Epicardial Adiposity in Relation to Metabolic Abnormality, Circulating Adipocyte FABP, and Preserved Ejection Fraction Heart Failure
Abstract
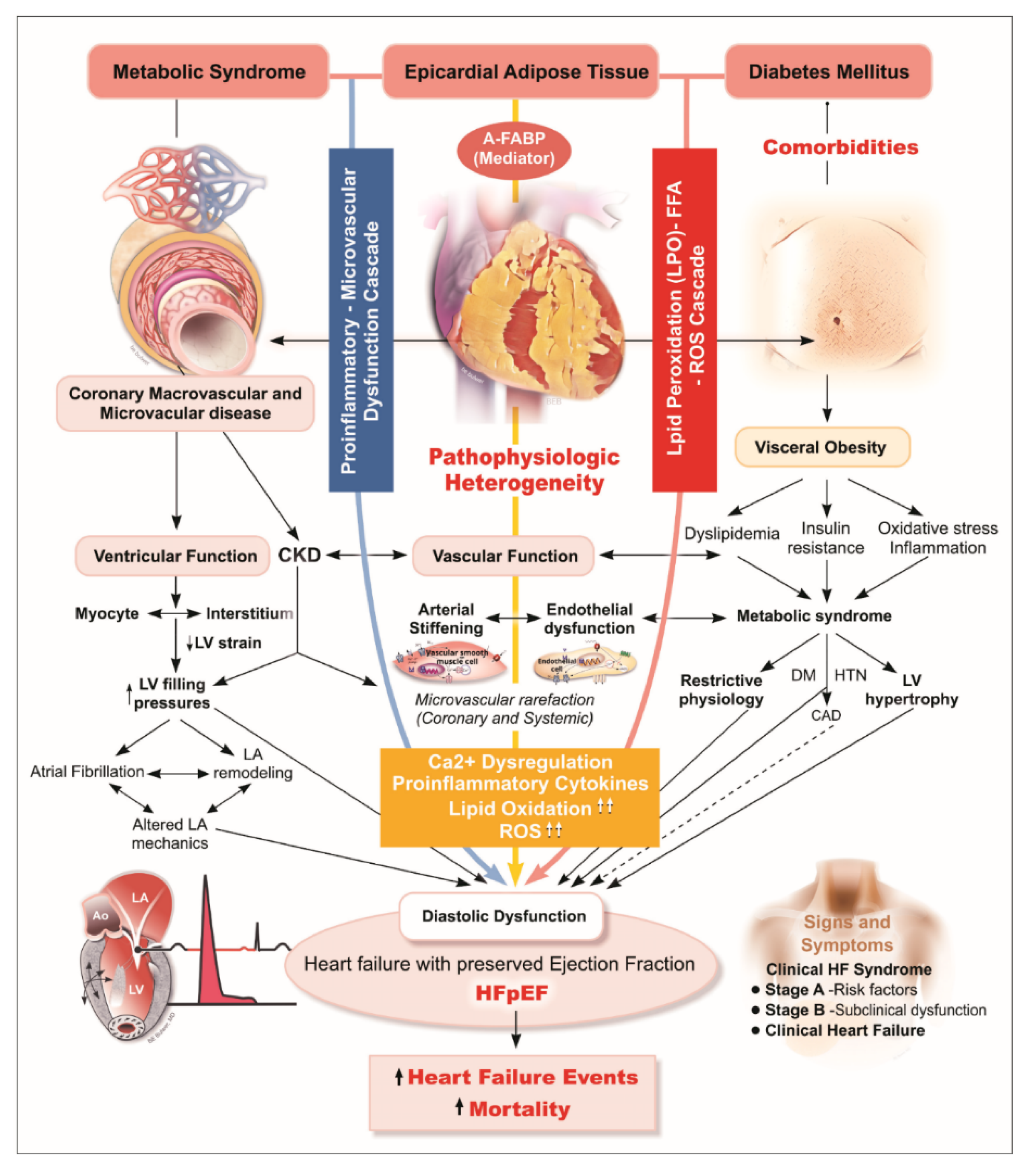
:1. Introduction
2. Methods
2.1. Study Subjects
2.2. Anthropometric Measurements
2.3. Biochemical Analysis of Pro-Inflammatory and HF Markers
2.4. Measures of EAT, and Cardiac Structure and Function
2.5. Validating EAT with CT-Based PCF Measurement
2.6. Statistical Analysis
3. Results
3.1. Clinical Demographical and Metabolic Relevance of EAT
3.2. Associations of EAT with Pro-Inflammatory/HF Markers
3.3. Associations of EAT with Cardiac Structure and Function
3.4. Association of EAT with Incident HF: Mediator Analysis
4. Discussion
4.1. Functional and Prognostic Significance of EAT as a Surrogate of Visceral Obesity
4.2. Associations of EAT with Circulating A-FABP
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.H.; Deswal, A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bank, I.E.M.; Gijsberts, C.M.; Teng, T.K.; Benson, L.; Sim, D.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Leong, G.K.T.; Ling, L.H.; et al. Prevalence and clinical significance of diabetes in Asian versus white patients with heart failure. JACC Heart Fail. 2017, 5, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hajer, G.R.; Van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef] [Green Version]
- Yatagai, T.; Nagasaka, S.; Taniguchi, A.; Fukushima, M.; Nakamura, T.; Kuroe, A.; Nakai, Y.; Ishibashi, S. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003, 52, 1274–1278. [Google Scholar] [CrossRef]
- Lopes, H.F.; Correa-Giannella, M.L.; Consolim-Colombo, F.M.; Egan, B.M. Visceral adiposity syndrome. Diabetol. Metab. Syndr. 2016, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the Asia-Pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [Green Version]
- Haass, M.; Kitzman, D.W.; Anand, I.S.; Miller, A.; Zile, M.R.; Massie, B.M.; Carson, P.E. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-preserve) trial. Circ. Heart Fail. 2011, 4, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tromp, J.; Tay, W.T.; Ouwerkerk, W.; Teng, T.K.; Yap, J.; MacDonald, M.R.; Leineweber, K.; McMurray, J.J.V.; Zile, M.R.; Anand, I.S.; et al. Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry. PLoS Med. 2018, 15, e1002541. [Google Scholar]
