Local Bone Mineral Density, Subcutaneous and Visceral Adipose Tissue Measurements in Routine Multi Detector Computed Tomography—Which Parameter Predicts Incident Vertebral Fractures Best?
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
2. Materials and Methods
2.1. Participants and Setting
2.2. Study Design
2.3. Multi Detector Computed Tomography (MDCT) Imaging
2.4. Quantitative Variables
2.4.1. Bone Mineral Density (BMD) Measurements
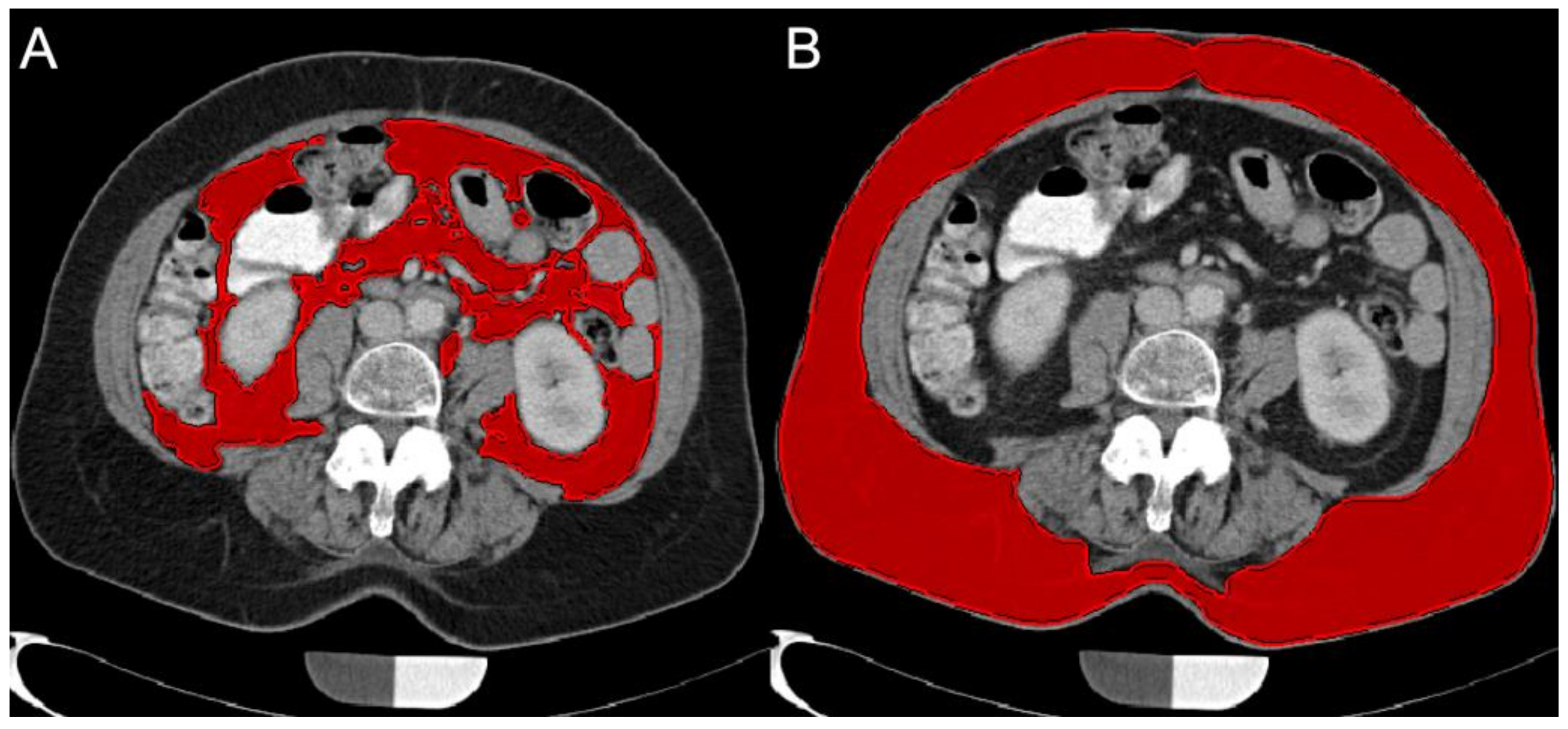
2.4.2. Subcutaneous Adipose Tissue (SAT) and Visceral Adipose Tissue (VAT) Volume Quantification
2.5. Statistical Methods
3. Results
3.1. Participants
3.2. Descriptive Data
3.3. Outcome Data
3.4. Main Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef]
- Ioannidis, G.; Papaioannou, A.; Hopman, W.M.; Akhtar-Danesh, N.; Anastassiades, T.; Pickard, L.; Kennedy, C.C.; Prior, J.C.; Olszynski, W.P.; Davison, K.S.; et al. Relation between fractures and mortality: Results from the Canadian Multicentre Osteoporosis Study. Can. Med. Assoc. J. 2009, 181, 265–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melton, L.J.; Atkinson, E.J.; Cooper, C.; O’Fallon, W.M.; Riggs, B.L. Vertebral fractures predict subsequent fractures. Osteoporos Int. 1999, 10, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J.; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; McCloskey, E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008, 19, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A.; Initiative, T.F.O.T.F.; Hans, D.; Cooper, C.; Baim, S.; Bilezikian, J.P.; Binkley, N.; Cauley, J.A.; Compston, J.E.; Dawson-Hughes, B.; et al. Interpretation and use of FRAX in clinical practice. Osteoporos. Int. 2011, 22, 2395–2411. [Google Scholar] [CrossRef]
- Crandall, C.J.; Schousboe, J.T.; Morin, S.N.; Lix, L.M.; Leslie, W. Performance of FRAX and FRAX-Based Treatment Thresholds in Women Aged 40 Years and Older: The Manitoba BMD Registry. J. Bone Miner. Res. 2019, 34, 1419–1427. [Google Scholar] [CrossRef]
- Löffler, M.T.; Sollmann, N.; Mei, K.; Valentinitsch, A.; Noël, P.; Kirschke, J.; Baum, T. X-ray-based quantitative osteoporosis imaging at the spine. Osteoporos. Int. 2019, 31, 233–250. [Google Scholar] [CrossRef]
- Löffler, M.T.; Jacob, A.; Valentinitsch, A.; Rienmüller, A.; Zimmer, C.; Ryang, Y.-M.; Baum, T.; Kirschke, J.S. Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. Eur. Radiol. 2019, 29, 4980–4989. [Google Scholar] [CrossRef] [Green Version]
- Engelke, K. Quantitative Computed Tomography—Current Status and New Developments. J. Clin. Densitom. 2017, 20, 309–321. [Google Scholar] [CrossRef]
