Development of a New Chemiluminescent Enzyme Immunoassay Using a Two-Step Sandwich Method for Measuring Aldosterone Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Radioimmunoassay
2.3. CLEIA Method Using the Current Reagent
2.4. CLEIA Method Using the Improved Reagent
2.5. LC-MS/MS
2.6. Statistical Analysis
3. Results
3.1. Basal Clinical Characteristics of the Patients
3.2. Validation of Aldosterone Concentrations Using the Improved Reagent
3.3. Serum and Plasma Aldosterone Concentrations Using the Improved Reagent
3.4. Correlation of Aldosterone Concentrations between the Improved Reagent and RIA
3.5. Correlation of Aldosterone Concentrations Obtained with the Improved and Current Reagents
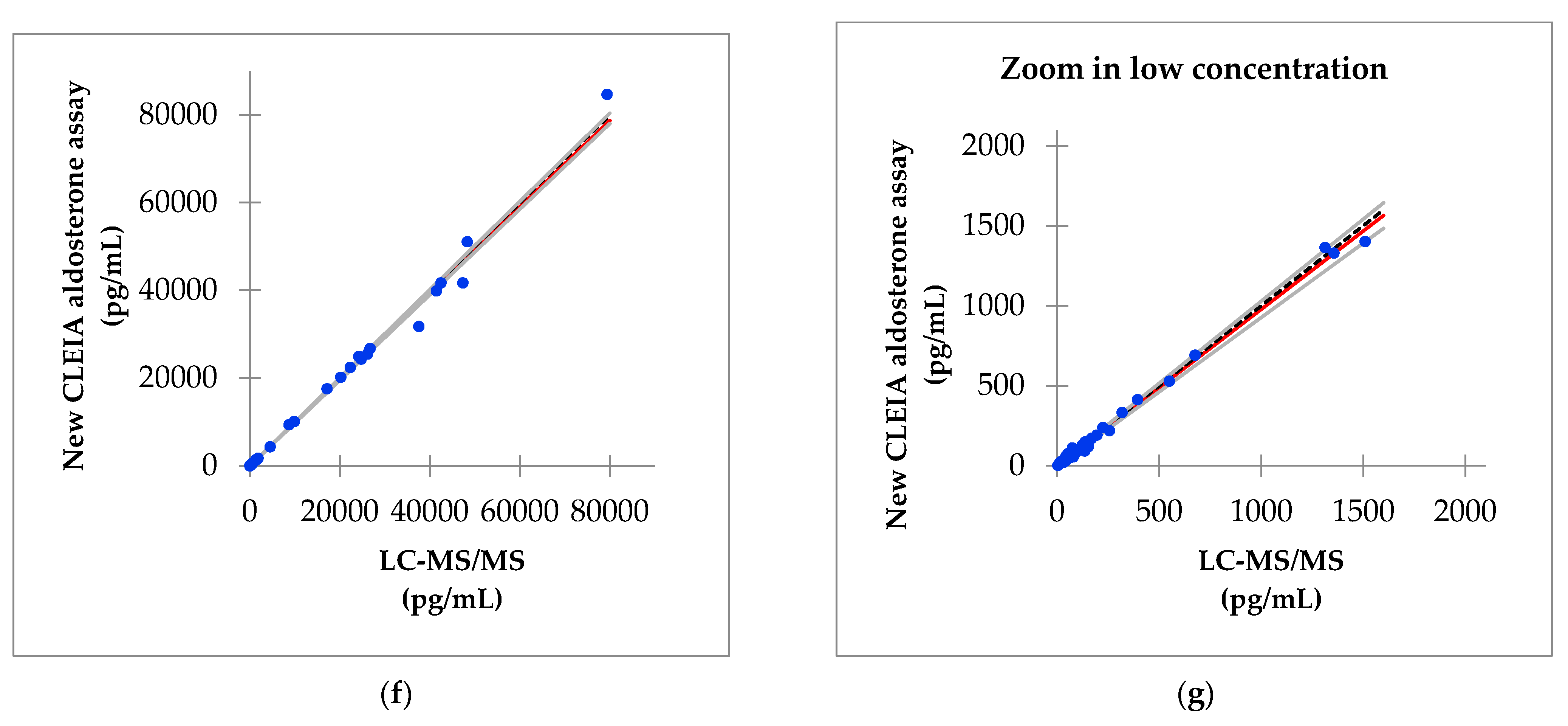
3.6. Correlation of Aldosterone Concentrations between the Improved Reagent and LC-MS/MS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Funder, J.W.; Carey, R.M.; Fardella, C.; Gomez-Sanchez, C.E.; Mantero, F.; Stowasser, M.; Young, W.F., Jr.; Montori, V.M. Case detection, diagnosis, and treatment of patients with primary aldosteronism: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008, 93, 3266–3281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, R.; Omata, K.; Ito, S.; Satoh, F. Progress in the Management of Primary Aldosteronism. Am. J. Hypertens 2018, 31, 522–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, W.F., Jr. Diagnosis and treatment of primary aldosteronism: Practical clinical perspectives. J. Intern. Med. 2019, 285, 126–148. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, A.; Mulatero, P.; Baudrand, R.; Adler, G.K. The Expanding Spectrum of Primary Aldosteronism: Implications for Diagnosis, Pathogenesis, and Treatment. Endocr. Rev. 2018, 39, 1057–1088. [Google Scholar] [CrossRef] [Green Version]
- Shibata, H.; Itoh, H. Mineralocorticoid receptor-associated hypertension and its organ damage: Clinical relevance for resistant hypertension. Am. J. Hypertens 2012, 25, 514–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, T.; Hirata, Y. Aldosterone as a cardiovascular risk hormone. Endocr. J. 2007, 54, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Byrd, J.B.; Turcu, A.F.; Auchus, R.J. Primary Aldosteronism: Practical Approach to Diagnosis and Management. Circulation 2018, 138, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Savard, S.; Amar, L.; Plouin, P.F.; Steichen, O. Cardiovascular