Arrhythmic Mitral Valve Prolapse: Introducing an Era of Multimodality Imaging-Based Diagnosis and Risk Stratification
Abstract
1. Introduction
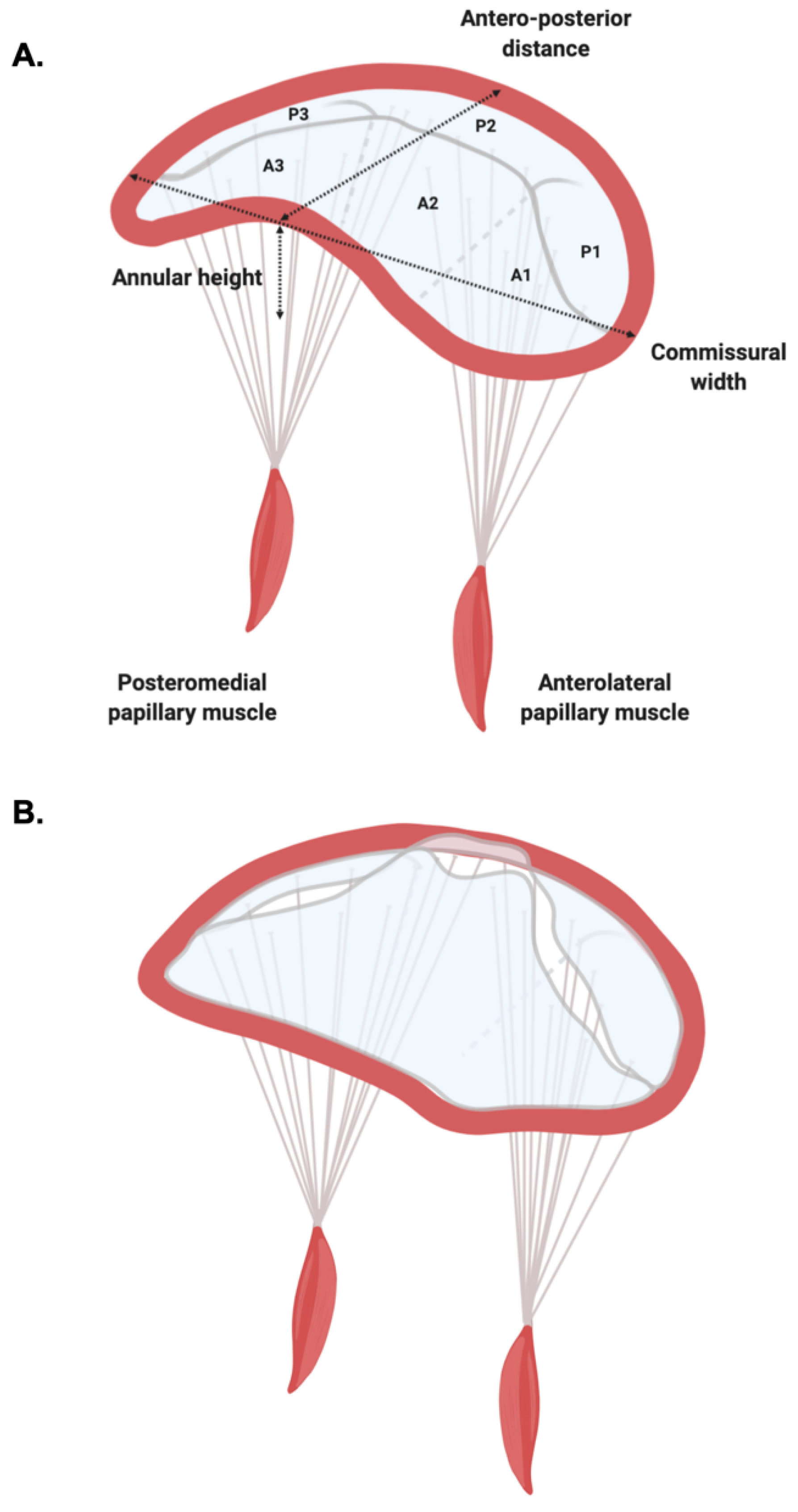
1.1. Mitral Valve Anatomy
1.2. Mitral Valve Prolapse
2. Mitral Valve Prolapse: A Historical Perspective
3. Diagnosis of MVP
3.1. Echocardiography
3.2. Cardiovascular Magnetic Resonance
3.3. Cardiac Computed Tomography
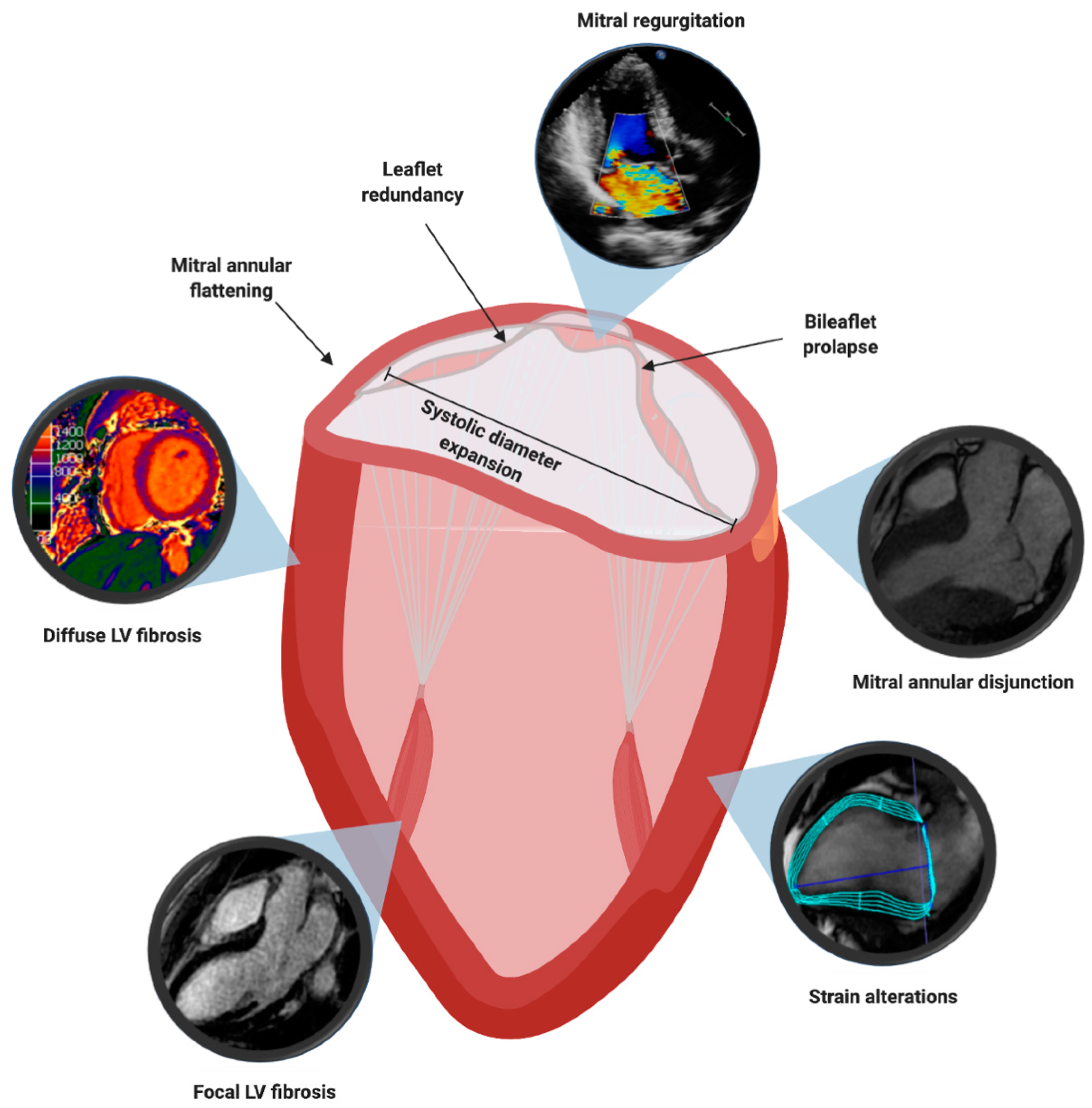
4. Multimodality Imaging Features Associated to Sudden Cardiac Death
4.1. Mitral Valve Leaflet Alterations
4.2. Mitral Valve Annulus Alterations
4.3. Myocardial Structural Abnormalities
4.4. Myocardial Contraction Abnormalities
4.5. Electrophysiologic Risk Factors
5. Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, R.A.; Handschumacher, M.D.; Sanfilippo, A.J.; Hagege, A.A.; Harrigan, P.; Marshall, J.E.; Weyman, A.E. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation 1989, 80, 589–598. [Google Scholar] [CrossRef]
- Jiang, L.; Owais, K.; Matyal, R.; Khabbaz, K.R.; Liu, D.C.; Montealegre-Gallegos, M.; Hess, P.E.; Mahmood, F. Dynamism of the mitral annulus: A spatial and temporal analysis. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1191–1197. [Google Scholar] [CrossRef]
- Garbi, M.; Monaghan, M.J. Quantitative mitral valve anatomy and pathology. Echo Res. Pract. 2015, 2. [Google Scholar] [CrossRef]
- Stephenson, A.; Adams, J.W.; Vaccarezza, M. The vertebrate heart: An evolutionary perspective. J. Anat. 2017, 231, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Salgo, I.S.; Gorman, J.H.; Gorman, R.C.; Jackson, B.M.; Bowen, F.W.; Plappert, T.; St John Sutton, M.G.; Edmunds, L.H.J. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation 2002, 106, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, J.J. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am. Heart J. 2012, 164, 163–176. [Google Scholar] [CrossRef]
- McCarthy, K.P.; Ring, L.; Rana, B.S. Anatomy of the mitral valve: Understanding the mitral valve complex in mitral regurgitation. Eur. J. Echocardiogr. 2010, 11, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A. Cardiac valve surgery—the “French correction”. J. Thorac. Cardiovasc. Surg. 1983, 86, 323–337. [Google Scholar] [CrossRef]
- Lam, J.H.; Ranganathan, N.; Wigle, E.D.; Silver, M.D. Morphology of the human mitral valve—Chordae tendineae: A new classification. Circulation 1970, 41, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Stathogiannis, E.; Newell, J.B.; Harrigan, P.; Weyman, A.E. Reconsideration of echocardiographic standards for mitral valve prolapse: Lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J. Am. Coll. Cardiol. 1988, 11, 1010–1019. [Google Scholar] [CrossRef]