- Jang, S.; Graffy, P.M.; Ziemlewicz, T.J.; Lee, S.J.; Summers, R.M.; Pickhardt, P.J. Opportunistic Osteoporosis Screening at Routine Abdominal and Thoracic CT: Normative L1 Trabecular Attenuation Values in More than 20 000 Adults. Radiol. 2019, 291, 360–367. [Google Scholar] [CrossRef]
- Baum, T.; Müller, D.; Dobritz, M.; Wolf, P.; Rummeny, E.J.; Link, T.M.; Bauer, J.S. Converted Lumbar BMD Values Derived from Sagittal Reformations of Contrast-Enhanced MDCT Predict Incidental Osteoporotic Vertebral Fractures. Calcif. Tissue Int. 2012, 90, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Shahnazari, M.; Orwoll, E.S.; Lane, N.E. Osteoporosis in men: Findings from the Osteoporotic Fractures in Men Study (MrOS). Ther. Adv. Musculoskelet. Dis. 2016, 8, 15–27. [Google Scholar] [CrossRef]
- Nielson, C.M.; Marshall, L.M.; Adams, A.L.; Leblanc, E.S.; Cawthon, P.M.; Ensrud, K.; Stefanick, M.L.; Barrett-Connor, E.; Orwoll, E.S.; the Osteoporotic Fractures in Men Study (MrOS) Research Group. BMI and fracture risk in older men: The osteoporotic fractures in men study (MrOS). J. Bone Miner. Res. 2011, 26, 496–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheu, Y.; Marshall, L.M.; Holton, K.F.; Caserotti, P.; Boudreau, R.M.; Strotmeyer, E.S.; Cawthon, P.; Cauley, J.A. Abdominal body composition measured by quantitative computed tomography and risk of non-spine fractures: The Osteoporotic Fractures in Men (MrOS) study. Osteoporos. Int. 2013, 24, 2231–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollmann, N.; Franz, D.; Burian, E.; Löffler, M.T.; Probst, M.; Gersing, A.; Schwaiger, B.J.; Pfeiffer, D.; Kirschke, J.; Baum, T.; et al. Assessment of paraspinal muscle characteristics, lumbar BMD, and their associations in routine multi-detector CT of patients with and without osteoporotic vertebral fractures. Eur. J. Radiol. 2020, 125, 108867. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Mendes, N.; Miller, K.K.; Rosen, C.J.; Lee, H.; Klibanski, A.; Misra, M. Visceral Fat Is a Negative Predictor of Bone Density Measures in Obese Adolescent Girls. J. Clin. Endocrinol. Metab. 2010, 95, 1247–1255. [Google Scholar] [CrossRef]
- Paula, F.; De Araújo, I.M.; Carvalho, A.L.; Elias, J.; Salmon, C.E.G.; Nogueira-Barbosa, M.H. The Relationship of Fat Distribution and Insulin Resistance with Lumbar Spine Bone Mass in Women. PLoS ONE 2015, 10, e0129764. [Google Scholar] [CrossRef]
