Association of Circulating miRNA Expression with Preeclampsia, Its Onset, and Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Sampling
2.2. RNA Extraction and Reverse Transcription
2.3. miRNA Expression Analysis by Quantitative Real-Time PCR
2.4. Cp-Value Analysis
2.5. Statistical Analysis
3. Results
3.1. Characterisation of the Study Group
3.2. Selection of Reference Genes for Normalisation
3.3. Different miRNA Expression in Plasma of PE Patients
3.4. miRNA Expression in Context with Severity and Onset of Preeclampsia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gathiram, P.; Moodley, J. Pre-Eclampsia: Its Pathogenesis and Pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef] [Green Version]
- North, R.A.; McCowan, L.M.E.; Dekker, G.A.; Poston, L.; Chan, E.H.Y.; Stewart, A.W.; Black, M.A.; Taylor, R.S.; Walker, J.J.; Baker, P.N.; et al. Clinical Risk Prediction for Pre-Eclampsia in Nulliparous Women: Development of Model in International Prospective Cohort. BMJ 2011, 342, d1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diagnosing Preeclampsia—Key Definitions and ACOG Guidelines. Available online: https://www.obgproject.com/2017/01/08/diagnosing-preeclampsia-key-definitions/ (accessed on 18 August 2020).
- Lisonkova, S.; Joseph, K.S. Incidence of Preeclampsia: Risk Factors and Outcomes Associated with Early-versus Late-Onset Disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef] [PubMed]
- Uzan, J.; Carbonnel, M.; Piconne, O.; Asmar, R.; Ayoubi, J.-M. Pre-Eclampsia: Pathophysiology, Diagnosis, and Management. Vasc. Health Risk Manag. 2011, 7, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Silasi, M.; Cohen, B.; Karumanchi, S.A.; Rana, S. Abnormal Placentation, Angiogenic Factors, and the Pathogenesis of Preeclampsia. Obstet. Gynecol. Clin. N. Am. 2010, 37, 239–253. [Google Scholar] [CrossRef]
- McMaster, M.T.; Zhou, Y.; Fisher, S.J. Abnormal Placentation and the Syndrome of Preeclampsia. Semin. Nephrol. 2004, 24, 540–547. [Google Scholar] [CrossRef]
- Aplin, J.D. Developmental Cell Biology of Human Villous Trophoblast: Current Research Problems. Int. J. Dev. Biol. 2010, 54, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Fu, G.; Brkić, J.; Hayder, H.; Peng, C. MicroRNAs in Human Placental Development and Pregnancy Complications. Int. J. Mol. Sci. 2013, 14, 5519–5544. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ge, Q.; Guo, L.; Lu, Z. Maternal Plasma MiRNAs Expression in Preeclamptic Pregnancies. BioMed Res. Int. 2013, 2013, 970265. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Ospina-Prieto, S.; Schmidt, A.; Chaiwangyen, W.; Markert, U.R. Elsevier Trophoblast Research Award Lecture: Origin, Evolution and Future of Placenta MiRNAs. Placenta 2014, 35, S39–S45. [Google Scholar] [CrossRef]
- Lycoudi, A.; Mavreli, D.; Mavrou, A.; Papantoniou, N.; Kolialexi, A. MiRNAs in Pregnancy-Related Complications. Expert Rev. Mol. Diagn. 2015, 15, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Moley, K.H.; Gronowski, A.M. Diagnostic Potential for MiRNAs as Biomarkers for Pregnancy-Specific Diseases. Clin. Biochem. 2013, 46, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Lasabova, Z.; Zigo, I.; Svecova, I.; Szabo, G.; Stanclova, A.; Skerenova, M.; Zubor, P.; Biskupska-Bodova, K.; Rigo, J.; Nagy, B.; et al. Association of Specific Diplotypes Defined by Common Rs1800682 and Rare Rs34995925 Single Nucleotide Polymorphisms within the STAT1 Transcription Binding Site of the FAS Gene Promoter with Preeclampsia. Gen. Physiol. Biophys. 2014, 33, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Small-for-Gestational-Age Fetus, Investigation and Management (Green-Top Guideline No. 31). Available online: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg31/ (accessed on 23 February 2021).