- Delling, F.N.; Rong, J.; Larson, M.G.; Lehman, B.; Osypiuk, E.; Stantchev, P.; Slaugenhaupt, S.A.; Benjamin, E.J.; Levine, R.A.; Vasan, R.S. Familial clustering of mitral valve prolapse in the community. Circulation 2015, 131, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.L.; Fagan, L.F. Possible X-linked congenital heart disease. Circulation 1969, 39, 611–614. [Google Scholar] [CrossRef][Green Version]
- Kyndt, F.; Gueffet, J.-P.; Probst, V.; Jaafar, P.; Legendre, A.; Le Bouffant, F.; Toquet, C.; Roy, E.; McGregor, L.; Lynch, S.A.; et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation 2007, 115, 40–49. [Google Scholar] [CrossRef]
- Disse, S.; Abergel, E.; Berrebi, A.; Houot, A.M.; Le Heuzey, J.Y.; Diebold, B.; Guize, L.; Carpentier, A.; Corvol, P.; Jeunemaitre, X. Mapping of a first locus for autosomal dominant myxomatous mitral-valve prolapse to chromosome 16p11.2-p12.1. Am. J. Hum. Genet. 1999, 65, 1242–1251. [Google Scholar] [CrossRef]
- Freed, L.A.; Acierno, J.S.J.; Dai, D.; Leyne, M.; Marshall, J.E.; Nesta, F.; Levine, R.A.; Slaugenhaupt, S.A. A locus for autosomal dominant mitral valve prolapse on chromosome 11p15.4. Am. J. Hum. Genet. 2003, 72, 1551–1559. [Google Scholar] [CrossRef]
- Nesta, F.; Leyne, M.; Yosefy, C.; Simpson, C.; Dai, D.; Marshall, J.E.; Hung, J.; Slaugenhaupt, S.A.; Levine, R.A. New locus for autosomal dominant mitral valve prolapse on chromosome 13: Clinical insights from genetic studies. Circulation 2005, 112, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Durst, R.; Sauls, K.; Peal, D.S.; deVlaming, A.; Toomer, K.; Leyne, M.; Salani, M.; Talkowski, M.E.; Brand, H.; Perrocheau, M.; et al. Mutations in DCHS1 cause mitral valve prolapse. Nature 2015, 525, 109–113. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kramer-Fox, R.; Spitzer, M.C. Comparison of cardiovascular and skeletal features of primary mitral valve prolapse and Marfan syndrome. Am. J. Cardiol. 1989, 63, 317–321. [Google Scholar] [CrossRef]
- van de Laar, I.M.B.H.; Oldenburg, R.A.; Pals, G.; Roos-Hesselink, J.W.; de Graaf, B.M.; Verhagen, J.M.A.; Hoedemaekers, Y.M.; Willemsen, R.; Severijnen, L.-A.; Venselaar, H.; et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 2011, 43, 121–126. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Geltman, E.M.; Rodey, G.E.; Uitto, J. Mitral valve prolapse: A consistent manifestation of type IV Ehlers-Danlos syndrome. The pathogenetic role of the abnormal production of type III collagen. Circulation 1981, 64, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hortop, J.; Tsipouras, P.; Hanley, J.A.; Maron, B.J.; Shapiro, J.R. Cardiovascular involvement in osteogenesis imperfecta. Circulation 1986, 73, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Rosenhek, R.; Falk, V. Degenerative mitral valve regurgitation: Best practice revolution. Eur. Heart J. 2010, 31, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.B.; Bosman, C.K. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. Am. Heart J. 1966, 71, 166–178. [Google Scholar] [CrossRef]