- Vural, B.; Atalar, F.; Ciftci, C.; Demirkan, A.; Susleyici-Duman, B.; Gunay, D.; Akpinar, B.; Sagbas, E.; Ozbek, U.; Buyukdevrim, A.S. Presence of fatty-acid-binding protein 4 expression in human epicardial adipose tissue in metabolic syndrome. Cardiovasc Pathol. 2008, 17, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Utz, W.; Haufe, S.; Lamounier-Zepter, V.; Pofahl, M.; Traber, J.; Janke, J.; Luft, F.C.; Boschmann, M.; Schulz-Menger, J.; et al. Fatty acid binding protein 4 predicts left ventricular mass and longitudinal function in overweight and obese women. Heart 2013, 99, 944–948. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.; Wong, W.K.; Lam, K.S. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Lamounier-Zepter, V.; Look, C.; Alvarez, J.; Christ, T.; Ravens, U.; Schunck, W.H.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Morano, I. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: A new link between obesity and heart disease. Circ. Res. 2009, 105, 326–334. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.E.; Ma, S.; Wai, D.; Chew, S.K.; Tai, E.S. Can we apply the national cholesterol education program adult treatment panel definition of the metabolic syndrome to Asians? Diabetes Care 2004, 27, 1182–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.N.; Sung, K.T.; Yen, C.H.; Su, C.H.; Lee, P.Y.; Hung, T.C.; Huang, W.H.; Chien, S.C.; Tsai, J.P.; Yun, C.H.; et al. Carotid arterial mechanics as useful biomarker of extracellular matrix turnover and preserved ejection fraction heart failure. ESC Heart Fail. 2020, 7, 1615–1625. [Google Scholar] [CrossRef]
- Lai, Y.H.; Yun, C.H.; Yang, F.S.; Liu, C.C.; Wu, Y.J.; Kuo, J.Y.; Yeh, H.I.; Lin, T.Y.; Bezerra, H.G.; Shih, S.C.; et al. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition: A study validated with computed tomography. J. Am. Soc. Echocardiogr. 2012, 25, 234–241. [Google Scholar] [CrossRef]
- Hung, C.L.; Goncalves, A.; Lai, Y.J.; Lai, Y.H.; Sung, K.T.; Lo, C.I.; Liu, C.C.; Kuo, J.Y.; Hou, C.J.; Chao, T.F.; et al. Light to moderate habitual alcohol consumption is associated with subclinical ventricular and left atrial mechanical dysfunction in an asymptomatic population: Dose-response and propensity analysis. J. Am. Soc. Echocardiogr. 2016, 29, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.H.; Lin, T.Y.; Wu, Y.J.; Liu, C.C.; Kuo, J.Y.; Yeh, H.I.; Yang, F.S.; Chen, S.C.; Hou, C.J.; Bezerra, H.G.; et al. Pericardial and thoracic peri-aortic adipose tissues contribute to systemic inflammation and calcified coronary atherosclerosis independent of body fat composition, anthropometric measures and traditional cardiovascular risks. Eur. J. Radiol. 