Abstract
We present a 71-year-old female patient who underwent 18F-PSMA-1007 PET/CT for suspected metastatic renal cell carcinoma (RCC), as RCC also shows high PSMA-expression in tumor neovascularization. 18F-PSMA-1007 PET/CT showed a high PSMA-avidity in the renal tumor, enlarged intra-abdominal and mediastinal lymph nodes. Moreover, PSMA-positive pleural, pulmonal and osseous lesions were found. However, histopathology revealed an immature plasma cell myeloma with an endothelial PSMA-expression of the neovasculature. This case illustrates the increased PSMA-avidity in multiple myeloma and highlights PSMA as a potential theragnostic target in multiple myeloma. For clinical routine, lymphatic diseases such as extramedullary myeloma should be considered as differential diagnosis in PSMA-avid renal masses on PET/CT.
A 71-year-old woman presented to the uro-oncological department with a newly diagnosed, left-sided renal tumor for further clinical workup and the subsequent initiation of therapy. Contrast-enhanced computed tomography (CT), initially performed at the pelvis and the lower limbs to rule out arterial occlusion, revealed an incidental finding of an extensive, inhomogeneous, marginal contrast-enhancing renal tumor of the left kidney. In addition, there were surrounding, pathologically enlarged abdominal lymph nodes (Figure 1A). The primary differential diagnosis consisted of renal cell carcinoma (RCC) with nodal abdominal spread. As the initial results highlighted the added clinical value of prostate-specific-antigen (PSMA)-targeted positron emission tomography (PET) imaging in metastatic RCC due to the PSMA-expression of tumor neovascularization [1,2,3,4,5], this patient underwent 18F-PSMA-1007 PET/CT for whole body staging before any further tumor-specific therapies. Here, the left renal lesion showed a markedly increased PSMA-expression (maximal standardized uptake value (SUVmax) 30.0; tumor-to-background ratio (TBR), SUVmax/SUVmean-liver 3,1; Figure 1B).
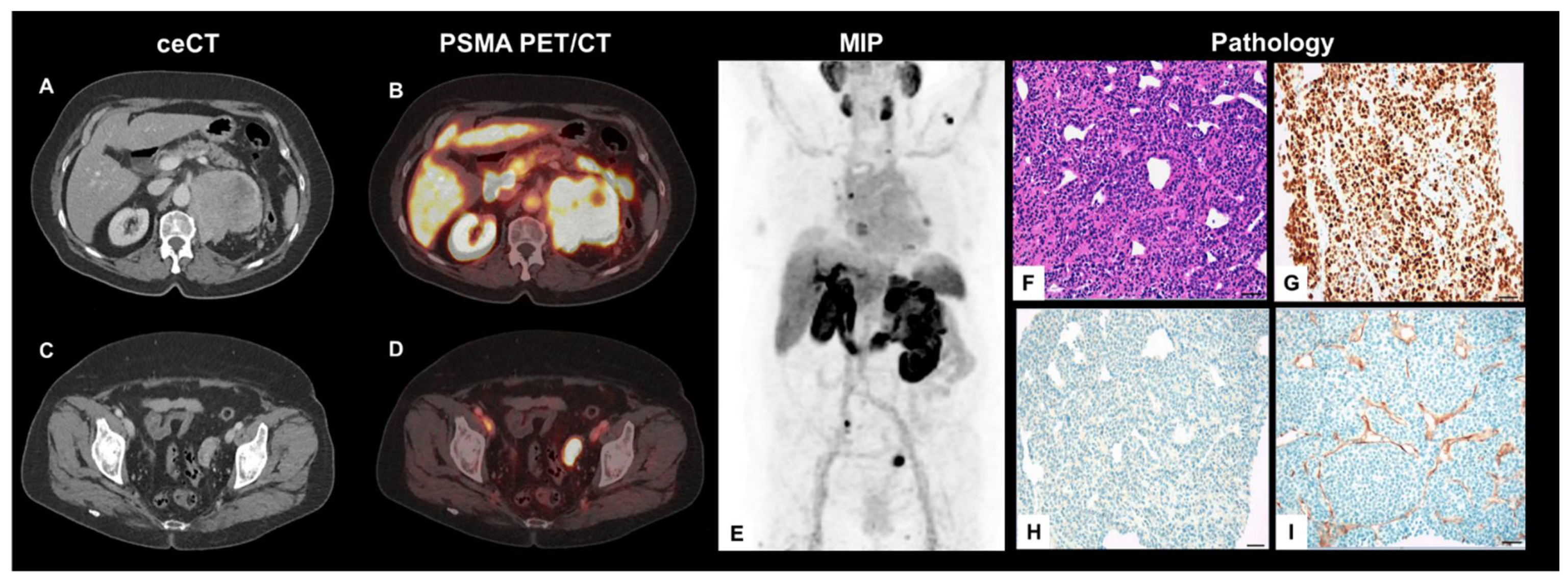
Figure 1.
Axial, contrast-enhanced CT (ceCT) (A,C) and fused PET/CT planes (B,D), MIP (E) as well as histology findings from ultrasound-guided biopsy (F–I) (Scale bar = 50 µm).