- Lees, C.C.; Stampalija, T.; Baschat, A.A.; Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and Management of Small-for-gestational-age Fetus and Fetal Growth Restriction. Ultrasound Obs. Gynecol 2020, 56, 298–312. [Google Scholar] [CrossRef] [PubMed]
- McCowan, L.M.; Figueras, F.; Anderson, N.H. Evidence-Based National Guidelines for the Management of Suspected Fetal Growth Restriction: Comparison, Consensus, and Controversy. Am. J. Obs. Gynecol. 2018, 218, S855–S868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- R Core Team R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 July 2020).
- Eklund, A. Beeswarm: The Bee Swarm Plot, an Alternative to Stripchart. Available online: https://CRAN.R-project.org/package=beeswarm (accessed on 16 July 2020).
- Maechler, M.; Rousseeuw, P.; Croux, C.; Todorov, V.; Ruckstuhl, A.; Salibian-Barrera, M.; Verbeke, T.; Koller, M.; Conceicao, E.L.T.; Palma, M.A. di Robustbase: Basic Robust Statistics. Available online: https://CRAN.R-project.org/package=robustbase (accessed on 16 July 2020).
- Ishwaran, H.; Kogalur, U.B. RandomForestSRC: Fast Unified Random Forests for Survival, Regression, and Classification (RF-SRC). Available online: https://CRAN.R-project.org/package=randomForestSRC (accessed on 16 July 2020).
- Ehrlinger, J. GgRandomForests: Visually Exploring Random Forests. Available online: https://CRAN.R-project.org/package=ggRandomForests (accessed on 16 July 2020).
- Donker, R.B.; Mouillet, J.-F.; Nelson, D.M.; Sadovsky, Y. The Expression of Argonaute2 and Related MicroRNA Biogenesis Proteins in Normal and Hypoxic Trophoblasts. Mol. Hum. Reprod. 2007, 13, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Brkić, J.; Liu, M.; Fu, G.; Peng, C.; Wang, Y.-L. Placental Trophoblast Cell Differentiation: Physiological Regulation and Pathological Relevance to Preeclampsia. Mol. Asp. Med. 2013, 34, 981–1023. [Google Scholar] [CrossRef]
- Seitz, H.; Royo, H.; Bortolin, M.-L.; Lin, S.-P.; Ferguson-Smith, A.C.; Cavaillé, J. A Large Imprinted MicroRNA Gene Cluster at the Mouse Dlk1-Gtl2 Domain. Genome Res. 2004, 14, 1741–1748. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Liu, M.; Yu, H.; Zhang, J.; Zhou, R. Circulating MicroRNAs as Biomarkers for Diagnosis and Prediction of Preeclampsia: A Systematic Review and Meta-Analysis. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 253, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fei, M.; Xue, G.; Zhou, Q.; Jia, Y.; Li, L.; Xin, H.; Sun, S. Elevated Levels of Hypoxia-Inducible MicroRNA-210 in Pre-Eclampsia: New Insights into Molecular Mechanisms for the Disease. J. Cell. Mol. Med. 2012, 16, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Olarerin-George, A.O.; Schwartz, N.; Srinivas, S.; Bastek, J.; Hogenesch, J.B.; Elovitz, M.A. MiR-210 Inhibits Trophoblast Invasion and Is a Serum Biomarker for Preeclampsia. Am. J. Pathol. 2013, 183, 1437–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineles, B.L.; Romero, R.; Montenegro, D.; Tarca, A.L.; Han, Y.M.; Kim, Y.M.; Draghici, S.; Espinoza, J.; Kusanovic, J.P.; Mittal, P.; et al. Distinct Subsets of MicroRNAs Are Expressed Differentially in the Human Placentas of Patients with Preeclampsia. Am. J. Obs. Gynecol. 2007, 196, 261.e1–261.e6. [Google Scholar] [CrossRef]
- Zhu, X.; Han, T.; Sargent, I.L.; Yin, G.; Yao, Y. Differential Expression Profile of MicroRNAs in Human Placentas from Preeclamptic Pregnancies vs Normal Pregnancies. Am. J. Obs. Gynecol. 2009, 200, 661.e1–661.e7. [Google Scholar] [CrossRef]
