Importance of Cardiopulmonary Exercise Testing amongst Subjects Recovering from COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Measurements
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.; Balbi, B.; Prince, I.; Cattaneo, D.; Masocco, F.; Zaccaria, S.; Bertalli, L.; Cattini, F.; Lomazzo, A.; Negro, F.D.; et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur. Respir. J. 2020, 56, 2002096. [Google Scholar] [CrossRef]
- Society, A.T. American College of Chest Physicians ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Phillips, D.B.; Collins, S.É.; Stickland, M.K. Measurement and Interpretation of Exercise Ventilatory Efficiency. Front. Physiol. 2020, 11, 659. [Google Scholar] [CrossRef]
- Neder, J.A.; Berton, D.C.; Arbex, F.F.; Alencar, M.C.; Rocha, A.; Sperandio, P.A.; Palange, P.; O’Donnell, D.E. Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur. Respir. J. 2017, 49, 1602036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.-G.; Hansen, J.E.; Garatachea, N.; Storer, T.W.; Wasserman, K. Ventilatory Efficiency during Exercise in Healthy Subjects. Am. J. Respir. Crit. Care Med. 2002, 166, 1443–1448. [Google Scholar] [CrossRef]
- Naeije, R.; Faoro, V. The great breathlessness of cardiopulmonary diseases. Eur. Respir. J. 2018, 51, 1702517. [Google Scholar] [CrossRef]
- Barbosa, G.W.; Neder, J.A.; Utida, K.; O’Donnell, D.E.; Müller, P.D.T. Impaired exercise ventilatory efficiency in smokers with low transfer factor but normal spirometry. Eur. Respir. J. 2017, 49, 1602511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Quanyer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993, 16, 5–40. [Google Scholar]
- Cotes, J.E.; Chinn, D.J.; Quanjer, P.H.; Roca, J.; Yernault, J.C. Standardisation of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993, 16, 41–52. [Google Scholar]
