Synchrotron Radiation-Based Refraction-Contrast Tomographic Images Using X-ray Dark-Field Imaging Optics in Human Lung Adenocarcinoma and Histologic Correlations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lung Tissue Preparation
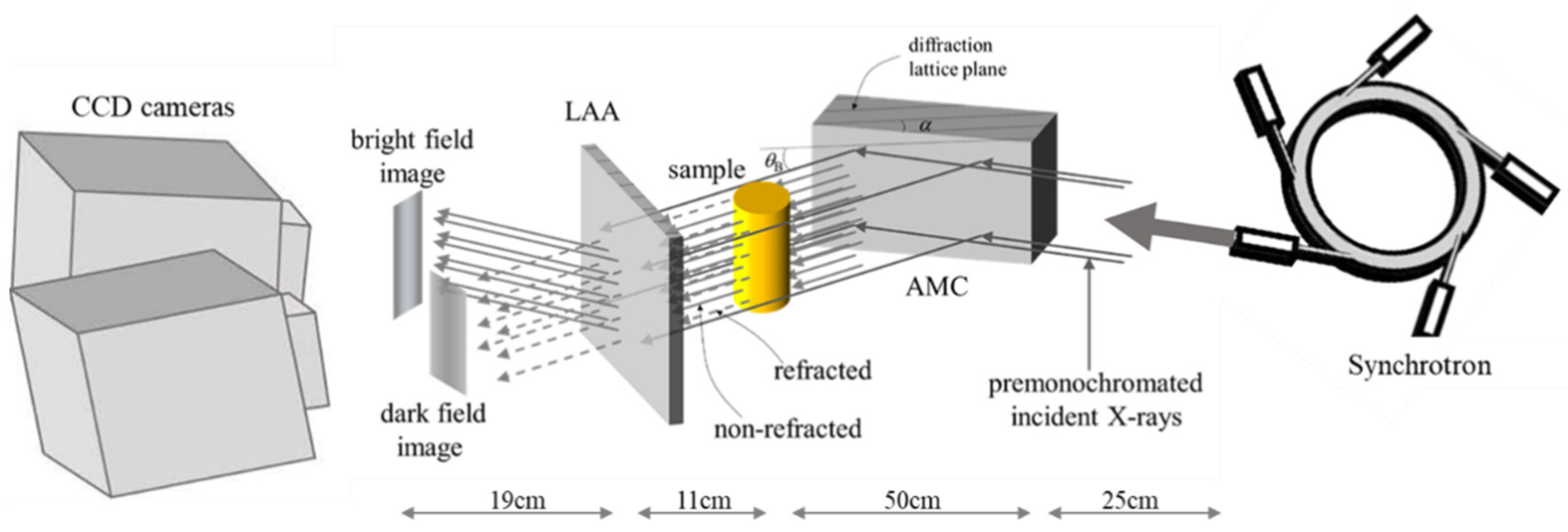
2.2. X-ray Source and Experimental Setup
2.3. Acquisition and Comprehension of Imaging Data
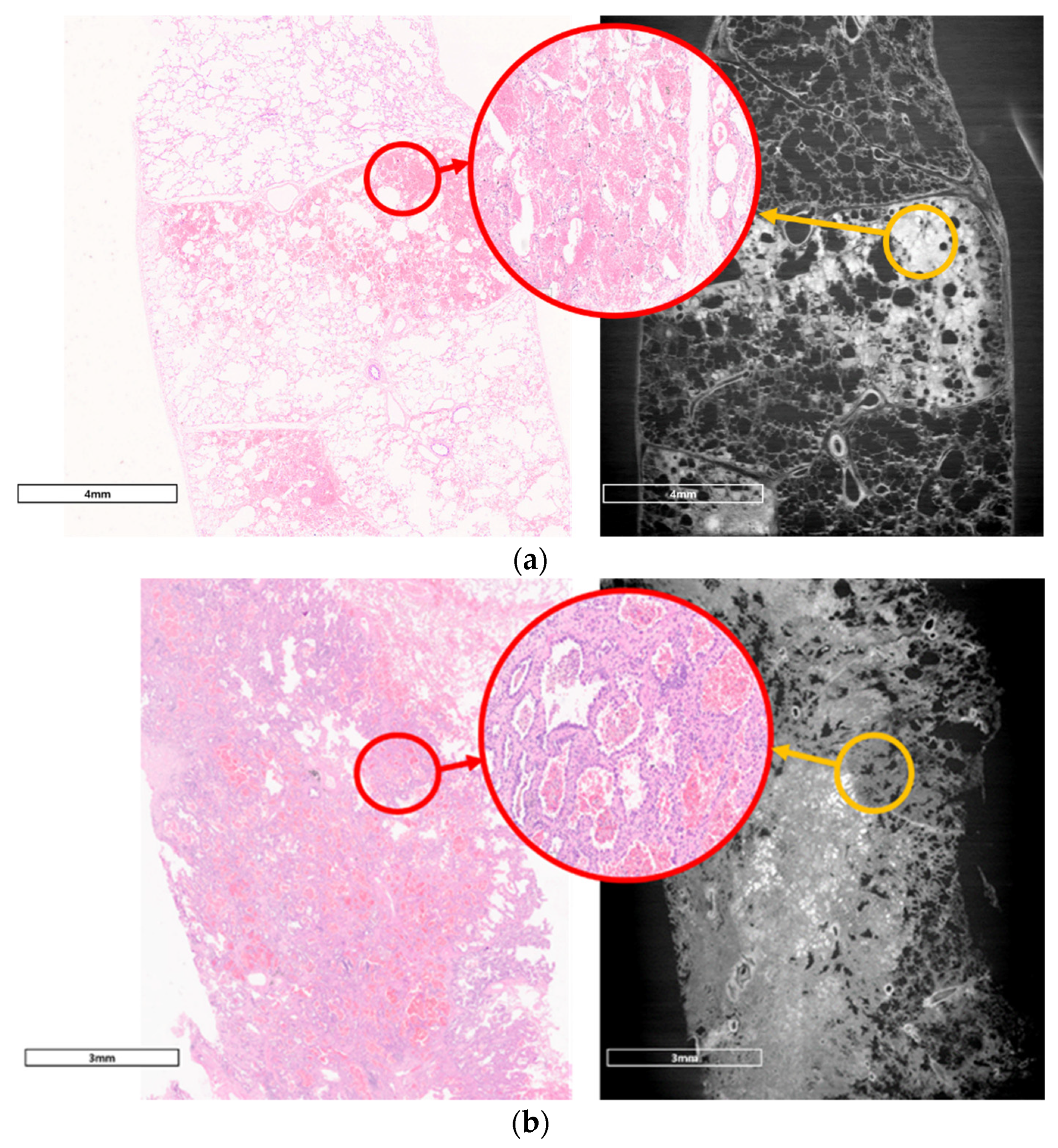
2.4. Comparison with Pathologic Examination
3. Results
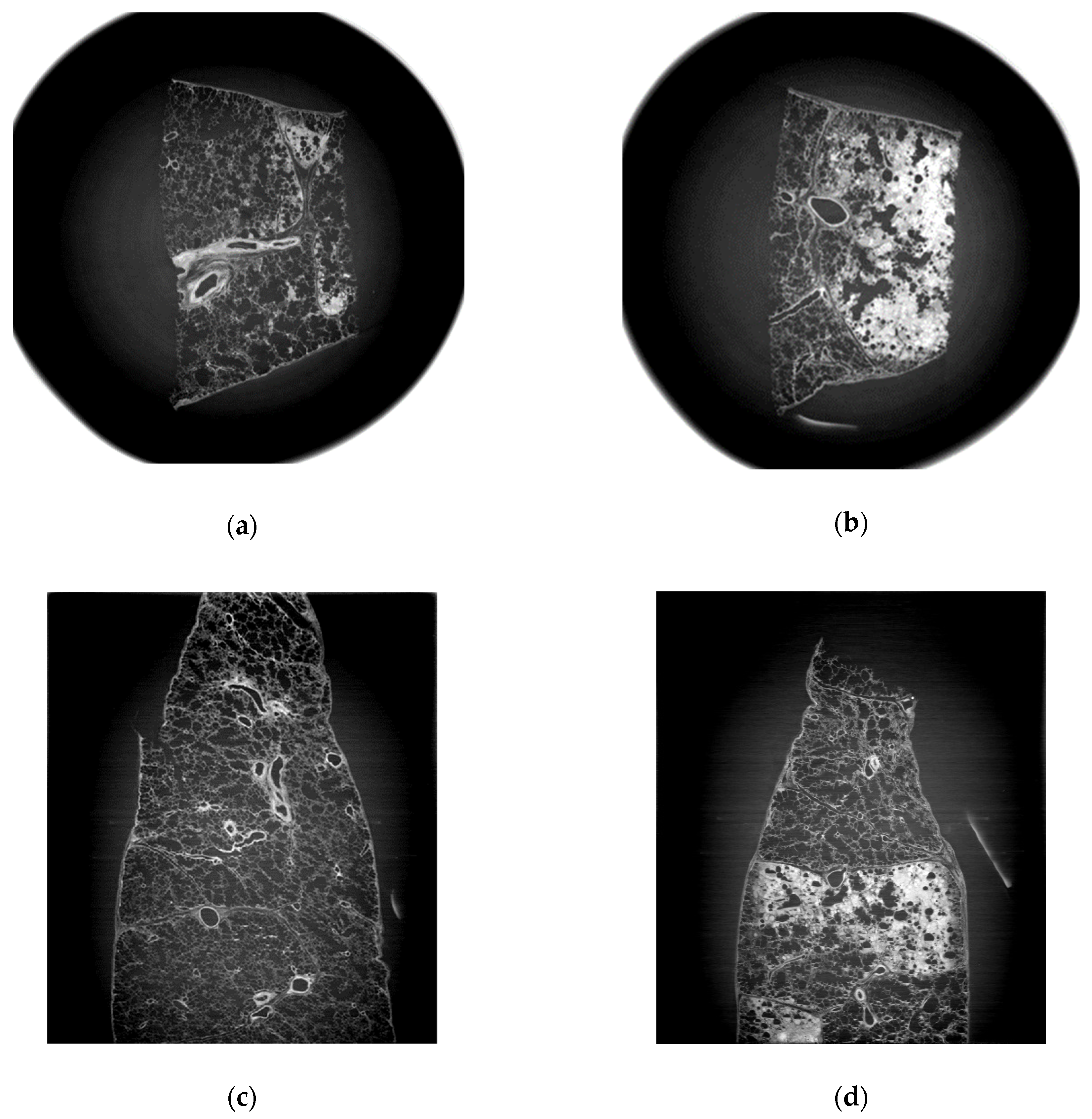
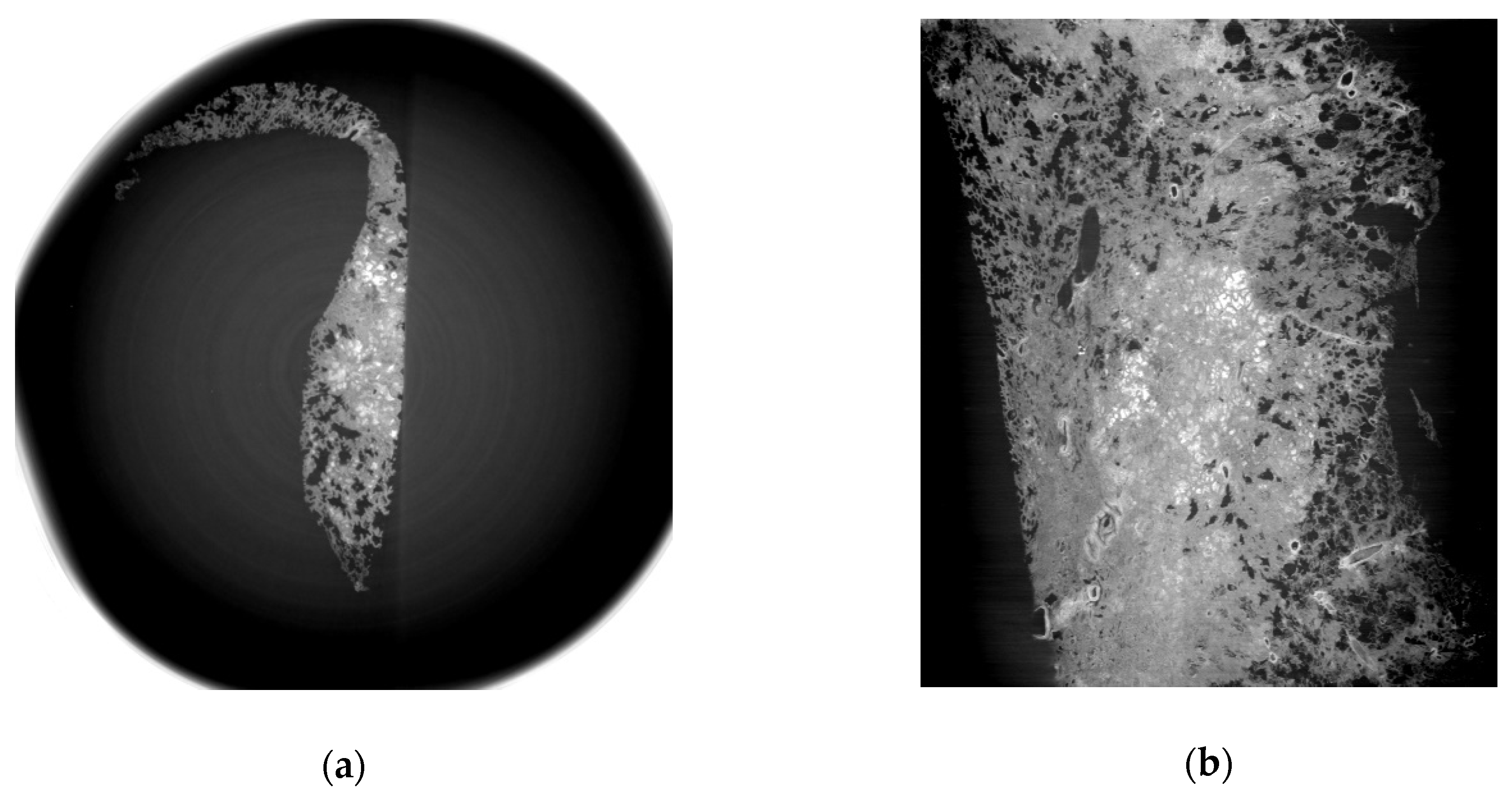
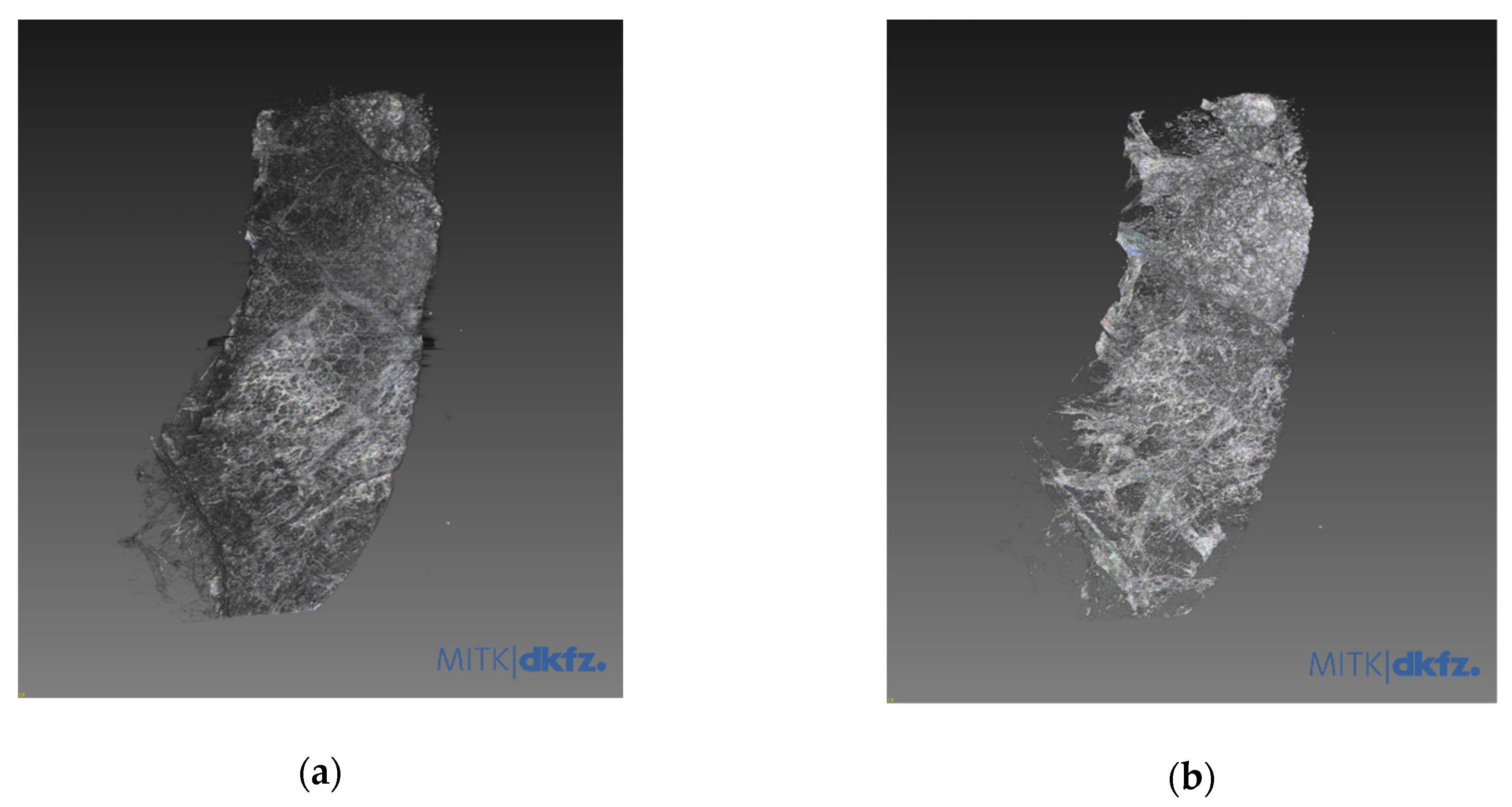
3.1. Refraction-Contrast Synchrotron Tomographic Images of Lung Tissue Including Cancer and 3D Reconstruction
3.2. Comparison with Pathologic Examination
3.3. Special Staining for Diagnosis of Lung Adenocarcinoma Including Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aberle, D.; Adams, A.; Adams, A.; Berg, C.; Black, W.; Clapp, J.; Fagerstrom, R.; Gareen, I.; Gatsonis, C.; Marcus, P. National Lung Screening Trial Research T. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Walters, S.; Maringe, C.; Coleman, M.P.; Peake, M.D.; Butler, J.; Young, N.; Bergström, S.; Hanna, L.; Jakobsen, E.; Kölbeck, K. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004–2007. Thorax 2013, 68, 551–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Sun, J.; Guan, Y.; Yue, W.; Xu, L.X.; Li, Y.; Zhang, G.; Hwu, Y.; Je, J.H.; Margaritondo, G. Morphological study of early-stage lung cancer using synchrotron radiation. J. Synchrotron Radiat. 2008, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sunaguchi, N.; Yuasa, T.; Huo, Q.; Ichihara, S.; Ando, M. X-ray refraction-contrast computed tomography images using dark-field imaging optics. Appl. Phys. Lett. 2010, 97, 153701. [Google Scholar] [CrossRef]
- Umetani, K.; Itoh, H.; Kawata, Y.; Niki, N. High-resolution wide-field synchrotron radiation micro-CT for large human lung specimen imaging. In Proceedings of the Advanced Optical Imaging Technologies, Beijing, China, 11–13 October 2018; Volume 10816, p. 108160A. [Google Scholar] [CrossRef]
- Ando, M.; Nakao, Y.; Jin, G.; Sugiyama, H.; Sunaguchi, N.; Sung, Y.; Suzuki, Y.; Sun, Y.; Tanimoto, M.; Kawashima, K.; et al. Improving contrast and spatial resolution in crystal analyzer-based X-ray dark-field imaging: Theoretical considerations and experimental demonstration. Med. Phys. 2020, 47, 5505–5513. [Google Scholar] [CrossRef]
- Sunaguchi, N.; Yuasa, T.; Gupta, R.; Ando, M. An efficient reconstruction algorithm for differential phase-contrast tomographic images from a limited number of views. Appl. Phys. Lett. 2015, 107, 253701. [Google Scholar] [CrossRef]
- Hausmann, R. Methods of Lung Fixation. In Forensic Pathology Reviews; Springer: Cham, Switzerland, 2006; pp. 437–451. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Li, A.; Zhang, Y.; Fan, C. Synchrotron-based X-ray microscopy for sub-100 nm resolution cell imaging. Curr. Opin. Chem. Biol. 2017, 39, 11–16. [Google Scholar] [CrossRef]
- Jheon, S.; Youn, H.-S.; Kim, H.-T.; Choi, G.-H.; Kim, J.-K. High-resolution X-ray refraction imaging of rat lung and histological correlations. Microsc. Res. Tech. 2006, 69, 656–659. [Google Scholar] [CrossRef]