- Biró, O.; Alasztics, B.; Molvarec, A.; Joó, J.; Nagy, B.; Rigó, J. Various Levels of Circulating Exosomal Total-MiRNA and MiR-210 HypoxamiR in Different Forms of Pregnancy Hypertension. Pregnancy Hypertens 2017, 10, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Zhao, Y.; Liu, M.; Wang, Y.; Wang, H.; Li, Y.-X.; Zhu, X.; Yao, Y.; Wang, H.; Qiao, J.; et al. Variations of MicroRNAs in Human Placentas and Plasma from Preeclamptic Pregnancy. Hypertension 2014, 63, 1276–1284. [Google Scholar] [CrossRef]
- Munaut, C.; Tebache, L.; Blacher, S.; Noël, A.; Nisolle, M.; Chantraine, F. Dysregulated Circulating MiRNAs in Preeclampsia. Biomed. Rep. 2016, 5, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.S.-Q.; Casselman, R.C.; Tayade, C.; Smith, G.N. Differential Expression of Plasma MicroRNA in Preeclamptic Patients at Delivery and 1 Year Postpartum. Am. J. Obstet. Gynecol. 2015, 213, 367.e1–367.e9. [Google Scholar] [CrossRef]
- Mayor-Lynn, K.; Toloubeydokhti, T.; Cruz, A.C.; Chegini, N. Expression Profile of MicroRNAs and MRNAs in Human Placentas From Pregnancies Complicated by Preeclampsia and Preterm Labor. Reprod. Sci. 2011, 18, 46–56. [Google Scholar] [CrossRef]
- Ura, B.; Feriotto, G.; Monasta, L.; Bilel, S.; Zweyer, M.; Celeghini, C. Potential Role of Circulating MicroRNAs as Early Markers of Preeclampsia. Taiwan J. Obs. Gynecol. 2014, 53, 232–234. [Google Scholar] [CrossRef] [Green Version]
- Granger, J.P.; Alexander, B.T.; Llinas, M.T.; Bennett, W.A.; Khalil, R.A. Pathophysiology of Hypertension during Preeclampsia Linking Placental Ischemia with Endothelial Dysfunction. Hypertension 2001, 38, 718–722. [Google Scholar] [CrossRef]
- Tkachenko, A.; Illarionov, R.; Vashukova, E.; Glotov, A. Publication-Based Analysis of MiR-210 Dependent Biomarkers of Pre-Eclampsia. Biol. Commun. 2020, 65, 163–177. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 Is Induced during the Macrophage Inflammatory Response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef] [Green Version]
- Gan, L.; Liu, Z.; Wei, M.; Chen, Y.; Yang, X.; Chen, L.; Xiao, X. MiR-210 and MiR-155 as Potential Diagnostic Markers for Pre-Eclampsia Pregnancies. Medicine 2017, 96, e7515. [Google Scholar] [CrossRef]
- Jairajpuri, D.S.; Malalla, Z.H.; Mahmood, N.; Almawi, W.Y. Circulating MicroRNA Expression as Predictor of Preeclampsia and Its Severity. Gene 2017, 627, 543–548. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Chaiwangyen, W.; Ospina-Prieto, S.; Schneider, U.; Herrmann, J.; Gruhn, B.; Markert, U.R. MicroRNA Expression Profiles of Trophoblastic Cells. Placenta 2012, 33, 725–734. [Google Scholar] [CrossRef]
- Luo, S.-S.; Ishibashi, O.; Ishikawa, G.; Ishikawa, T.; Katayama, A.; Mishima, T.; Takizawa, T.; Shigihara, T.; Goto, T.; Izumi, A.; et al. Human Villous Trophoblasts Express and Secrete Placenta-Specific MicroRNAs into Maternal Circulation via Exosomes. Biol. Reprod. 2009, 81, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Maccani, M.A.; Padbury, J.F.; Marsit, C.J. MiR-16 and MiR-21 Expression in the Placenta Is Associated with Fetal Growth. PLoS ONE 2011, 6, e21210. [Google Scholar] [CrossRef]
- Lasabová, Z.; Vazan, M.; Zibolenova, J.; Svecova, I. Overexpression of MiR-21 and MiR-122 in Preeclamptic Placentas. Neuro Endocrinol. Lett. 2015, 36, 695–699. [Google Scholar]