2012, 81, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Rengo, G.; Perrone-Filardi, P.; Pagano, G.; Femminella, G.D.; Paolillo, S.; Petraglia, L.; Gambino, G.; Caruso, A.; Grimaldi, M.G.; et al. Increased epicardial adipose tissue volume correlates with cardiac sympathetic denervation in patients with heart failure. Circ. Res. 2016, 118, 1244–1253. [Google Scholar] [CrossRef] [Green Version]
- Schejbal, V. Epicardial fatty tissue of the right ventricle--morphology, morphometry and functional significance. Pneumologie. 1989, 43, 490–499. [Google Scholar]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef]
- Iacobellis, G.; Ribaudo, M.C.; Assael, F.; Vecci, E.; Tiberti, C.; Zappaterreno, A.; Di Mario, U.; Leonetti, F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 2003, 88, 5163–5168. [Google Scholar] [CrossRef] [Green Version]
- Mookadam, F.; Goel, R.; Alharthi, M.S.; Jiamsripong, P.; Cha, S. Epicardial fat and its association with cardiovascular risk: A cross-sectional observational study. Heart Views. 2010, 11, 103–108. [Google Scholar] [PubMed]
- Iacobellis, G.; Leonetti, F.; Singh, N.; Sharma, A.M. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 2007, 115, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.T.; De Mol, P.; De Vries, S.T.; Widya, R.L.; Hammer, S.; Van Schinkel, L.D.; Van der Meer, R.W.; Gans, R.O.; Webb, A.G.; Kan, H.E.; et al. Exercise and type 2 diabetes mellitus: Changes in tissue-specific fat distribution and cardiac function. Radiology 2013, 269, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Van Woerden, G.; Gorter, T.M.; Westenbrink, B.D.; Willems, T.P.; Van Veldhuisen, D.J.; Rienstra, M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 1559–1566. [Google Scholar] [CrossRef] [Green Version]
- Perez-Belmonte, L.M.; Moreno-Santos, I.; Gomez-Doblas, J.J.; Garcia-Pinilla, J.M.; Morcillo-Hidalgo, L.; Garrido-Sanchez, L.; Santiago-Fernandez, C.; Crespo-Leiro, M.G.; Carrasco-Chinchilla, F.; Sanchez-Fernandez, P.L.; et al. Expression of epicardial adipose tissue thermogenic genes in patients with reduced and preserved ejection fraction heart failure. Int. J. Med. Sci. 2017, 14, 891–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, H.S.; Fain, J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Tamarappoo, B.K.; Hachamovitch, R.; Kwon, D.H.; Alraies, M.C.; Halliburton, S.; Schoenhagen, P.; Dey, D.; Berman, D.S.; Marwick, T.H. Association of epicardial fat, hypertension, subclinical coronary artery disease, and metabolic syndrome with left ventricular diastolic dysfunction. Am. J. Cardiol. 