- Yi, E.; Han, S.-M.; Chang, J.-E.; Kim, H.-T.; Kim, J.-K.; Seo, S.-J.; Chung, J.-H.; Jheon, S. Synchrotron tomographic images from human lung adenocarcinoma: Three-dimensional reconstruction and histologic correlations. Microsc. Res. Tech. 2017, 80, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Hausermann, D.; Hall, C.; Maksimenko, A.; Campbell, C. The imaging and medical beam line at the Australian Synchrotron. AIP Conf. Proc. 2010, 1266, 3. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.L.; Wuennemann, F.; Pacilé, S.; Albers, J.; Arfelli, F.; Dreossi, D.; Biederer, J.; Konietzke, P.; Stiller, W.; Wielpütz, M.O. Towards synchrotron phase-contrast lung imaging in patients—A proof-of-concept study on porcine lungs in a human-scale chest phantom. J. Synchrotron Radiat. 2018, 25, 1827–1832. [Google Scholar] [CrossRef]
- Di Lillo, F.; Mettivier, G.; Sarno, A.; Castriconi, R.; Russo, P. Towards breast cancer rotational radiotherapy with synchrotron radiation. Phys. Med. 2017, 41, 20–25. [Google Scholar] [CrossRef]
- Verry, C.; Sancey, L.; Dufort, S.; Le Duc, G.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.L.; Villa, J.; et al. Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol. BMJ Open 2019, 9, e023591. [Google Scholar] [CrossRef]
- Castelli, E.; Tonutti, M.; Arfelli, F.; Longo, R.; Quaia, E.; Rigon, L.; Sanabor, D.; Zanconati, F.; Dreossi, D.; Abrami, A.; et al. Mammography with Synchrotron Radiation: First Clinical Experience with Phase-Detection Technique. Radiology 2011, 259, 684–694. [Google Scholar] [CrossRef]
- Ando, M.; Sunaguchi, N.; Shimao, D.; Pan, A.; Yuasa, T.; Mori, K.; Suzuki, Y.; Jin, G.; Kim, J.-K.; Lim, J.-H.; et al. Dark-Field Imaging: Recent Developments and Potential Clinical Applications. Phys. Med. 2016, 32, 1801–1812. [Google Scholar] [CrossRef]
- Ando, M.; Yamasaki, K.; Ohbayashi, C.; Esumi, H.; Hyodo, K.; Sugiyama, H.; Li, G.; Maksimenko, A.; Kawai, T. Attempt at Two-Dimensional Mapping of X-ray Fluorescence from Breast Cancer Tissue. Jpn. J. Appl. Phys. 2005, 44, L998–L1001. [Google Scholar] [CrossRef]