- Zhou, F.; Sun, Y.; Gao, Q.; Wang, H. MicroRNA-21 Regulates the Proliferation of Placental Cells via FOXM1 in Preeclampsia. Exp. Ther. Med. 2020, 20, 1871–1878. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Yun, J.; Lee, O.-J.; Han, H.-S.; Yeo, M.-K.; Lee, M.-A.; Suh, K.-S. MicroRNA Expression Profiles in Placenta with Severe Preeclampsia Using a PNA-Based Microarray. Placenta 2013, 34, 799–804. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, X.; Ma, L.; Gao, N.; Tang, H.; Jian, F.; Ma, Y. Downregulations of Circulating MiR-31 and MiR-21 Are Associated with Preeclampsia. Pregnancy Hypertens. 2019, 17, 59–63. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Stearns, A.T.; Park, P.J.; Dreyfuss, J.M.; Ashley, S.W.; Rhoads, D.B.; Tavakkolizadeh, A. MicroRNA Mir-16 Is Anti-Proliferative in Enterocytes and Exhibits Diurnal Rhythmicity in Intestinal Crypts. Exp. Cell Res. 2010, 316, 3512–3521. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, P.; Dong, Y.; Cai, X.; Hou, D.; Guo, J.; Yin, Y.; Zhang, Y.; Li, J.; Liang, H.; et al. A Microarray-Based Approach Identifies ADP Ribosylation Factor-like Protein 2 as a Target of MicroRNA-16. J. Biol. Chem. 2011, 286, 9468–9476. [Google Scholar] [CrossRef] [Green Version]
- Bandi, N.; Zbinden, S.; Gugger, M.; Arnold, M.; Kocher, V.; Hasan, L.; Kappeler, A.; Brunner, T.; Vassella, E. MiR-15a and MiR-16 Are Implicated in Cell Cycle Regulation in a Rb-Dependent Manner and Are Frequently Deleted or down-Regulated in Non-Small Cell Lung Cancer. Cancer Res. 2009, 69, 5553–5559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linsley, P.S.; Schelter, J.; Burchard, J.; Kibukawa, M.; Martin, M.M.; Bartz, S.R.; Johnson, J.M.; Cummins, J.M.; Raymond, C.K.; Dai, H.; et al. Transcripts Targeted by the MicroRNA-16 Family Cooperatively Regulate Cell Cycle Progression. Mol. Cell. Biol. 2007, 27, 2240–2252. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fan, H.; Zhao, G.; Liu, D.; Du, L.; Wang, Z.; Hu, Y.; Hou, Y. MiR-16 Inhibits the Proliferation and Angiogenesis-Regulating Potential of Mesenchymal Stem Cells in Severe Pre-Eclampsia. FEBS J. 2012, 279, 4510–4524. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, H.; Huo, Z.; Ma, Z.; Dang, J.; Dang, W.; Pan, L.; Chen, J.; Zhong, H. MicroRNA-16 Inhibits Feto-Maternal Angiogenesis and Causes Recurrent Spontaneous Abortion by Targeting Vascular Endothelial Growth Factor. Sci. Rep. 2016, 6, 35536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Cardiovascular and Cerebrovascular Disease Associated MicroRNAs Are Dysregulated in Placental Tissues Affected with Gestational Hypertension, Preeclampsia and Intrauterine Growth Restriction. PLoS ONE 2015, 10, e0138383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hromadnikova, I.; Kotlabova, K.; Doucha, J.; Dlouha, K.; Krofta, L. Absolute and Relative Quantification of Placenta-Specific MicroRNAs in Maternal Circulation with Placental Insufficiency–Related Complications. J. Mol. Diagn. 2012, 14, 160–167. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, H.; Lin, H.; Qi, J.; Zhu, C.; Gao, Z.; Wang, H. Circulating MicroRNAs Are Elevated in Plasma from Severe Preeclamptic Pregnancies. Reproduction 2012, 143, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Xie, Y.; Zhang, H.; Wu, Y. Down-Regulation of NDRG2 Gene Expression in Human Colorectal Cancer Involves Promoter Methylation and MicroRNA-650. Biochem. Biophys. Res. Commun. 2011, 406, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Li, F.; Gao, F.; Sun, D.; Yao, L. Upregulation of MiR-650 Is Correlated with down-Regulation of ING4 and Progression of Hepatocellular Carcinoma. J. Surg. Oncol. 2013, 107, 105–110. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, W.; Zhang, J.; Huo, S.; Zhou, L.; Gu, Z.; Zhang, M. MicroRNA-650 Targets ING4 to Promote Gastric Cancer Tumorigenicity. Biochem. Biophys. Res. Commun. 2010, 395, 275–280. [Google Scholar] [CrossRef]