2012, 110, 1793–1798. [Google Scholar] [CrossRef] [Green Version]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.Y.; Lin, L.Y.; Tseng, Y.H.; Chang, C.C.; Wu, C.K.; Lin, J.L.; Tseng, W.Y. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC. Cardiovasc Imaging 2014, 7, 991–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, J.; Dauch, J.R.; Hinder, L.M.; Hayes, J.M.; Backus, C.; Pennathur, S.; Kretzler, M.; Brosius, F.C., 3rd; Feldman, E.L. The metabolic syndrome and microvascular complications in a murine model of type 2 diabetes. Diabetes 2015, 64, 3294–3304. [Google Scholar] [CrossRef] [Green Version]
- Turak, O.; Ozcan, F.; Canpolat, U.; Isleyen, A.; Cebeci, M.; Oksuz, F.; Mendi, M.A.; Cagli, K.; Golbasi, Z.; Aydogdu, S. Increased echocardiographic epicardial fat thickness and high-sensitivity CRP level indicate diastolic dysfunction in patients with newly diagnosed essential hypertension. Blood Press Monitor. 2013, 18, 259–264. [Google Scholar] [CrossRef]
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; Van Goor, H.; Sanjabi, B.; Van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Tsutamoto, T.; Wada, A.; Tsutsui, T.; Ishii, C.; Ohno, K.; Fujii, M.; Taniguchi, A.; Hamatani, T.; Nozato, Y.; et al. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation 2003, 107, 2559–2565. [Google Scholar] [PubMed] [Green Version]
- Zhang, J.; Qiao, C.; Chang, L.; Guo, Y.; Fan, Y.; Villacorta, L.; Chen, Y.E.; Zhang, J. Cardiomyocyte overexpression of fabp4 aggravates pressure overload-induced heart hypertrophy. PLoS ONE 2016, 11, e0157372. [Google Scholar] [CrossRef] [Green Version]
- Djousse, L.; Bartz, T.M.; Ix, J.H.; Kochar, J.; Kizer, J.R.; Gottdiener, J.S.; Tracy, R.P.; Mozaffarian, D.; Siscovick, D.S.; Mukamal, K.J.; et al. Fatty acid-binding protein 4 and incident heart failure: The cardiovascular health study. Eur. J. Heart Fail. 2013, 15, 394–399. [Google Scholar] [CrossRef]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Aspects Med. 2013, 34, 1–11. [Google Scholar] [CrossRef] [Green Version]


| Metabolic Score Categories | All Subjects (N = 252) | Epicardial Adipose Tissue (EAT) | p for Trend | R Value (All Variable Correlated with EAT) | p Value (Pearson Correlation) | ANOVA/ꭓ2 | ||