- Criley, J.M.; Lewis, K.B.; Humphries, J.O.; Ross, R.S. Prolapse of the mitral valve: Clinical and cine-angiocardiographic findings. Br. Heart J. 1966, 28, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.B.; Bosman, C.K.; Pocock, W.A.; Marchand, P. Late systolic murmurs and non-ejection (“mid-late”) systolic clicks. An analysis of 90 patients. Br. Heart J. 1968, 30, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Warth, D.C.; King, M.E.; Cohen, J.M.; Tesoriero, V.L.; Marcus, E.; Weyman, A.E. Prevalence of mitral valve prolapse in normal children. J. Am. Coll. Cardiol. 1985, 5, 1173–1177. [Google Scholar] [CrossRef]
- Levine, R.A.; Triulzi, M.O.; Harrigan, P.; Weyman, A.E. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation 1987, 75, 756–767. [Google Scholar] [CrossRef]
- Delling, F.N.; Rong, J.; Larson, M.G.; Lehman, B.; Fuller, D.; Osypiuk, E.; Stantchev, P.; Hackman, B.; Manning, W.J.; Benjamin, E.J.; et al. Evolution of Mitral Valve Prolapse: Insights From the Framingham Heart Study. Circulation 2016, 133, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Kvasnicka, J.H.; Gardin, J.M.; Gidding, S.S.; Manolio, T.A.; Jacobs, D.R.J. Anthropometric and physiologic correlates of mitral valve prolapse in a biethnic cohort of young adults: The CARDIA study. Am. Heart J. 1999, 138, 486–492. [Google Scholar] [CrossRef]
- Devereux, R.B.; Jones, E.C.; Roman, M.J.; Howard, B.V.; Fabsitz, R.R.; Liu, J.E.; Palmieri, V.; Welty, T.K.; Lee, E.T. Prevalence and correlates of mitral valve prolapse in a population-based sample of American Indians: The Strong Heart Study. Am. J. Med. 2001, 111, 679–685. [Google Scholar] [CrossRef]
- Theal, M.; Sleik, K.; Anand, S.; Yi, Q.; Yusuf, S.; Lonn, E. Prevalence of mitral valve prolapse in ethnic groups. Can. J. Cardiol. 2004, 20, 511–515. [Google Scholar]
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; McGoon, M.D.; Shub, C.; Miller, F.A.J.; Ilstrup, D.M.; Tajik, A.J. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N. Engl. J. Med. 1985, 313, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Tamborini, G.; Maltagliati, A.; Galli, C.A.; Sisillo, E.; Salvi, L.; Naliato, M.; Porqueddu, M.; Parolari, A.; Zanobini, M.; et al. Head-to-head comparison of two and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J. Am. Coll. Cardiol. 