- A Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. American Thoracic Society statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158 Pt 1, 1384–1387. [Google Scholar] [CrossRef] [Green Version]
- Mannocci, A.; Di Thiene, D.; Del Cimmuto, A.; Masala, D.; Boccia, A.; De Vito, E.; La Torre, G. International Physical Activity Questionnaire: Validation and assessment in an Italian sample. Ital. J. Public Health 2010, 7, 369–376. [Google Scholar]
- Gläser, S.; Obst, A.; Opitz, C.F.; Dörr, M.; Felix, S.B.; Empen, K.; Völzke, H.; Ewert, R.; Schäper, C.; Koch, B. Peripheral endothelial dysfunction is associated with gas exchange inefficiency in smokers. Respir. Res. 2011, 12, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brischetto, M.J.; Millman, R.P.; Peterson, D.D.; Silage, D.A.; Pack, A.I. Effect of aging on ventilatory response to exercise and CO2. J. Appl. Physiol. 1984, 56, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- McGurk, S.P.; Blanksby, B.A.; Anderson, M.J. The Relationship of Hypercapnic Ventilatory Responses to Age, Gender and Athleticism. Sports Med. 1995, 19, 173–183. [Google Scholar] [CrossRef]
- Salazar-Martínez, E.; De Matos, T.R.; Arrans, P.; Santalla, A.; Orellana, J.N. Ventilatory efficiency response is unaffected by fitness level, ergometer type, age or body mass index in male athletes. Biol. Sport 2018, 35, 393–398. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-Rate Recovery Immediately after Exercise as a Predictor of Mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Crisafulli, E.; Scelfo, C.; Tzani, P.; Aiello, M.; Bertorelli, G.; Chetta, A. Asymptomatic peripheral artery disease can limit maximal exercise capacity in chronic obstructive pulmonary disease patients regardless of airflow obstruction and lung hyperinflation. Eur. J. Prev. Cardiol. 2017, 24, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Vigna, M.; Ielpo, A.; Tzani, P.; Mangia, A.; Teopompi, E.; Aiello, M.; Alfieri, V.; Bertorelli, G.; Palange, P.; et al. Heart rate recovery is associated with ventilatory constraints and excess ventilation during exercise in patients with chronic obstructive pulmonary disease. Eur. J. Prev. Cardiol. 