- Beaudoin, C.; Moore, L.; Gagné, M.; Bessette, L.; Ste-Marie, L.G.; Brown, J.P.; Jean, S. Performance of predictive tools to identify individuals at risk of non-traumatic fracture: A systematic review, meta-analysis, and meta-regression. Osteoporos. Int. 2019, 30, 721–740. [Google Scholar] [CrossRef]
- Kaze, A.D.; Rosen, H.N.; Paik, J.M. A meta-analysis of the association between body mass index and risk of vertebral fracture. Osteoporos. Int. 2017, 29, 31–39. [Google Scholar] [CrossRef]
- Bauer, J.S.; Henning, T.D.; Müeller, D.; Lu, Y.; Majumdar, S.; Link, T. Volumetric Quantitative CT of the Spine and Hip Derived from Contrast-Enhanced MDCT: Conversion Factors. Am. J. Roentgenol. 2007, 188, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Baum, T.; Müller, D.; Dobritz, M.; Rummeny, E.; Link, T.; Bauer, J.S. BMD measurements of the spine derived from sagittal reformations of contrast-enhanced MDCT without dedicated software. Eur. J. Radiol. 2011, 80, e140–e145. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.B.; Demontiero, O.; Vogrin, S.; Ng, A.; Duque, G. Marrow Adipose Tissue in Older Men: Association with Visceral and Subcutaneous Fat, Bone Volume, Metabolism, and Inflammation. Calcif. Tissue Int. 2018, 103, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Muehlematter, U.J.; Mannil, M.; Becker, A.; Vokinger, K.N.; Finkenstaedt, T.; Osterhoff, G.; Fischer, M.A.; Guggenberger, R. Vertebral body insufficiency fractures: Detection of vertebrae at risk on standard CT images using texture analysis and machine learning. Eur. Radiol. 2019, 29, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Valentinitsch, A.; Trebeschi, S.; Kaesmacher, J.; Lorenz, C.; Löffler, M.T.; Zimmer, C.; Baum, T.; Kirschke, J. Opportunistic osteoporosis screening in multi-detector CT images via local classification of textures. Osteoporos. Int. 2019, 30, 1275–1285. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Allaire, B.T.; Lu, D.; Johannesdottir, F.; Kopperdahl, D.; Keaveny, T.M.; Jarraya, M.; Guermazi, A.; Bredella, M.A.; Samelson, E.J.; Kiel, D.P.; et al. Prediction of incident vertebral fracture using CT-based finite element analysis. Osteoporos. Int. 2019, 30, 323–331. [Google Scholar] [CrossRef]
- Guise, T.A. Bone Loss and Fracture Risk Associated with Cancer Therapy. Oncology 2006, 11, 1121–1131. [Google Scholar] [CrossRef]
- Paik, J.M.; Rosen, H.N.; Katz, J.N.; Rosner, B.A.; Rimm, E.B.; Gordon, C.M.; Curhan, G.C. BMI, Waist Circumference, and Risk of Incident Vertebral Fracture in Women. Obesity 2019, 27, 1513–1519. [Google Scholar] [CrossRef]