2006, 48, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Biaggi, P.; Jedrzkiewicz, S.; Gruner, C.; Meineri, M.; Karski, J.; Vegas, A.; Tanner, F.C.; Rakowski, H.; Ivanov, J.; David, T.E.; et al. Quantification of mitral valve anatomy by three-dimensional transesophageal echocardiography in mitral valve prolapse predicts surgical anatomy and the complexity of mitral valve repair. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2012, 25, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Gripari, P.; Mapelli, M.; Bellacosa, I.; Piazzese, C.; Milo, M.; Fusini, L.; Muratori, M.; Ali, S.G.; Tamborini, G.; Pepi, M. Transthoracic echocardiography in patients undergoing mitral valve repair: Comparison of new transthoracic 3D techniques to 2D transoesophageal echocardiography in the localization of mitral valve prolapse. Int. J. Cardiovasc. Imaging 2018, 34, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, CIR0000000000000923. [Google Scholar] [CrossRef]
- Kamoen, V.; El Haddad, M.; De Backer, T.; De Buyzere, M.; Timmermans, F. Insights into functional mitral regurgitation using the average pixel intensity method. Int. J. Cardiovasc. Imaging 2019, 35, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Swift, A.J.; Zhong, L.; Carlhäll, C.-J.; Ebbers, T.; Westenberg, J.; Hope, M.D.; Bucciarelli-Ducci, C.; Bax, J.J.; Myerson, S.G. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat. Rev. Cardiol. 2020, 17, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Peters, D.C.; Salton, C.J.; Bzymek, D.; Nezafat, R.; Goddu, B.; Kissinger, K.V.; Zimetbaum, P.J.; Manning, W.J.; Yeon, S.B. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC. Cardiovasc. Imaging 2008, 1, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Delling, F.N.; Kang, L.L.; Yeon, S.B.; Kissinger, K.V.; Goddu, B.; Manning, W.J.; Han, Y. CMR predictors of mitral regurgitation in mitral valve prolapse. JACC. Cardiovasc. Imaging 2010, 3, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Buchner, S.; Debl, K.; Poschenrieder, F.; Feuerbach, S.; Riegger, G.A.J.; Luchner, A.; Djavidani, B. Cardiovascular magnetic resonance for direct assessment of anatomic regurgitant orifice in mitral regurgitation. Circ. Cardiovasc. Imaging 2008, 1, 148–155. [Google Scholar] [CrossRef]
- Scatteia, A.; Pascale, C.E.; Gallo, P.; Pezzullo, S.; America, R.; Cappelletti, A.M.; Dalla Vecchia, L.A.; Guarini, P.; Dellegrottaglie, S. Abnormal Papillary Muscle Signal on Cine MRI As a Typical Feature of Mitral Valve Prolapse. Sci. Rep. 2020, 10, 9166. [Google Scholar] [CrossRef]