2018, 25, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Subjects N = 28 | Subjects with EVef N = 20 | Subjects with EVin N = 8 | p-Value |
|---|---|---|---|---|
| Age, y | 55.3 [52.3; 61.9] | 55.1 [53.6; 59.2] | 58.4 [48.7; 63.7] | 0.576 |
| Male, n (%) | 22 (79) | 15 (75) | 7 (87) | 0.640 |
| BMI, kg/m2 | 25.9 ± 3.4 | 25.8 ± 3.2 | 26.2 ± 4.1 | 0.765 |
| FFMI, kg/m2 | 19 ± 2.2 | 19 ± 2.4 | 18.9 ± 1.9 | 0.907 |
| Smoking habit, no/current or former, n (%) | 19 (68)/9 (32) | 13 (65)/7 (35) | 6 (75)/2 (25) | >0.999 |
| Arterial hypertension, yes, n (%) | 9 (32) | 6 (30) | 3 (37) | >0.999 |
| FEV1, % pred. | 118.1 ± 13.6 | 118.9 ± 14 | 116.1 ± 13.1 | 0.629 |
| FEV1/FVC, % | 101 ± 6.1 | 101.3 ± 6.5 | 100.2 ± 5.6 | 0.679 |
| TLC, % predicted | 104.2 ± 12 | 105.6 ± 13 | 100.6 ± 8.8 | 0.333 |
| IC/TLC at rest | 0.50 ± 0.08 | 0.49 ± 0.09 | 0.51 ± 0.06 | 0.649 |
| DLCO, % predicted | 89.9 ± 13.5 | 90.2 ± 13.9 | 89.4 ± 13.3 | 0.888 |
| PaO2/FiO2 | 484.6 ± 37.6 | 477.7 ± 40.0 | 500 ± 27.7 | 0.169 |
| PaCO2, mmHg | 38.2 ± 3 | 38.4 ± 2.6 | 37.8 ± 3.9 | 0.699 |
| 6MWD, meters | 604.5 ± 67.1 | 598.2 ± 56.1 | 620.4 ± 91.9 | 0.440 |
| 6MWD, % predicted | 103 ± 15.2 | 101.8 ± 15.4 | 106.2 ± 15.1 | 0.502 |
| IPAQ (inactive, minimally active, HEPA active), n (%) | 4(14)/15(54)/9(32) | 4(20)/12(60)/4(20) | 0(0)/3(37)/5(63) | 0.101 |
| METs, vigorous | 0 [0; 1320] | 0 [0; 420] | 1520 [120; 6120] | 0.018 |
| METs, total | 1912.5 [1015.5; 3410.2] | 1372 [838.5; 2497] | 2805 [1698.7; 10,865.5] | 0.053 |
| Workload, watts | 187.7 ± 64 | 181.7 ± 56 | 202.7 ± 83.4 | 0.444 |
| RER | 1.19 [1.11; 1.25] | 1.20 [1.13; 1.27] | 1.12 [1.10; 1.20] | 0.062 |
| VO2 at peak, mL/kg/min | 29.2 ± 8.3 | 27.6 ± 5.2 | 32.9 ± 13.1 | 0.137 |