|---|---|---|---|---|---|---|---|---|
| Q1 (n = 84) ≤7.5 mm | Q2 (n = 84) 7.6–9.0 mm | Q3 (n = 84) ≥9.1 mm | ||||||
| Baseline Demographics | ||||||||
| Age, y | 65.8 ± 9.87 | 61.7 ± 7.79 | 65.8 ± 10.1 * | 69.7 ± 10.0 *,# | <0.001 | 0.381 | <0.001 | <0.001 |
| Female sex, n (%) | 165 (65.5%) | 47 (56.0%) | 53 (63.1%) | 65 (77.4%) | 0.004 | 0.153 | 0.02 | 0.01 |
| Systolic blood pressure, mm Hg | 140.3 ± 20.0 | 133.4 ± 19.1 | 140.3 ± 19.3 * | 147.2 ± 19.3 * | <0.001 | 0.303 | <0.001 | <0.001 |
| Diastolic blood pressure, mm Hg | 80.8 ± 12.3 | 78.6 ± 11.6 | 80.1 ± 12.1 | 83.6 ± 12.6 * | 0.01 | 0.169 | 0.01 | 0.03 |
| Heart rate, min−1 | 75.7 ± 11.3 | 73.3 ± 11.0 | 76.9 ± 12.1 | 77.1 ± 10.3 | 0.03 | 0.170 | 0.01 | 0.049 |
| Waist circumference, cm | 89.7 ± 11.8 | 85.0 ± 10.6 | 91.1 ± 11.4 * | 92.9 ± 12.0 * | <0.001 | 0.361 | <0.001 | <0.001 |
| Weight, kg | 66.3 ± 13.0 | 63.0 ± 11.8 | 68.0 ± 13.2 * | 68.0 ± 13.6 * | 0.01 | 0.213 | 0.001 | 0.02 |
| BMI, kg/m2 | 26.5 ± 4.24 | 24.6 ± 3.88 | 26.7 ± 4.04 * | 28.1 ± 4.06 * | <0.001 | 0.400 | <0.001 | <0.001 |
| Body fat, % | 34.3 ± 9.29 | 29.3 ± 8.68 | 34.9 ± 8.43 * | 38.9 ± 8.13 *,# | <0.001 | 0.465 | <0.001 | <0.001 |
| Laboratory Data | ||||||||
| Fasting glucose, mg/dL | 113.7 ± 2.39 | 105.3 ± 37.6 | 116.4 ± 34.0 | 119.2 ± 40.6 | 0.01 | 0.237 | <0.001 | 0.04 |
| Total cholesterol, mg/dL | 198.8 ± 42.9 | 202.1 ± 42.6 | 199.5 ± 39.7 | 194.9 ± 46.3 | 0.27 | 0.037 | 0.55 | 0.54 |
| Triglyceride, mg/dL | 115.0 ± 86.4 | 88.4 ± 55.3 | 132.6 ± 114.8 * | 123.9 ± 72.4 * | 0.01 | 0.227 | <0.001 | 0.002 |
| HDL, mg/dL | 54.9 ± 19.3 | 61.1 ± 23.1 | 53.6 ± 18.2 * | 49.9 ± 13.8 * | <0.001 | 0.229 | <0.001 | 0.001 |
| LDL, mg/dL | 119.9 ± 35.8 | 120.6 ± 35.0 | 120.5 ± 35.5 | 118.5 ± 37.2 | 0.71 | 0.018 | 0.78 | 0.91 |
| Uric acid, mg/dL | 6.04 ± 1.51 | 5.58 ± 1.40 | 5.99 ± 1.41 * | 6.45 ± 1.58 * | 0.001 | 0.301 | <0.001 | 0.003 |
| e-GFR, mL/min/1.73 m2 | 79.1 ± 25.9 | 87.3 ± 23.0 | 81.8 ± 22.4 | 68.2 ± 28.1 *,# | <0.001 | 0.342 | <0.001 | <0.001 |
| Biomarkers | ||||||||
| hs-CRP (median, 25th–75th), mg/L | 0.22 ± 0.24 | 0.17 ± 0.19 | 0.21 ± 0.26 | 0.27 ± 0.26 * | 0.01 | 0.255 | <0.001 | 0.03 |
| BNP (median, 25th–75th), pg/mL | 62.2 ± 125.0 | 34.7 ± 80.0 | 55.5 ± 99.0 | 95.4 ± 169.8 * | 0.002 | 0.207 | 0.001 | 0.01 |
| Galectin-3, ng/mL | 2.74 ± 2.36 | 2.16 ± 1.96 | 2.74 ± 2.16 | 3.32 ± 2.77 * | 0.001 | 0.248 | <0.001 | 0.001 |
| PIIINP, ng/mL | 0.98 ± 0.39 | 0.84 ± 0.26 | 0.98 ± 0.36 * | 1.13 ± 0.46 *,# | <0.001 | 0.363 | <0.001 | <0.001 |
