-Rosa, J.M.; Corominas, M.; Gonzalez, A.; Palacios, J.; Prat, J. Dedifferentiated endometrial carcinomas with neuroendocrine features: A clinicopathologic, immunohistochemical, and molecular genetic study. Hum. Pathol. 2018, 72, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Bellone, S.; Lopez, S.; Thakral, D.; Schwab, C.; English, D.P.; Black, J.; Cocco, E.; Choi, J.; Zammataro, L.; et al. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2016, 113, 12238–12243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Carmen, M.G.; Birrer, M.; Schorge, J.O. Carcinosarcoma of the ovary: A review of the literature. Gynecol. Oncol. 2012, 125, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Berton-Rigaud, D.; Devouassoux-Shisheboran, M.; Ledermann, J.A.; Leitao, M.M.; Powell, M.A.; Poveda, A.; Beale, P.; Glasspool, R.M.; Creutzberg, C.L.; Harter, P.; et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Uterine and Ovarian Carcinosarcoma. Int. J. Gynecol. Cancer 2014, 24, S55–S60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, R.A.; Nijman, H.W.; Wijbrandi, T.F.; Reyners, A.K.L.; Boezen, H.M.; Hollema, H. Molecular markers and clinical behavior of uterine carcinosarcomas: Focus on the epithelial tumor component. Mod. Pathol. 2011, 24, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Stransky, N.; McCord, C.L.; Cerami, E.; Lagowski, J.; Kelly, D.; Angiuoli, S.V.; Sausen, M.; Kann, L.; Shukla, M.; et al. Genomic analyses of gynaecologic carcinosarcomas reveal frequent mutations in chromatin remodelling genes. Nat. Commun. 2014, 5, 5006. [Google Scholar] [CrossRef] [Green Version]
- Cherniack, A.D.; Shen, H.; Walter, V.; Stewart, C.; Murray, B.A.; Bowlby, R.; Hu, X.; Ling, S.; Soslow, R.A.; Broaddus, R.R.; et al. Integrated Molecular Characterization of Uterine Carcinosarcoma. Cancer Cell 2017, 31, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Segura, S.E.; Pedra Nobre, S.; Hussein, Y.R.; Abu-Rustum, N.R.; Weigelt, B.; Soslow, R.A.; DeLair, D.F. DNA Mismatch Repair–deficient Endometrial Carcinosarcomas Portend Distinct Clinical, Morphologic, and Molecular Features Compared With Traditional Carcinosarcomas. Am. J. Surg. Pathol. 2020, 44, 1573–1579. [Google Scholar] [CrossRef]



| Molecular Markers | Clinical Significance | Immunohistochemistry Available/ Currently Used in Clinical Practice |
|---|---|---|
| TP53 | Found in >95% HGSCs | Yes/Yes * |
| BRCA1/2 | Better prognosis, eligible for PARPi therapy | Yes/No |
| PD1/PD-L1 | Better prognosis in late-stage HGSCs | Yes/No |
| Molecular Markers | Clinical Significance | Immunohistochemistry Available/ Currently Used in Clinical Practice |
|---|---|---|
| BRAFV600E | Good prognosis, candidate for Trametinib therapy | Yes/Yes |
| Hormone receptors | Candidate for HRT (only advanced-stage LGSC) | Yes/Yes |
| KRAS | Poor prognosis | No/No |
| ERBB2 | Unknown | Yes/No |
| TCGA-EEC Class | Molecular Surrogate | Clinical Significance | Immunohistochemistry Available/ Currently Used in Clinical Practice |
|---|---|---|---|
| Hypermutated | POLE sequencing (Sanger) | Excellent prognosis, candidate for checkpoint inhibition | No/Yes |
| Ultramutated | MSI assay | Intermediate prognosis, candidate for checkpoint inhibition | Yes/Yes |
| CN-high | TP53 sequencing | Poor prognosis | Yes/Yes |
| CN-low | POLE/MSI/TP53 wild-type | Intermediate prognosis | Yes/Yes |
| Molecular Markers | Clinical Significance | Immunohistochemistry Available/ Currently Used in Clinical Practice |
|---|---|---|
| PD1/PD-L1 | Candidate for checkpoint inhibition | Yes/Yes (selected cases) |
| Hormone receptors | Better prognosis, candidate for HRT | Yes/Yes |
| PTEN | Target for PI3K/AKT inhibition | Yes/No * |
| ARID1A | Target for EZH2 and HDAC inhibition | Yes/No * |
| CTNNB1 | Good prognosis | Yes/No |
| CDKN2A | Worse prognosis | Yes/No |
| Molecular Markers | Histotype | Immunohistochemistry Surrogate Available | Comments |
|---|---|---|---|
| TP53 | HGSC, ENOC, CCC, CS | Yes | Mutated in 96% HGSCs Worse prognosis in ENOCs |
| BRCA1, BRCA2 | HGSC, CS | Yes | Better prognosis, PARPi eligible |
| BRAF | LGSC | Yes | Lower recurrence rate, better prognosis possibility of targeted therapy in advanced LGSC |
| KRAS | LGSC, MLA, CS | No | Higher recurrence rate, worse prognosis |
| ERBB2 | LGSC, MC | Yes | Better prognosis in MC, no data available in LGSC |
| Hormone receptors | LGSC, ENOC | Yes | Diffuse expression associated with better prognosis, possibility of HRT in LGSC and low-grade ENOC |
| MLH1, PMS2, MSH2, MSH6 | ENOC, CCC, MC, DEDC/UC | Yes (MLH1 methylation analysis required for MLH1/PMS2 loss) | Better prognosis, 80% cases due to somatic MLH1 hypermethylation, germline mutations associated with Lynch Syndrome, high PD-L1 expression (checkpoint inhibition candidate) |
| POLE | ENOC, CCC, DEDC/UC | No | Excellent prognosis, high PD-L1 expression (checkpoint inhibition candidate) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santandrea, G.; Piana, S.; Valli, R.; Zanelli, M.; Gasparini, E.; De Leo, A.; Mandato, V.D.; Palicelli, A. Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature. Diagnostics 2021, 11, 199. https://doi.org/10.3390/diagnostics11020199
Santandrea G, Piana S, Valli R, Zanelli M, Gasparini E, De Leo A, Mandato VD, Palicelli A. Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature. Diagnostics. 2021; 11(2):199. https://doi.org/10.3390/diagnostics11020199
Chicago/Turabian StyleSantandrea, Giacomo, Simonetta Piana, Riccardo Valli, Magda Zanelli, Elisa Gasparini, Antonio De Leo, Vincenzo Dario Mandato, and Andrea Palicelli. 2021. "Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature" Diagnostics 11, no. 2: 199. https://doi.org/10.3390/diagnostics11020199
APA StyleSantandrea, G., Piana, S., Valli, R., Zanelli, M., Gasparini, E., De Leo, A., Mandato, V. D., & Palicelli, A. (2021). Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature. Diagnostics, 11(2), 199. https://doi.org/10.3390/diagnostics11020199