| VO2 at AT, mL/kg/min | 17.6 [15.9; 22.4] | 17.6 [16.2; 20.4] | 20 [13.5; 29.7] | 0.684 |
| O2 pulse at rest, mL/beat/min | 7.3 [5.8; 7.8] | 7.5 [6.9; 7.9] | 6.2 [5.4; 7.5] | 0.169 |
| O2 pulse at peak, mL/beat/min | 14.5 ± 3.9 | 13.8 ± 3.8 | 16.1 ± 4 | 0.168 |
| PETCO2 change 1 | 3.1 ± 4.4 | 3.7 ± 4.7 | 1.5 ± 3.6 | 0.235 |
| VE at rest | 16.9 ± 4.1 | 16.6 ± 4.4 | 17.8 ± 3.2 | 0.470 |
| VE at peak | 95.2 ± 33.4 | 89.2 ± 27.3 | 110.4 ± 43.9 | 0.131 |
| RR at rest, bpm | 15.9 ± 3.5 | 15.7 ± 3.8 | 16.5 ± 2.8 | 0.637 |
| RR at peak, bpm | 36.4 ± 8.9 | 34.4 ± 7.4 | 41.4 ± 10.8 | 0.057 |
| VO2/Watts, mL/min/watts | 11.8 [11.5; 12.6] | 11.8 [11.4; 12.3] | 12.2 [11.6; 13.9] | 0.263 |
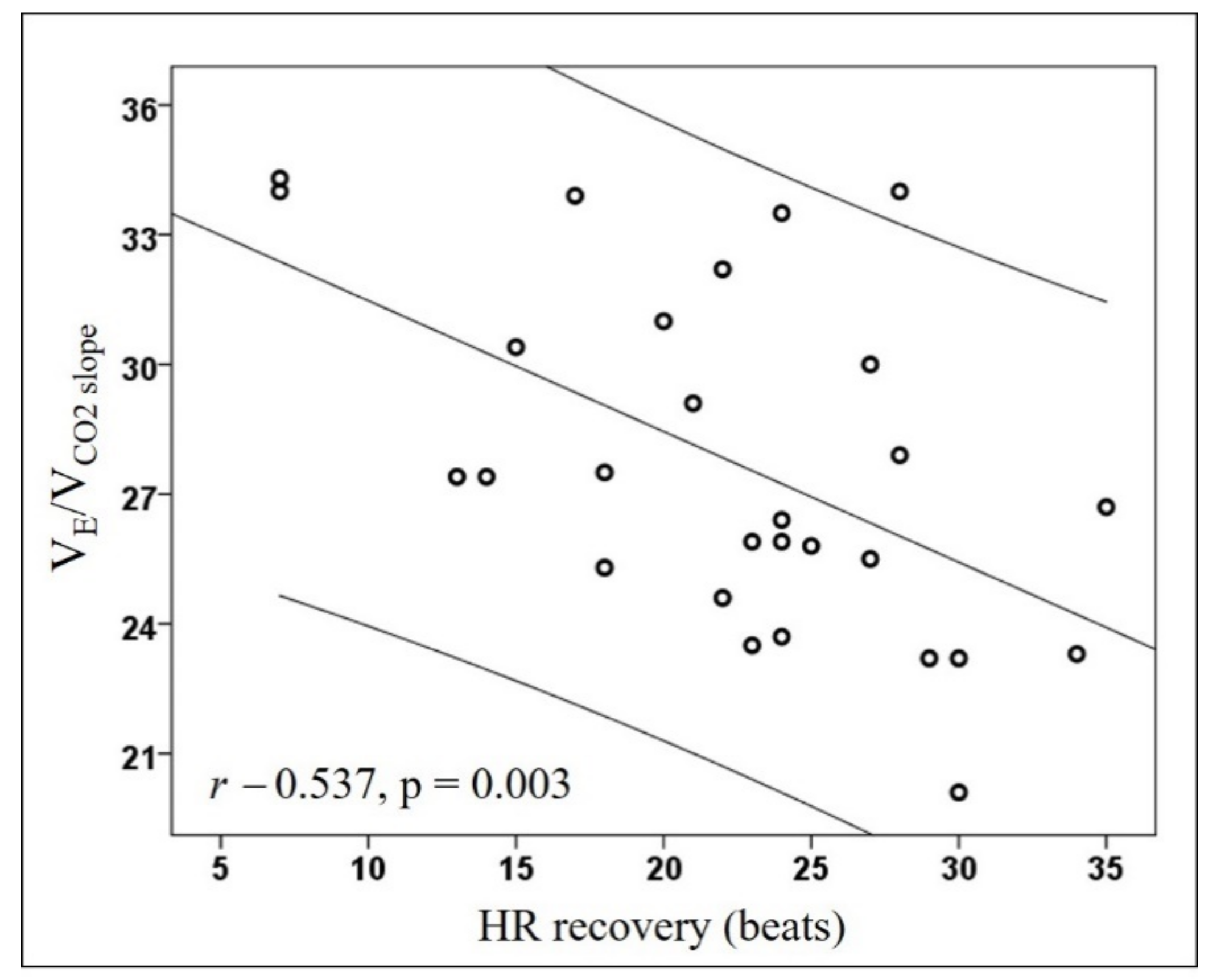
| VE/VCO2 slope | 27.7 ± 3.9 | 25.6 ± 2.3 | 32.9 ± 1.5 | <0.001 |
| VE/VCO2 at AT | 28.9 ± 2.9 | 28.2 ± 2.7 | 30.5 ± 3 | 0.066 |
| VE/VCO2 intercept | 2.35 [0.12; 5.37] | 3.65 [1.75; 5.87] | −1.10 [−3.52; 0.57] | <0.001 |
| HR/VO2 slope, L−1 | 44.5 [38.2; 70] | 47.2 [39.8; 74.9] | 37.2 [34.4; 59.1] | 0.060 |
| Brething reserve, % | 36.5 ± 14.7 | 39.8 ± 13 | 28.1 ± 16.3 | 0.054 |
| VD/VT | 0.26 ± 0.02 | 0.26 ± 0.02 | 0.27 ± 0.02 | 0.151 |
| SBP at rest, mmHg | 120 [115; 125] | 120 [116.2; 125] | 120 [111.2; 125] | 0.853 |
| SBP at peak, mmHg | 183.7 ± 18.4 | 185.7 ± 19.1 | 178.7 ± 16.4 | 0.373 |
| DBP at rest, mmHg | 80 [70; 80] | 80 [70; 83.7] | 80 [70; 80] | 0.625 |
| DBP at peak, mmHg | 95.4 ± 10.3 | 94.5 ± 9.9 | 97.5 ± 11.3 | 0.495 |
| HR at rest, beats/min | 69.7 ± 8.9 | 70.1 ± 10.1 | 68.9 ± 5.2 | 0.749 |
| HR at peak, beats/min | 156.6 ± 18.7 | 158.4 ± 17.6 | 152.1 ± 21.7 | 0.429 |
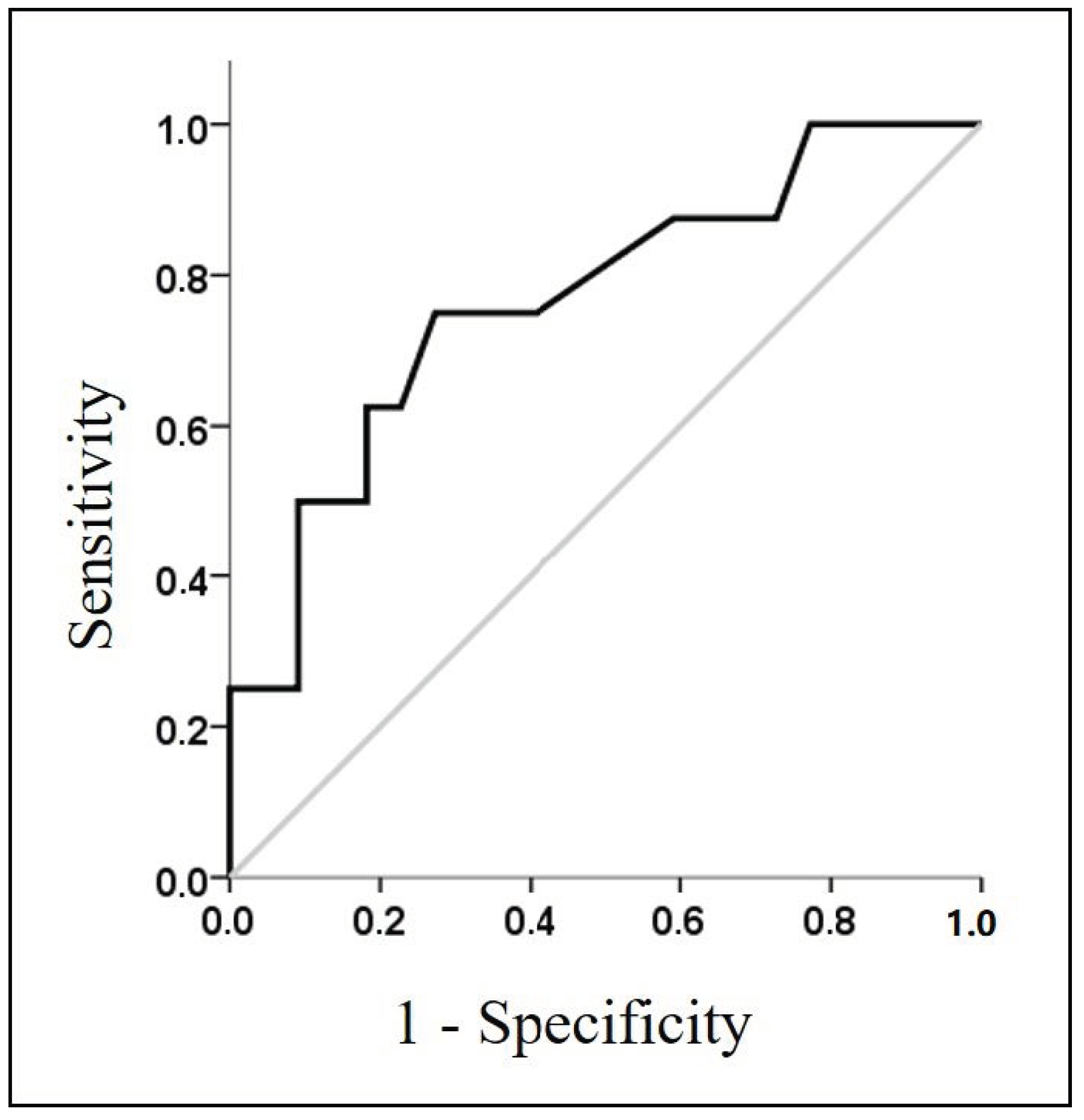
| HR recovery, beats/min | 22.4 ± 7 | 24.4 ± 5.8 | 17.5 ± 7.6 | 0.015 |
| DP reserve | 21060 [16,515; 22,013] | 21030 [17,647; 22,445] | 21,060 [12,630; 21,952] | 0.647 |
| RPEdyspnea, score | 16.2 ± 2.6 | 16 ± 2.4 | 16.8 ± 3 | 0.430 |
| RPEfatigue, score | 17.5 [16.2; 19] | 17.5 [16.2; 19] | 18 [15.5; 19.7] | 0.796 |