- Gunel, T.; Hosseini, M.K.; Gumusoglu, E.; Kisakesen, H.I.; Benian, A.; Aydinli, K. Expression Profiling of Maternal Plasma and Placenta MicroRNAs in Preeclamptic Pregnancies by Microarray Technology. Placenta 2017, 52, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Hornakova, A.; Kolkova, Z.; Holubekova, V.; Loderer, D.; Lasabova, Z.; Biringer, K.; Halasova, E. Diagnostic Potential of MicroRNAs as Biomarkers in the Detection of Preeclampsia. Genet. Test. Mol. Biomark. 2020, 24, 321–327. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; da Silva, A.M.; Calin, G.; Pantel, K. Data Normalization Strategies for MicroRNA Quantification. Clin. Chem. 2015, 61, 1333–1342. [Google Scholar] [CrossRef]
- Xiang, M.; Zeng, Y.; Yang, R.; Xu, H.; Chen, Z.; Zhong, J.; Xie, H.; Xu, Y.; Zeng, X. U6 Is Not a Suitable Endogenous Control for the Quantification of Circulating MicroRNAs. Biochem. Biophys. Res. Commun. 2014, 454, 210–214. [Google Scholar] [CrossRef]
- Shen, Y.; Tian, F.; Chen, Z.; Li, R.; Ge, Q.; Lu, Z. Amplification-Based Method for MicroRNA Detection. Biosens. Bioelectron. 2015, 71, 322–331. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, N.; Wright, D.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; de Alvarado, M.; Carbone, I.F.; Dutemeyer, V.; Fiolna, M.; Frick, A.; et al. Multicenter Screening for Pre-Eclampsia by Maternal Factors and Biomarkers at 11-13 Weeks’ Gestation: Comparison with NICE Guidelines and ACOG Recommendations. Ultrasound. Obs. Gynecol 2017, 49, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.Y.; Syngelaki, A.; Poon, L.C.; Rolnik, D.L.; O’Gorman, N.; Delgado, J.L.; Akolekar, R.; Konstantinidou, L.; Tsavdaridou, M.; Galeva, S.; et al. Screening for Pre-Eclampsia by Maternal Factors and Biomarkers at 11–13 Weeks’ Gestation. Ultrasound. Obs. Gynecol 2018, 52, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.Y.; Wright, D.; Syngelaki, A.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Greco, E.; Wright, A.; Maclagan, K.; et al. Comparison of Diagnostic Accuracy of Early Screening for Pre-Eclampsia by NICE Guidelines and a Method Combining Maternal Factors and Biomarkers: Results of SPREE. Ultrasound. Obs. Gynecol 2018, 51, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Sovio, U.; Gaccioli, F.; Cook, E.; Hund, M.; Charnock-Jones, D.S.; Smith, G.C.S. Prediction of Preeclampsia Using the Soluble Fms-Like Tyrosine Kinase 1 to Placental Growth Factor Ratio: A Prospective Cohort Study of Unselected Nulliparous Women. Hypertension 2017, 69, 731–738. [Google Scholar] [CrossRef]
- Bian, X.; Biswas, A.; Huang, X.; Lee, K.J.; Li, T.K.-T.; Masuyama, H.; Ohkuchi, A.; Park, J.S.; Saito, S.; Tan, K.H.; et al. Short-Term Prediction of Adverse Outcomes Using the SFlt-1 (Soluble Fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women With Suspected Preeclampsia. Hypertension 2019, 74, 164–172. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Cathrine Staff, A.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the SFlt-1:PlGF Ratio in Women With Suspected Preeclampsia. Obstet. Anesth. Dig. 2016, 36, 145–146. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). PlGF-Based Testing to Help Diagnose Suspected Pre-Eclampsia (Triage PlGF Test, Elecsys Immunoassay SFlt-1/PlGF Ratio, DELFIA Xpress PlGF 1-2-3 Test, and BRAHMS SFlt-1 Kryptor/BRAHMS PlGF plus Kryptor PE Ratio); NICE: London, UK, 2016. [Google Scholar]
- German Society of Obstetrics and Gynecology (DGGG); OEGG; SGGG. Guidelines for Hypertensive Disorders in Pregnancy. Diagnosis and Therapy. Available online: https://www.awmf.org/leitlinien/detail/ll/015-018.html (accessed on 16 February 2021).