complications associated with primary aldosteronism: A controlled cross-sectional study. Hypertension 2013, 61, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Dick, S.M.; Queiroz, M.; Bernardi, B.L.; Dall’Agnol, A.; Brondani, L.A.; Silveiro, S.P. Update in diagnosis and management of primary aldosteronism. Clin. Chem Lab. Med. 2018, 56, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Saito, J.; Omura, M. Prevalence of primary aldosteronism: Should we screen for primary aldosteronism before treating hypertensive patients with medication? Endocr. J. 2007, 54, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Morera, J.; Reznik, Y. MANAGEMENT OF ENDOCRINE DISEASE: The role of confirmatory tests in the diagnosis of primary aldosteronism. Eur. J. Endocrinol. 2019, 180, R45–R58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzolo, F.; Salvagno, G.; Caruso, B.; Cocco, C.; Zorzi, F.; Zaltron, C.; Castagna, A.; Bertolone, L.; Morandini, F.; Lippi, G.; et al. Fully automated chemiluminescence vs RIA aldosterone assay in primary aldosteronism work-up. J. Hum. Hypertens 2017, 31, 826–830. [Google Scholar] [CrossRef]
- Schirpenbach, C.; Seiler, L.; Maser-Gluth, C.; Beuschlein, F.; Reincke, M.; Bidlingmaier, M. Automated chemiluminescence-immunoassay for aldosterone during dynamic testing: Comparison to radioimmunoassays with and without extraction steps. Clin. Chem. 2006, 52, 1749–1755. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, R.; Ono, Y.; Tezuka, Y.; Kudo, M.; Yamamoto, S.; Arai, T.; Gomez-Sanchez, C.E.; Sasano, H.; Satoh, F. Rapid Screening of Primary Aldosteronism by a Novel Chemiluminescent Immunoassay. Hypertension 2017, 70, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M. Steroid measurement with LC-MS/MS. Application examples in pediatrics. J. Steroid Biochem. Mol. Biol. 2010, 121, 520–527. [Google Scholar] [CrossRef]
- Nishikawa, T.; Omura, M.; Kawaguchi, M.; Takatsu, A.; Satoh, F.; Ito, S.; Kurihara, I.; Itoh, H.; Yanase, T.; Shibata, H.; et al. Calibration and evaluation of routine methods by serum certified reference material for aldosterone measurement in blood. Endocr. J. 2016, 63, 1065–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan. Available online: https://unit.aist.go.jp/qualmanmet/refmate/index.html (accessed on 15 February 2021).