| Variables related to COVID-19 hospitalisation | ||||
| PaO2/FiO2 2 ≤ 300, n (%) | 13 (46) | 9 (45) | 4 (50) | >0.999 |
| ICU/medical ward 3, n (%) | 5 (18)/23 (82) | 2 (10)/18 (90) | 3 (37)/5 (63) | 0.123 |
| Length of stay, d | 5.9 [4.2; 10.5] | 5.9 [4.2; 9.7] | 5.5 [3.6; 21.9] | 0.779 |
| Pulmonary embolism, n (%) | 2 (7.1) | 1 (5) | 1 (12.5) | 0.497 |
| Oxygen-therapy, n (%) | 16 (57) | 10 (50) | 6 (75) | 0.401 |
| Ventilatory support 4, n (%) | 10 (36) | 6 (30) | 4 (50) | 0.400 |
| Lopinavir/ritonavir, n (%) | 22 (79) | 16 (80) | 6 (75) | >0.999 |
| Hydroxychloroquine, n (%) | 26 (93) | 18 (90) | 8 (100) | >0.999 |
| Antibiotics, n (%) | 9 (32) | 7 (35) | 2 (25) | >0.999 |
| Tocilizumab, n (%) | 8 (29) | 5 (25) | 3 (37) | 0.651 |
| Steroids, n (%) | 13 (46) | 8 (40) | 5 (62) | 0.410 |
| Prophylactic LMWH, n (%) | 8 (29) | 6 (30) | 2 (25) | >0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorelli, G.; Braggio, M.; Gabbiani, D.; Busti, F.; Caminati, M.; Senna, G.; Girelli, D.; Laveneziana, P.; Ferrari, M.; Sartori, G.; et al. Importance of Cardiopulmonary Exercise Testing amongst Subjects Recovering from COVID-19. Diagnostics 2021, 11, 507. https://doi.org/10.3390/diagnostics11030507
Dorelli G, Braggio M, Gabbiani D, Busti F, Caminati M, Senna G, Girelli D, Laveneziana P, Ferrari M, Sartori G, et al. Importance of Cardiopulmonary Exercise Testing amongst Subjects Recovering from COVID-19. Diagnostics. 2021; 11(3):507. https://doi.org/10.3390/diagnostics11030507
Chicago/Turabian StyleDorelli, Gianluigi, Michele Braggio, Daniele Gabbiani, Fabiana Busti, Marco Caminati, Gianenrico Senna, Domenico Girelli, Pierantonio Laveneziana, Marcello Ferrari, Giulia Sartori, and et al. 2021. "Importance of Cardiopulmonary Exercise Testing amongst Subjects Recovering from COVID-19" Diagnostics 11, no. 3: 507. https://doi.org/10.3390/diagnostics11030507