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the Management of Cardiovascular Diseases during Pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef] [PubMed]


| miRBase ID | Assay Catalog Number | Coordinates (GRCh38) | miRNA Sequence |
|---|---|---|---|
| hsa-miR-16-5p | YP00205702 | chr13: 50048973-50049061 [−] | 5‘-UAGCAGCACGUAAAUAUUGGCG-3‘ |
| hsa-miR-21-5p | YP00204230 | chr17: 59841266-59841337 [+] | 5‘-UAGCUUAUCAGACUGAUGUUGA-3‘ |
| hsa-miR-103a-3p | YP00204063 | chr20: 3917494-3917571 [+] | 5‘-AGCAGCAUUGUACAGGGCUAUGA-3‘ |
| hsa-miR-155-5p | YP00204308 | chr21: 25573980-25574044 [+] | 5‘-UUAAUGCUAAUCGUGAUAGGGGUU-3‘ |
| hsa-miR-188-5p | YP00204239 | chrX: 50003503-50003588 [+] | 5‘-CAUCCCUUGCAUGGUGGAGGG-3‘ |
| hsa-miR-191-5p | YP00204306 | chr3: 49020618-49020709 [−] | 5‘-CAACGGAAUCCCAAAAGCAGCUG-3‘ |
| hsa-miR-210-5p | YP00204321 | chr11: 568089-568198 [−] | 5‘-AGCCCCUGCCCACCGCACACUG-3‘ |
| hsa-miR-222-3p | YP00204551 | chrX: 45747015-45747124 [−] | 5‘-AGCUACAUCUGGCUACUGGGU-3‘ |
| hsa-miR-650 | YP00204233 | chr22: 22822776-22822871 [+] | 5‘-AGGAGGCAGCGCUCUCAGGAC-3‘ |
| Parameter | Healthy Pregnant Women | PE Patients | p Value |
|---|---|---|---|
| (n = 32) | (n = 27) | ||
| Age (years) | 30 (25–37) | 27 (21–50) | 0.072 |
| Blood pressure (mmHg) | |||
| Systolic | 128 (99–147) | 157 (125–198) | <0.001 |
| Diastolic | 79 (54–91) | 102.5 (75–120) | < 0.001 |
| Proteinuria (g/24 h) | None | 1.54 (0.126–9.86) | - |
| Pregnancy body mass index | 26.5 (20.4–40.4) | 30.8 (20.2–44.6) | <0.001 |
| Gestational age at delivery (weeks) | 40 (37–42) | 37.5 (27–42) | <0.001 |
| Mode of delivery | 0.01 | ||
| Vaginal | 20 (62.5%) | 9 (33.3%) | |
| Caesarean section | 9 (28.1%) | 15 (55.5%) | |
| NA | 3 (9.4%) | 3 (11.1%) | |
| Foetal birth weight (grams) | 3535 (2630–4540) | 2800 (590–4290) | 0.002 |
| Foetal growth restriction early late SGA Foetal sex | − − − − | 8 (29.6%) 2 (7.4%) 6 (22.2%) 3 (11.1%) | |