- Ando, M.; Sunaguchi, N.; Wu, Y.; Do, S.; Sung, Y.; Louissaint, A.; Yuasa, T.; Ichihara, S.; Gupta, R. Crystal analyser-based X-ray phase contrast imaging in the dark field: Implementation and evaluation using excised tissue specimens. Eur. Radiol. 2014, 24, 423–433. [Google Scholar] [CrossRef]
- Willer, K.; Fingerle, A.A.; Gromann, L.B.; De Marco, F.; Herzen, J.; Achterhold, K.; Gleich, B.; Muenzel, D.; Scherer, K.; Renz, M.; et al. X-ray dark-field imaging of the human lung—A feasibility study on a deceased body. PLoS ONE 2018, 13, e0204565. [Google Scholar] [CrossRef]
- Meinel, F.G.; Schwab, F.; Yaroshenko, A.; Velroyen, A.; Bech, M.; Hellbach, K.; Fuchs, J.; Stiewe, T.; Yildirim, A.Ö.; Bam-berg, F.; et al. Lung tumors on multimodal radiographs derived from grating-based X-ray imaging—A feasibility study. Phys. Med. 2014, 30, 352–357. [Google Scholar] [CrossRef]
- Gradl, R.; Morgan, K.S.; Dierolf, M.; Jud, C.; Hehn, L.; Gunther, B.; Moller, W.; Kutschke, D.; Yang, L.; Stoeger, T.; et al. Dynamic In Vivo Chest X-ray Dark-Field Imaging in Mice. IEEE Trans. Med. Imaging 2018, 38, 649–656. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, E.; Sunaguchi, N.; Lee, J.H.; Kim, C.-Y.; Lee, S.; Jheon, S.; Ando, M.; Seok, Y. Synchrotron Radiation-Based Refraction-Contrast Tomographic Images Using X-ray Dark-Field Imaging Optics in Human Lung Adenocarcinoma and Histologic Correlations. Diagnostics 2021, 11, 487. https://doi.org/10.3390/diagnostics11030487
Yi E, Sunaguchi N, Lee JH, Kim C-Y, Lee S, Jheon S, Ando M, Seok Y. Synchrotron Radiation-Based Refraction-Contrast Tomographic Images Using X-ray Dark-Field Imaging Optics in Human Lung Adenocarcinoma and Histologic Correlations. Diagnostics. 2021; 11(3):487. https://doi.org/10.3390/diagnostics11030487
Chicago/Turabian StyleYi, Eunjue, Naoki Sunaguchi, Jeong Hyeon Lee, Chul-Yong Kim, Sungho Lee, Sanghoon Jheon, Masami Ando, and Yangki Seok. 2021. "Synchrotron Radiation-Based Refraction-Contrast Tomographic Images Using X-ray Dark-Field Imaging Optics in Human Lung Adenocarcinoma and Histologic Correlations" Diagnostics 11, no. 3: 487. https://doi.org/10.3390/diagnostics11030487