- Koo, H.J.; Kang, J.-W.; Oh, S.Y.; Kim, D.-H.; Song, J.-M.; Kang, D.-H.; Song, J.-K.; Kim, J.B.; Jung, S.-H.; Choo, S.J.; et al. Cardiac computed tomography for the localization of mitral valve prolapse: Scallop-by-scallop comparisons with echocardiography and intraoperative findings. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-C.; Ha, F.J.; Teh, A.W.; Calafiore, P.; Jones, E.F.; Johns, J.; Koshy, A.N.; O’Donnell, D.; Hare, D.L.; Farouque, O.; et al. Mitral Valve Prolapse and Sudden Cardiac Death: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e010584. [Google Scholar] [CrossRef]
- Chahal, C.A.A.; Bouatia-Naji, N. Genetics of mitral valve prolapse and its clinical impact. E-J. Cardiol. Pract. 2019, 16, 35. [Google Scholar]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef]
- Jeresaty, R.M. Sudden death in the mitral valve prolapse-click syndrome. Am. J. Cardiol. 1976, 37, 317–318. [Google Scholar] [CrossRef]
- Marks, A.R.; Choong, C.Y.; Sanfilippo, A.J.; Ferré, M.; Weyman, A.E. Identification of high-risk and low-risk subgroups of patients with mitral-valve prolapse. N. Engl. J. Med. 1989, 320, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Zuppiroli, A.; Mori, F.; Favilli, S.; Barchielli, A.; Corti, G.; Montereggi, A.; Dolara, A. Arrhythmias in mitral valve prolapse: Relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. Am. Heart J. 1994, 128, 919–927. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.-T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and Outcome of Arrhythmic Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef]
- Zouridakis, E.G.; Parthenakis, F.I.; Kochiadakis, G.E.; Kanoupakis, E.M.; Vardas, P.E. QT dispersion in patients with mitral valve prolapse is related to the echocardiographic degree of the prolapse and mitral leaflet thickness. Europace 2001, 3, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Avierinos, J.-F.; Gersh, B.J.; Melton, L.J., III; Bailey, K.R.; Shub, C.; Nishimura, R.A.; Tajik, A.J.; Enriquez-Sarano, M. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002, 106, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rubio, A.; Schwammenthal, Y.; Schwammenthal, E.; Block, M.; Reinhardt, L.; Garcia-Alberola, A.; Sierra, G.; Shenasa, M.; Haverkamp, W.; Scheld, H.H.; et al. Patients with valvular heart disease presenting with sustained ventricular tachyarrhythmias or syncope: Results of programmed ventricular stimulation and long-term follow-up. Circulation 1997, 96, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, C.; Mischke, K.; Schimpf, T.; Neef, P.; Schauerte, P. Ventricular fibrillation due to severe mitral valve prolapse. Int. J. Cardiol. 2007, 116, e101–e102. [Google Scholar] [CrossRef] [PubMed]
- Sriram, C.S.; Syed, F.F.; Ferguson, M.E.; Johnson, J.N.; Enriquez-Sarano, M.; Cetta, F.; Cannon, B.C.; Asirvatham, S.J.; Ackerman, M.J. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2013, 62, 222–230. [Google Scholar] [CrossRef]