The pre-known enlarged lymph nodes also showed a high PSMA-avidity on PET (SUVmax 17.0). In addition, mediastinal lymph nodes, multilocular pulmonary, pleural, and osseous lesions presented with PSMA-avidity, partially even with extra-osseous soft tissue extension and mixed sclerotic/lytic correlate on CT. Beyond this, a left para-ovarian soft tissue bulk with highly increased PSMA-expression was found (Figure 1C,D). A MIP projection demonstrates the whole tumor burden (Figure 1E). For further evaluation, histological specimens of the left renal tumor were obtained using ultrasound-guided biopsy.
The microscopic examination of the biopsy revealed dense aggregates of mature and immature plasma cells in hematoxylin and eosin (H&E)-stained tissue specimens (Figure 1F) with positivity for CD38 and CD138 and strong nuclear staining for multiple myeloma oncogene 1 (MUM1) (Figure 1G). Staining for pan-cytokeratin (KL1 staining) was negative, ruling out a plasmacytoid urothelial carcinoma of the renal pelvis (Figure 1H). The lesion was negative for CD20, cytokeratin-7 (CK7), paired box gene 8 (Pax8), and GATA3. Limitations in biopsy quantity and quality prevented the immuno-histochemical analysis of kappa and lambda light chains; however, based on histomorphology and the typical expression profile the diagnosis was confirmed as an immature plasma cell myeloma independently by two experienced pathologists. Immunohistochemical staining for PSMA showed a strong expression in tumor-associated microvasculature (Figure 1I).
To date, increased PSMA-avidity in multiple myeloma has been described only sporadically in the literature [6,7,8] Of note, in a single case, PSMA expression was additionally shown to decrease after systemic therapy, highlighting PSMA-expression as a potential theranostic target for multiple myeloma, e.g., during radioligand therapy using 177Lu- or 225Ac-labeled PSMA-ligands [9]. To date, however, there is no literature describing PSMA-avid masses suggestive of renal RCC, which was then confirmed to be highly PSMA-avid multiple myeloma. For clinical routine, this case underlines that lymphatic diseases such as extramedullary myeloma should be considered as differential diagnosis in PSMA-avid renal masses on PET/CT despite their rare occurrence, as manifestations of multiple myeloma may also show a highly endothelial PSMA-expression.
Author Contributions
L.M.M.: clinical management, draft manuscript; S.T.L.: draft manuscript, histopathological workup; D.M. and C.W.: supervision, histopathological workup; M.S. (Melanie Schott), S.T. and M.S. (Michael Staehler): clinical management, increased intellectual content; L.B., H.I. and M.B.: clinical management, increased intellectual content; W.G.K., J.R. and P.B.: revision manuscript, increased intellectual content; M.U.: manuscript draft, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The ethics committee waives additional approval for case reports from clinical routine.
Consent for Publication
The patient gave written consent prior to the PET/CT exam.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meyer, A.R.; Carducci, M.A.; Denmeade, S.R.; Markowski, M.C.; Pomper, M.G.; Pierorazio, P.M.; Allaf, M.E.; Rowe, S.P.; Gorin, M.A. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 2019, 33, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Mittlmeier, L.M.; Unterrainer, M.; Todica, A.; Cyran, C.C.; Rodler, S.; Bartenstein, P.; Stief, C.G.; Ilhan, H.; Staehler, M. PSMA PET/CT for tyrosine-kinase inhibitor monitoring in metastatic renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2216–2217. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Blazak, J.; Tham, C.M.; Ng, K.L.; Shepherd, B.; Lawson, M.; Preston, J.; Vela, I.; Thomas, P.; Wood, S. Pilot study: Use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Callahan, J.; Pryor, D.; Martin, J.; Lawrentschuk, N.; Hofman, M.S. Utility of 68Ga prostate specific membrane antigen–positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J. Med. Imaging Radiat. Oncol. 2017, 61, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Mittlmeier, L.M.; Unterrainer, M.; Rodler, S.; Todica, A.; Albert, N.L.; Burgard, C.; Cyran, C.C.; Kunz, W.G.; Ricke, J.; Bartenstein, P.; et al. 18F-PSMA-1007 PET/CT for response assessment in patients with metastatic renal cell carcinoma undergoing tyrosine kinase or checkpoint inhibitor therapy: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Steiger, K.; Schwaiger, M.; Eiber, M. Image of the Month: Multifocal 68Ga Prostate-Specific Membrane Antigen Ligand Uptake in the Skeleton in a Man with Both Prostate Cancer and Multiple Myeloma. Clin. Nucl. Med. 2017, 42, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.; Joy, A.; Pillai, M.R.; Nanabala, R.; Thomas, B. 68Ga-PSMA PET/CT imaging in multiple myeloma. Clin. Nucl. Med. 2017, 42, e126–e127. [Google Scholar] [CrossRef] [PubMed]
- Veerasuri, S.; Redman, S.; Graham, R.; Meehan, C.; Little, D. Non-prostate uptake on 18F-PSMA-1007 PET/CT: A case of Myeloma. BJR Case Rep. 2020, 6, 20200102. [Google Scholar] [CrossRef]
- Pan, Q.; Luo, Y.; Ma, Y.; Li, F. The Change of 68Ga-Prostate-Specific Membrane Antigen Uptake in Myeloma After Chemotherapy in a Patient with Multiple Myeloma and Concurrent Prostate Cancer. Clin. Nucl. Med. 2020, 45, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).