- Teruyama, K.; Naruse, M.; Tsuiki, M.; Kobayashi, H. Novel chemiluminescent immunoassay to measure plasma aldosterone and plasma active renin concentrations for the diagnosis of primary aldosteronism. J. Hum. Hypertens 2021. [Google Scholar] [CrossRef] [PubMed]
- Lenders, J.W.M.; Eisenhofer, G.; Reincke, M. Subtyping of Patients with Primary Aldosteronism: An Update. Horm. Mwtab. Res. 2017, 49, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Buffolo, F.; Monticone, S.; Williams, T.A.; Rossato, D.; Burrello, J.; Tetti, M.; Veglio, F.; Mulatero, P. Subtype Diagnosis of Primary Aldosteronism: Is Adrenal Vein Sampling Always Necessary? Int. J. Mol. Sci. 2017, 18, 848. [Google Scholar] [CrossRef] [Green Version]
- Rossi, G.P.; Rossitto, G.; Amar, L.; Azizi, M.; Riester, A.; Reincke, M.; Degenhart, C.; Widimsky, J., Jr.; Naruse, M.; Deinum, J.; et al. Clinical Outcomes of 1625 Patients with Primary Aldosteronism Subtyped with Adrenal Vein Sampling. Hypertension 2019, 74, 800–808. [Google Scholar] [CrossRef]
- St-Jean, M.; Bourdeau, I.; Therasse, É.; Lacroix, A. Use of peripheral plasma aldosterone concentration and response to ACTH during simultaneous bilateral adrenal veins sampling to predict the source of aldosterone secretion in primary aldosteronism. Clin. Endocrinol. 2020, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Aono, D.; Kometani, M.; Karashima, S.; Usukura, M.; Gondo, Y.; Hashimoto, A.; Demura, M.; Furukawa, K.; Takeda, Y.; Kawashiri, M.; et al. Primary aldosteronism subtype discordance between computed tomography and adrenal venous sampling. Hypertens Res. 2019, 42, 1942–1950. [Google Scholar] [CrossRef] [PubMed]



| PA | Non-PA | p | |
|---|---|---|---|
| Age (years) | 51.5 ± 13.5 | 47.0 ± 16.3 | 0.45 |
| Male/Female | 6/9 | 5/7 | |
| BMI (kg/m2) | 23.5 ± 2.9 | 30.8 ± 8.1 | 0.01 |
| Systolic blood pressure (mmHg) | 129.2 ± 15.2 | 113.1 ± 11.2 | 0.004 |
| Diastolic blood pressure (mmHg) | 84.3 ± 11.3 | 72.3 ± 15.1 | 0.03 |
| HR (bpm) | 76.6 ± 13.5 | 78.9 ± 10.3 | 0.61 |
| BUN (mg/dL) | 11.2 ± 3.3 | 15.2 ± 5.9 | 0.05 |
| Creatinine (mg/dL) | 0.68 ± 0.11 | 0.89 ± 0.34 | 0.06 |
| Na (mmol/L) | 139.9 ± 1.7 | 139.3 ± 3.0 | 0.50 |
| K (mmol/L) | 3.8 ± 0.3 | 4.0 ± 0.4 | 0.23 |
| Cl (mmol/L) | 106.0 ± 2.0 | 104.4 ± 2.5 | 0.09 |
| eGFR (mL/min/1.73 m2) | 82.7 ± 12.2 | 70.9 ± 24.0 | 0.14 |
| Plasma aldosterone concentration (pg/mL) | 158.9 ± 57.0 | 170.0 ± 84.6 | 0.70 |
| Active renin concentration (pg/mL) | 7.4 ± 19.2 | 28.0 ± 31.2 | 0.06 |
| ARR | 103.2 ± 76.3 | 12.5 ± 9.5 | <0.001 |
| Antihypertensive drugs | |||
| Calcium channel blocker | 10/15 (67%) | 6/12 (50%) | |
| Angiotensin II Receptor Blocker | 0/15 (0%) | 5/12 (42%) | |
| Mineralocorticoid receptor antagonist | 1/15 (7%) | 0/12 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozeki, Y.; Tanimura, Y.; Nagai, S.; Nomura, T.; Kinoshita, M.; Shibuta, K.; Matsuda, N.; Miyamoto, S.; Yoshida, Y.; Okamoto, M.; et al. Development of a New Chemiluminescent Enzyme Immunoassay Using a Two-Step Sandwich Method for Measuring Aldosterone Concentrations. Diagnostics 2021, 11, 433. https://doi.org/10.3390/diagnostics11030433
Ozeki Y, Tanimura Y, Nagai S, Nomura T, Kinoshita M, Shibuta K, Matsuda N, Miyamoto S, Yoshida Y, Okamoto M, et al. Development of a New Chemiluminescent Enzyme Immunoassay Using a Two-Step Sandwich Method for Measuring Aldosterone Concentrations. Diagnostics. 2021; 11(3):433. https://doi.org/10.3390/diagnostics11030433
Chicago/Turabian StyleOzeki, Yoshinori, Yukie Tanimura, Satoshi Nagai, Takuya Nomura, Mizuki Kinoshita, Kanako Shibuta, Naoki Matsuda, Shotaro Miyamoto, Yuichi Yoshida, Mitsuhiro Okamoto, and et al. 2021. "Development of a New Chemiluminescent Enzyme Immunoassay Using a Two-Step Sandwich Method for Measuring Aldosterone Concentrations" Diagnostics 11, no. 3: 433. https://doi.org/10.3390/diagnostics11030433