| Boy | 14 (43.75%) | 9 (33.3%) | |
| Girl | 15 (46.88%) | 14 (51.9%) | |
| NA | 3 (9.4%) | 4 (14.8%) |
| Parameter | Patients with Mild PE | Patients with Severe PE | p Value |
|---|---|---|---|
| (n = 11) | (n = 13) | ||
| Age (years) | 24.5 (21–32) | 29 (22–50) | 0.051 |
| Blood pressure (mmHg) | |||
| Systolic | 144 (125–160) | 164 (134–198) | 0.002 |
| Diastolic | 96 (75–106) | 106 (84–120) | 0.02 |
| Proteinuria (g/24 h) | 1.41 (0.297–3.66) | 1.8 (0.126–9.86) | 0.7 |
| Pregnancy body mass index | 29.1 (20.2–44.6) | 31.5 (24.6–41.6) | 0.3 |
| Gestational age at delivery (weeks) | 38 (27–42) | 37 (28–41) | 0.8 |
| Mode of delivery | 0.2 | ||
| Vaginal | 6 (54.5%) | 3 (23.1%) | |
| Caesarean section | 5 (45.5%) | 10 (76.9%) | |
| Foetal birth weight (grams) | 2440 (590–3900) | 2930 (660–4290) | 0.6 |
| Foetal sex | 0.3 | ||
| Boy | 5 (45.5%) | 9 (69.2%) | |
| Girl | 6 (54.5%) | 3 (23.1%) | |
| NA | 0 | 1 (7.7%) |
| Parameter | Patients with Early Onset PE | Patients with Late-Onset PE | p Value |
|---|---|---|---|
| (n = 7) | (n = 17) | ||
| Age (years) | 27 (21–50) | 26.5 (22–34) | >0.9 |
| Blood pressure (mmHg) | |||
| Systolic | 155 (143–189) | 157 (125–180) | 0.6 |
| Diastolic | 102 (81–110) | 104 (75–116) | 0.5 |
| Proteinuria (g/24 h) | 1.8 (1.286–3.66) | 1.04 (0.126–9.86) | 0.3 |
| Pregnancy body mass index | 28.4 (20.2–44.6) | 31.1 (23.4–44.6) | 0.6 |
| Gestational age at delivery (weeks) | 31 (27–34) | 39 (35–41) | <0.001 |
| Mode of delivery | 0.01 | ||
| Vaginal | 0 (0%) | 9 (52.9%) | |
| Caesarean section | 7 (100%) | 8 (47.1%) | |
| Foetal birth weight (grams) | 1460 (590–1790) | 3140 (1600–4290) | <0.001 |
| Foetal sex | >0.9 | ||
| Boy | 4 (57.1%) | 10 (58.8%) | |
| Girl | 2 (28.6%) | 7 (41.2%) | |
| NA | 1 (14.3%) |
| miRNA | Median Fold Change | p Value |
|---|---|---|
| hsa-miR-16-5p | 0.78 | 0.0903 |
| hsa-miR-21-5p | 1.16 | 0.0015 |
| hsa-miR-155-5p | 1.62 | 0.0005 |
| hsa-miR-210-5p | 1.29 | 0.0735 |