| A-FABP, ng/mL | 26.1 ± 21.4 | 17.4 ± 8.31 | 25.4 ± 14.7 * | 35.7 ± 30.4 *,# | <0.001 | 0.392 | <0.001 | <0.001 |
| Medical Histories | ||||||||
| Hypertension, n (%) | 179 (71%) | 47 (56.0%) | 59 (70.2%) | 73 (86.9%) | <0.001 | — | — | <0.001 |
| Diabetes, n (%) | 75 (29.8%) | 12 (14.3%) | 25 (29.8%) | 38 (45.2%) | <0.001 | — | — | <0.001 |
| Cardiovascular diseases, n (%) | 34 (13.5%) | 6 (7.1%) | 10 (11.9%) | 18 (21.4%) | 0.01 | — | — | 0.02 |
| Heart failure, n (%) | 51 (20.2%) | 5 (6.0%) | 14 (16.7%) | 32 (38.1%) | <0.001 | — | — | <0.001 |
| Metabolic score (median, 25th–75th) | 3 (2–4) | 2 (1–4) | 3 (2–4) * | 4 (3–5) *,# | <0.001 | — | — | <0.001 |
| Cardiac Structure and Function | ||||||||
| IVS, mm | 9.20 ± 1.46 | 8.84 ± 1.29 | 9.15 ± 1.33 | 9.60 ± 1.65 * | <0.001 | 0.292 | <0.001 | <0.001 |
| LVPW, mm | 9.19 ± 1.29 | 8.81 ± 1.12 | 9.33 ± 1.38 * | 9.42 ± 1.30 * | 0.002 | 0.302 | 0 | 0.004 |
| LVIDd, mm | 46.3 ± 3.93 | 46.3 ± 4.20 | 46.6 ± 3.89 | 45.9 ± 3.71 | 0.56 | 0.001 | 0.98 | 0.55 |
| LV mass, g | 144.6 ± 37.0 | 137.3 ± 34.4 | 147.5 ± 37.0 | 149.0 ± 38.8 | 0.04 | 0.214 | 0.001 | 0.08 |
| LV mass index, gm/m2 | 79.3 ± 18.8 | 76.9 ± 17.0 | 79.9 ± 20.6 | 81.2 ± 18.7 | 0.14 | 0.145 | 0.02 | 0.32 |
| Stroke volume, mL | 66.5 ± 12.4 | 67.0 ± 13.5 | 65.9 ± 11.4 | 66.5 ± 12.3 | 0.78 | 0.015 | 0.81 | 0.85 |
| LVEF, % | 67.1 ± 6.43 | 67.3 ± 6.20 | 65.8 ± 6.73 * | 68.2 ± 6.19 * | 0.38 | 0.040 | 0.52 | 0.05 |
| LVH, n (%) | 27 (10.7%) | 6 (7.1%) | 9 (10.7%) | 12 (14.3%) | 0.17 | 0.094 | 0.14 | 0.35 |
| E/A ratio | 0.92 ± 0.36 | 1.02 ± 0.394 | 0.89 ± 0.31 | 0.84 ± 0.34 * | 0.001 | 0.281 | <0.001 | 0.01 |
| TDI-e’ (average), cm/s | 7.71 ± 1.92 | 8.55 ± 1.98 | 7.76 ± 1.78 * | 6.82 ± 1.60 *,# | <0.001 | 0.441 | <0.001 | <0.001 |
| E/e’ (average) | 9.84 ± 3.60 | 8.15 ± 2.71 | 9.90 ± 3.40 * | 11.5 ± 3.85 *,# | <0.001 | 0.371 | <0.001 | <0.001 |
| LV SRe, s−1 | 1.08 ± 0.30 | 1.23 ± 0.31 | 1.07 ± 0.29 * | 0.96 ± 0.26 *# | <0.001 | 0.447 | <0.001 | <0.001 |
| LV SRa, s−1 | 1.19 ± 0.24 | 1.17 ± 0.25 | 1.21 ± 0.22 | 1.19 ± 0.25 | 0.61 | 0.062 | 0.33 | 0.63 |
| TDI-s’ (average), cm/s | 7.62 ± 1.45 | 7.99 ± 1.42 | 7.80 ± 1.55 | 7.07 ± 1.20 *,# | <0.001 | 0.285 | <0.001 | <0.001 |
| GCS, % | −20.6 ± 2.92 | −20.7 ± 2.84 | −20.9 ± 2.94 | −20.3 ± 3.00 | 0.41 | 0.104 | 0.11 | 0.48 |
| GLS, % | −19.5 ± 2.59 | −20.5 ± 2.37 | −19.4 ± 2.61 * | −18.5 ± 2.38 * | <0.001 | 0.408 | <0.001 | <0.001 |