- Vaidya, V.R.; DeSimone, C.V.; Damle, N.; Naksuk, N.; Syed, F.F.; Ackerman, M.J.; Ponamgi, S.P.; Nkomo, V.T.; Suri, R.M.; Noseworthy, P.A.; et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J. Interv. Card. Electrophysiol. 2016, 46, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Nordhues, B.D.; Siontis, K.C.; Scott, C.G.; Nkomo, V.T.; Ackerman, M.J.; Asirvatham, S.J.; Noseworthy, P.A. Bileaflet Mitral Valve Prolapse and Risk of Ventricular Dysrhythmias and Death. J. Cardiovasc. Electrophysiol. 2016, 27, 463–468. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; Tomsic, A.; Gripari, P.; van Wijngaarden, A.L.; van der Pas, S.L.; Palmen, M.; Klautz, R.J.M.; Pepi, M.; Bax, J.J.; Delgado, V.; et al. Evolution from mitral annular dysfunction to severe mitral regurgitation in Barlow’s disease. Interact. Cardiovasc. Thorac. Surg. 2020. [Google Scholar] [CrossRef]
- Hutchins, G.M.; Moore, G.W.; Skoog, D.K. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N. Engl. J. Med. 1986, 314, 535–540. [Google Scholar] [CrossRef]
- Eriksson, M.J.; Bitkover, C.Y.; Omran, A.S.; David, T.E.; Ivanov, J.; Ali, M.J.; Woo, A.; Siu, S.C.; Rakowski, H. Mitral annular disjunction in advanced myxomatous mitral valve disease: Echocardiographic detection and surgical correction. J. Am. Soc. Echocardiogr. 2005, 18, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, V.; Volpato, V.; Gripari, P.; Ghulam Ali, S.; Fusini, L.; Italiano, G.; Muratori, M.; Pontone, G.; Tamborini, G.; Pepi, M. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2021, 107, 25–32. [Google Scholar] [CrossRef]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, J.A. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef]
- Lee, A.P.-W.; Jin, C.-N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional Implication of Mitral Annular Disjunction in Mitral Valve Prolapse: A Quantitative Dynamic 3D Echocardiographic Study. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Konda, T.; Tani, T.; Suganuma, N.; Nakamura, H.; Sumida, T.; Fujii, Y.; Kawai, J.; Kitai, T.; Kim, K.; Kaji, S.; et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J. Echocardiogr. 2017, 15, 176–185. [Google Scholar] [CrossRef]
- Mantegazza, V.; Tamborini, G.; Muratori, M.; Gripari, P.; Fusini, L.; Italiano, G.; Volpato, V.; Sassi, V.; Pepi, M. Mitral Annular Disjunction in a Large Cohort of Patients With Mitral Valve Prolapse and Significant Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 2278–2280. [Google Scholar] [CrossRef] [PubMed]
- Dejgaard, L.A.; Skjølsvik, E.T.; Lie, Ø.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The Mitral Annulus Disjunction Arrhythmic Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef]