| miRNA | Mild PE | Severe PE | ||
|---|---|---|---|---|
| Median FC | p Value | Median FC | p Value | |
| hsa-miR-16-5p | 0.776 | 0.23 | 0.929 | 0.497 |
| hsa-miR-21-5p | 1.146 | 0.365 | 1.316 | 0.008 |
| hsa-miR-155-5p | 1.505 | 0.083 | 1.711 | 0.017 |
| hsa-miR-210-5p | 0.991 | 0.638 | 1.604 | 0.094 |
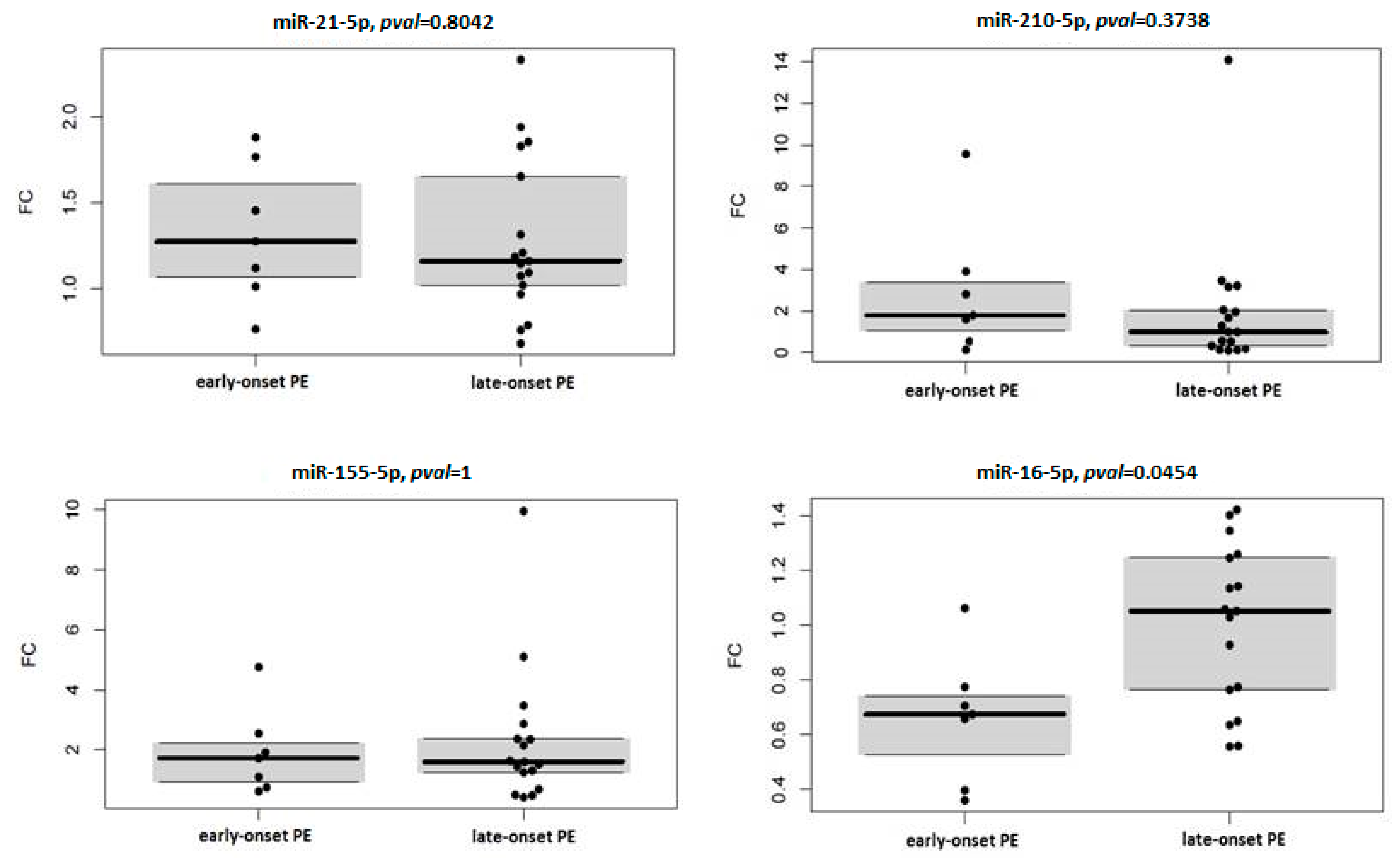
| miRNA | Early Onset PE | Late-Onset PE | ||
|---|---|---|---|---|
| Median FC | p Value | Median FC | p Value | |
| hsa-miR-16-5p | 0.675 | 0.031 | 1.052 | 1 |
| hsa-miR-21-5p | 1.275 | 0.078 | 1.162 | 0.035 |
| hsa-miR-155-5p | 1.711 | 0.156 | 1.597 | 0.011 |
| hsa-miR-210-5p | 1.792 | 0.156 | 0.991 | 0.344 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolkova, Z.; Holubekova, V.; Grendar, M.; Nachajova, M.; Zubor, P.; Pribulova, T.; Loderer, D.; Zigo, I.; Biringer, K.; Hornakova, A. Association of Circulating miRNA Expression with Preeclampsia, Its Onset, and Severity. Diagnostics 2021, 11, 476. https://doi.org/10.3390/diagnostics11030476
Kolkova Z, Holubekova V, Grendar M, Nachajova M, Zubor P, Pribulova T, Loderer D, Zigo I, Biringer K, Hornakova A. Association of Circulating miRNA Expression with Preeclampsia, Its Onset, and Severity. Diagnostics. 2021; 11(3):476. https://doi.org/10.3390/diagnostics11030476
Chicago/Turabian StyleKolkova, Zuzana, Veronika Holubekova, Marian Grendar, Marcela Nachajova, Pavol Zubor, Terezia Pribulova, Dusan Loderer, Imrich Zigo, Kamil Biringer, and Andrea Hornakova. 2021. "Association of Circulating miRNA Expression with Preeclampsia, Its Onset, and Severity" Diagnostics 11, no. 3: 476. https://doi.org/10.3390/diagnostics11030476
APA StyleKolkova, Z., Holubekova, V., Grendar, M., Nachajova, M., Zubor, P., Pribulova, T., Loderer, D., Zigo, I., Biringer, K., & Hornakova, A. (2021). Association of Circulating miRNA Expression with Preeclampsia, Its Onset, and Severity. Diagnostics, 11(3), 476. https://doi.org/10.3390/diagnostics11030476