- Liu, C.-T.; Broe, K.E.; Zhou, Y.; Boyd, S.K.; Cupples, L.A.; Hannan, M.T.; Lim, E.; McLean, R.R.; Samelson, E.J.; Bouxsein, M.L.; et al. Visceral Adipose Tissue Is Associated With Bone Microarchitecture in the Framingham Osteoporosis Study. J. Bone Miner. Res. 2017, 32, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Bering, T.; Diniz, K.G.; Coelho, M.P.P.; Vieira, D.A.; Soares, M.M.S.; Kakehasi, A.M.; Correia, M.I.T.; Teixeira, R.; Queiroz, D.M.; Rocha, G.A.; et al. Association between pre-sarcopenia, sarcopenia, and bone mineral density in patients with chronic hepatitis C. J. Cachex-Sarcopenia Muscle 2018, 9, 255–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapses, S.A.; Sukumar, D. Bone Metabolism in Obesity and Weight Loss. Annu. Rev. Nutr. 2012, 32, 287–309. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, I.; Parreiras-E-Silva, L.; Carvalho, A.; Elias, J.; Salmon, C.; De Paula, F. Insulin resistance negatively affects bone quality not quantity: The relationship between bone and adipose tissue. Osteoporos. Int. 2020, 31, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; Maffazioli, G.D.; Sokoloff, N.C.; Ackerman, K.E.; Lee, H.; Gupta, N.; Clarke, H.; Slattery, M.; Bredella, M.A.; Misra, M. Regional fat depots and their relationship to bone density and microarchitecture in young oligo-amenorrheic athletes. Bone 2015, 77, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickhardt, P.J.; Graffy, P.M.; Zea, R.; Lee, S.J.; Liu, J.; Sandfort, V.; Summers, R.M. Automated Abdominal CT Imaging Biomarkers for Opportunistic Prediction of Future Major Osteoporotic Fractures in Asymptomatic Adults. Radiology 2020, 297, 64–72. [Google Scholar] [CrossRef]
- Johnson, R.W.; Suva, L.J. Hallmarks of Bone Metastasis. Calcif. Tissue Int. 2018, 102, 141–151. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Status | Mean | SD | p |
|---|---|---|---|---|
| Age (years) | 0 | 66.7 | 9.0 | 0.972 |
| 1 | 65.9 | 9.4 | ||
| Follow-up (months) | 0 | 22.7 | 12.5 | 0.543 |
| 1 | 20.1 | 10.6 | ||
| Th5 (mg/mL) | 0 | 176.4 | 33.1 | 0.079 |
| 1 | 142.2 | 17.9 | ||
| Th6 (mg/mL) | 0 | 165.3 | 39.7 | 0.234 |
| 1 | 132.8 | 32.5 | ||
| Th7 (mg/mL) | 0 | 166.3 | 22.3 | 0.008 |
| 1 | 128.4 | 32.5 | ||
| Th8 (mg/mL) | 0 | 151.4 | 30.4 | 0.061 |
| 1 | 123.4 | 27.6 | ||
| Th9 (mg/mL) | 0 | 158.9 | 20.9 | 0.005 |
| 1 | 124.1 | 27.5 | ||
| Th10 (mg/mL) | 0 | 162.2 | 29.7 | 0.112 |
| 1 | 135.7 | 27.4 | ||
| Th11 (mg/mL) | 0 | 154.0 | 25.5 | 0.034 |
| 1 | 129.4 | 20.5 | ||
| Th12 (mg/mL) | 0 | 141.7 | 26.9 | 0.041 |
| 1 | 116.3 | 16.0 | ||
| L1 (mg/mL) | 0 | 136.8 | 32.4 | 0.121 |
| 1 | 115.1 | 25.1 | ||
| L2 (mg/mL) | 0 | 137.4 | 32.6 | 0.088 |
| 1 | 113.3 | 21.7 | ||