- Park, J.; Geirsson, A.; Bonde, P.N. Mathematical Blueprint of a Mitral Valve. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Blomme, B.; Deroanne, C.; Hulin, A.; Lambert, C.; Defraigne, J.-O.; Nusgens, B.; Radermecker, M.; Colige, A. Mechanical strain induces a pro-fibrotic phenotype in human mitral valvular interstitial cells through RhoC/ROCK/MRTF-A and Erk1/2 signaling pathways. J. Mol. Cell. Cardiol. 2019, 135, 149–159. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Lino Cardenas, C.L.; Lindsay, M.E. Hereditary Influence in Thoracic Aortic Aneurysm and Dissection. Circulation 2016, 133, 2516–2528. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hagler, M.A.; Hadley, T.M.; Zhang, H.; Mehra, K.; Roos, C.M.; Schaff, H.V.; Suri, R.M.; Miller, J.D. TGF-β signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc. Res. 2013, 99, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, M.A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients With Primary Mitral Regurgitation With and Without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.H.; Roujol, S.; Foppa, M.; Kissinger, K.V.; Goddu, B.; Hauser, T.H.; Zimetbaum, P.J.; Ngo, L.H.; Manning, W.J.; Nezafat, R.; et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017, 103, 204–209. [Google Scholar] [CrossRef]
- Guglielmo, M.; Fusini, L.; Muscogiuri, G.; Baessato, F.; Loffreno, A.; Cavaliere, A.; Rizzon, G.; Baggiano, A.; Rabbat, M.G.; Muratori, M.; et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur. Radiol. 2020. [Google Scholar] [CrossRef]
- Garbi, M.; Lancellotti, P.; Sheppard, M.N. Mitral valve and left ventricular features in malignant mitral valve prolapse. Open Hear. 2018, 5, e000925. [Google Scholar] [CrossRef]
- Muthukumar, L.; Jahangir, A.; Jan, M.F.; Perez Moreno, A.C.; Khandheria, B.K.; Tajik, A.J. Association Between Malignant Mitral Valve Prolapse and Sudden Cardiac Death: A Review. JAMA Cardiol. 2020, 5, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, L.; Rahman, F.; Jan, M.F.; Shaikh, A.; Kalvin, L.; Dhala, A.; Jahangir, A.; Tajik, A.J. The Pickelhaube Sign: Novel Echocardiographic Risk Marker for Malignant Mitral Valve Prolapse Syndrome. JACC.: Cardiovasc. Imaging 2017, 10, 1078–1080. [Google Scholar]
- Syed, F.F.; Ackerman, M.J.; McLeod, C.J.; Kapa, S.; Mulpuru, S.K.; Sriram, C.S.; Cannon, B.C.; Asirvatham, S.J.; Noseworthy, P.A. Sites of Successful Ventricular Fibrillation Ablation in Bileaflet Mitral Valve Prolapse Syndrome. Circ. Arrhythm. Electrophysiol. 2016, 9. [Google Scholar] [CrossRef]
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069. [Google Scholar] [CrossRef]
- Miller, M.A.; Dukkipati, S.R.; Turagam, M.; Liao, S.L.; Adams, D.H.; Reddy, V.Y. Arrhythmic Mitral Valve Prolapse: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2904–2914. [Google Scholar] [CrossRef]