| LV SRs, s−1 | −1.12 ± 0.15 | −1.19 ± 0.155 | −1.12 ± 0.13 * | −1.05 ± 0.13 *,# | <0.001 | 0.436 | <0.001 | <0.001 |
| Variables | EAT (mm) (Multi-Variate Regression Model) | |||
|---|---|---|---|---|
| Adjusted Coefficient | p Value | Adjusted Coefficient | p Value | |
| Age, years | 0.03 (0.01, 0.05) | 0.004 | 0.04 (0.01, 0.06) | 0.001 |
| Female sex, n (%) | 0.5 (0.07, 0.93) | 0.022 | 0.55 (0.12, 0.97) | 0.012 |
| Systolic blood pressure, mm Hg | 0.01 (−0.0003, 0.02) | 0.058 | N/A | N/A |
| Diastolic blood pressure, mm Hg | N/A | N/A | 0.02 (0.003, 0.03) | 0.02 |
| BMI, kg/m2 | 0.11 (0.06, 0.16) | <0.001 | 0.10 (0.06, 0.15) | <0.001 |
| Fasting glucose, mg/dL | - | - | - | - |
| HDL-c, mg/dL | −0.012 (−0.023, −0.0003) | 0.044 | −0.012 (−0.02, −0.0006) | 0.038 |
| e-GFR, mL/min/1.73 m2 | −0.012 (−0.02, −0.004) | 0.003 | −0.012 (−0.02, −0.004) | 0.003 |
| Hypertension, n (%) | - | - | - | - |
| Diabetes, n (%) | 0.78 (0.35–1.22) | <0.001 | 0.81 (0.37–1.24) | <0.001 |
| HFpEF, n (%) | 0.81 (0.30–1.33) | 0.002 | 0.83 (0.32–1.34) | 0.002 |
| Cox Regression Models | EAT (per 1 mm Increment) | |
|---|---|---|
| HR (95% CI) | p-Value | |
| Hospitalization for HF | ||
| Crude Model | 1.66 (1.45–1.91) | <0.001 |
| Multivariate model | 1.36 (1.14–1.64) | 0.001 |
| Composite HF/Death | ||
| Crude Model | 1.60 (1.41–1.83) | <0.001 |
| Multivariate model | 1.35 (1.14–1.60) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-L.; Sung, K.-T.; Lai, Y.-H.; Yen, C.-H.; Yun, C.-H.; Su, C.-H.; Kuo, J.-Y.; Liu, C.-Y.; Chien, C.-Y.; Cury, R.C.; et al. Epicardial Adiposity in Relation to Metabolic Abnormality, Circulating Adipocyte FABP, and Preserved Ejection Fraction Heart Failure. Diagnostics 2021, 11, 397. https://doi.org/10.3390/diagnostics11030397
Lin J-L, Sung K-T, Lai Y-H, Yen C-H, Yun C-H, Su C-H, Kuo J-Y, Liu C-Y, Chien C-Y, Cury RC, et al. Epicardial Adiposity in Relation to Metabolic Abnormality, Circulating Adipocyte FABP, and Preserved Ejection Fraction Heart Failure. Diagnostics. 2021; 11(3):397. https://doi.org/10.3390/diagnostics11030397
Chicago/Turabian StyleLin, Jiun-Lu, Kuo-Tzu Sung, Yau-Huei Lai, Chih-Hsuan Yen, Chun-Ho Yun, Cheng-Huang Su, Jen-Yuan Kuo, Chia-Yuan Liu, Chen-Yen Chien, Ricardo C. Cury, and et al. 2021. "Epicardial Adiposity in Relation to Metabolic Abnormality, Circulating Adipocyte FABP, and Preserved Ejection Fraction Heart Failure" Diagnostics 11, no. 3: 397. https://doi.org/10.3390/diagnostics11030397