| L3 (mg/mL) | 0 | 127.1 | 30.3 | 0.278 |
| 1 | 111.9 | 19.7 | ||
| L4 (mg/mL) | 0 | 128.8 | 31.7 | 0.569 |
| 1 | 113.6 | 25.6 | ||
| L5 (mg/mL) | 0 | 133.3 | 47.9 | 0.955 |
| 1 | 117.9 | 40.1 | ||
| L1-L3 (mg/mL) | 0 | 133.8 | 29.4 | 0.140 |
| 1 | 112.6 | 19.5 | ||
| VAT (cm3) | 0 | 266.3 | 262.7 | 0.589 |
| 1 | 245.0 | 260.2 | ||
| SAT (cm3) | 0 | 519.3 | 339.8 | 0.829 |
| 1 | 514.0 | 347.5 | ||
| VAT/SAT | 0 | 0.6 | 0.6 | 0.914 |
| 1 | 0.5 | 0.5 |
| Parameters | Odds Ratio | CI | AUC | SE | p |
|---|---|---|---|---|---|
| Th5 | 1.05 | 1.01–1.09 | 0.781 | 0.090 | 0.014 |
| Th6 | 1.03 | 1.00–1.05 | 0.722 | 0.097 | 0.051 |
| Th7 | 1.07 | 1.01–1.14 | 0.877 | 0.066 | 0.001 |
| Th8 | 1.04 | 1.01–1.07 | 0.738 | 0.099 | 0.036 |
| Th9 | 1.06 | 1.02–1.10 | 0.818 | 0.089 | 0.005 |
| Th10 | 1.03 | 1.01–1.06 | 0.754 | 0.100 | 0.025 |
| Th11 | 1.04 | 1.01–1.08 | 0.754 | 0.094 | 0.025 |
| Th12 | 1.05 | 1.01–1.10 | 0.749 | 0.096 | 0.029 |
| L1 | 1.03 | 1.00–1.05 | 0.668 | 0.106 | 0.138 |
| L2 | 1.03 | 1.01–1.06 | 0.658 | 0.104 | 0.165 |
| L3 | n.s. | 0.663 | 0.104 | 0.151 | |
| L4 | n.s. | 0.604 | 0.111 | 0.359 | |
| L5 | n.s. | 0.481 | 0.115 | 0.869 | |
| L1-L3 | 1.04 | 1.01–1.07 | 0.684 | 0.104 | 0.105 |
| VAT | n.s. | 0.578 | 0.112 | 0.495 | |
| SAT | n.s. | 0.497 | 0.116 | 0.981 | |
| VAT/SAT | n.s. | 0.519 | 0.118 | 0.869 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burian, E.; Grundl, L.; Greve, T.; Junker, D.; Sollmann, N.; Löffler, M.; Makowski, M.R.; Zimmer, C.; Kirschke, J.S.; Baum, T. Local Bone Mineral Density, Subcutaneous and Visceral Adipose Tissue Measurements in Routine Multi Detector Computed Tomography—Which Parameter Predicts Incident Vertebral Fractures Best? Diagnostics 2021, 11, 240. https://doi.org/10.3390/diagnostics11020240
Burian E, Grundl L, Greve T, Junker D, Sollmann N, Löffler M, Makowski MR, Zimmer C, Kirschke JS, Baum T. Local Bone Mineral Density, Subcutaneous and Visceral Adipose Tissue Measurements in Routine Multi Detector Computed Tomography—Which Parameter Predicts Incident Vertebral Fractures Best? Diagnostics. 2021; 11(2):240. https://doi.org/10.3390/diagnostics11020240
Chicago/Turabian StyleBurian, Egon, Lioba Grundl, Tobias Greve, Daniela Junker, Nico Sollmann, Maximilian Löffler, Marcus R. Makowski, Claus Zimmer, Jan S. Kirschke, and Thomas Baum. 2021. "Local Bone Mineral Density, Subcutaneous and Visceral Adipose Tissue Measurements in Routine Multi Detector Computed Tomography—Which Parameter Predicts Incident Vertebral Fractures Best?" Diagnostics 11, no. 2: 240. https://doi.org/10.3390/diagnostics11020240