- Franz, M.R. Mechano-electrical feedback. Cardiovasc. Res. 2000, 45, 263–266. [Google Scholar] [CrossRef]
- Quinn, T.A.; Kohl, P. Cardiac Mechano-Electric Coupling: Acute Effects of Mechanical Stimulation on Heart Rate and Rhythm. Physiol. Rev. 2021, 101, 37–92. [Google Scholar] [CrossRef]
- Basso, C.; Iliceto, S.; Thiene, G.; Perazzolo Marra, M. Mitral Valve Prolapse, Ventricular Arrhythmias, and Sudden Death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef]
- Enriquez, A.; Shirai, Y.; Huang, J.; Liang, J.; Briceño, D.; Hayashi, T.; Muser, D.; Fulton, B.; Han, Y.; Perez, A.; et al. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: Electrophysiologic substrate and catheter ablation outcomes. J. Cardiovasc. Electrophysiol. 2019, 30, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Fulton, B.L.; Liang, J.J.; Enriquez, A.; Garcia, F.C.; Supple, G.E.; Riley, M.P.; Schaller, R.D.; Dixit, S.; Callans, D.J.; Marchlinski, F.E.; et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J. Cardiovasc. Electrophysiol. 2018, 29, 146–153. [Google Scholar] [CrossRef]
- Lichstein, E. Site of origin of ventricular premature beats in patients with mitral valve prolapse. Am. Heart J. 1980, 100, 450–457. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukavica, D.; Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Muratori, M.; Tamborini, G.; Mantegazza, V.; Trancuccio, A.; Arnò, C.; et al. Arrhythmic Mitral Valve Prolapse: Introducing an Era of Multimodality Imaging-Based Diagnosis and Risk Stratification. Diagnostics 2021, 11, 467. https://doi.org/10.3390/diagnostics11030467
Kukavica D, Guglielmo M, Baggiano A, Muscogiuri G, Fusini L, Muratori M, Tamborini G, Mantegazza V, Trancuccio A, Arnò C, et al. Arrhythmic Mitral Valve Prolapse: Introducing an Era of Multimodality Imaging-Based Diagnosis and Risk Stratification. Diagnostics. 2021; 11(3):467. https://doi.org/10.3390/diagnostics11030467
Chicago/Turabian StyleKukavica, Deni, Marco Guglielmo, Andrea Baggiano, Giuseppe Muscogiuri, Laura Fusini, Manuela Muratori, Gloria Tamborini, Valentina Mantegazza, Alessandro Trancuccio, Carlo Arnò, and et al. 2021. "Arrhythmic Mitral Valve Prolapse: Introducing an Era of Multimodality Imaging-Based Diagnosis and Risk Stratification" Diagnostics 11, no. 3: 467. https://doi.org/10.3390/diagnostics11030467
APA StyleKukavica, D., Guglielmo, M., Baggiano, A., Muscogiuri, G., Fusini, L., Muratori, M., Tamborini, G., Mantegazza, V., Trancuccio, A., Arnò, C., Mazzanti, A., Pepi, M., Priori, S. G., & Pontone, G. (2021). Arrhythmic Mitral Valve Prolapse: Introducing an Era of Multimodality Imaging-Based Diagnosis and Risk Stratification. Diagnostics, 11(3), 467. https://doi.org/10.3390/diagnostics11030467











