Optical Biomarkers for the Diagnosis of Osteoarthritis through Raman Spectroscopy: Radiological and Biochemical Validation Using Ex Vivo Human Cartilage Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort and Sample Collection
2.2. Raman Spectra Obtainment and Data Processing
2.3. sGAGs and Total Collagen Biochemical Content Analysis
2.4. Statistical Analysis
3. Results
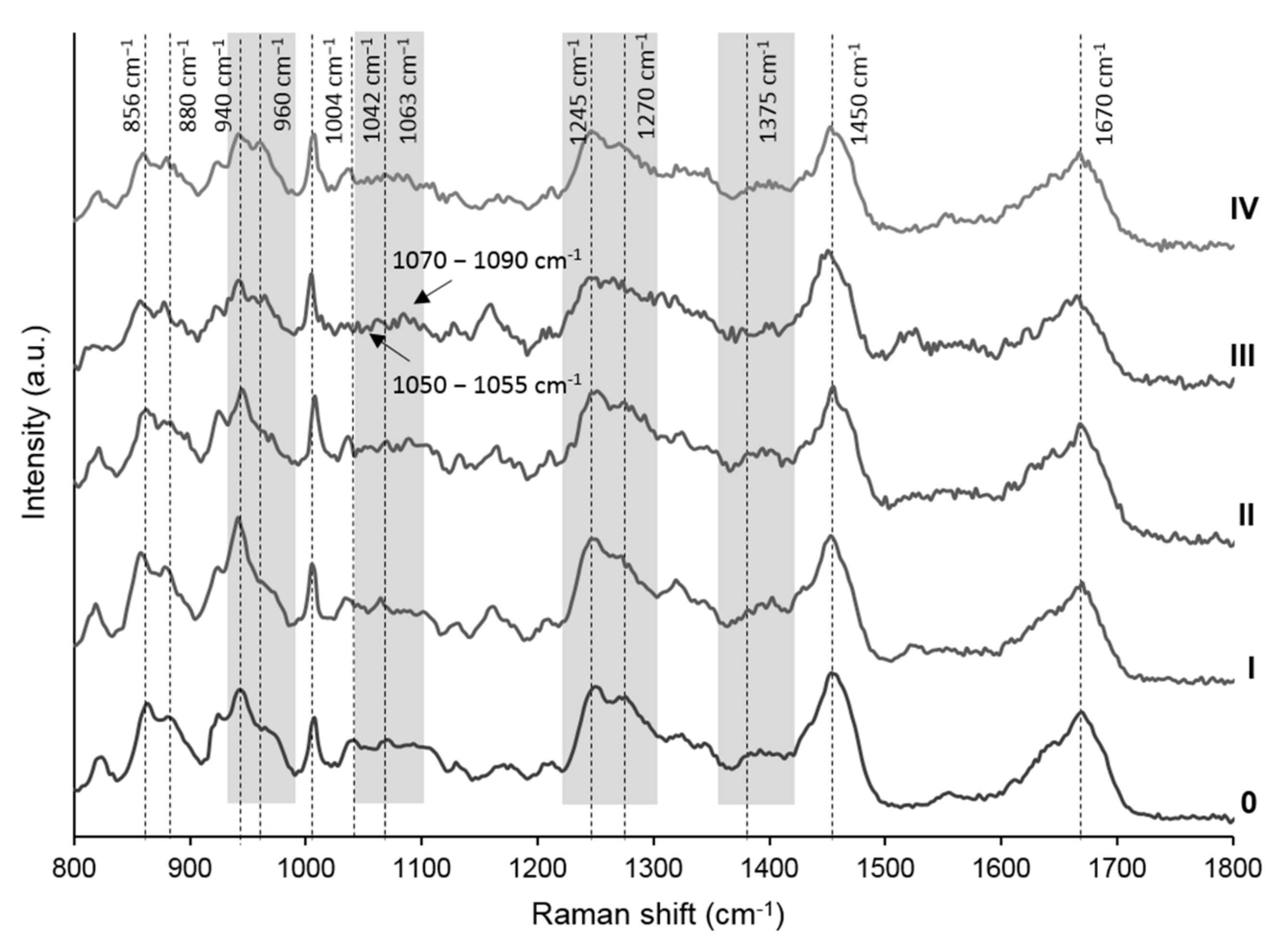
3.1. Molecular Alterations during Radiological OA Progression—Raman Spectra Analysis
3.2. Molecular Alterations during OA Progression—K-L Validation
3.3. Molecular Alterations during OA Progression—Biochemical Cross-Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone-Cartilage Interface Crosstalk in Osteoarthritis: Potential Pathways and Future Therapeutic Strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bay-Jensen, A.C.; Hoegh-Madsen, S.; Dam, E.; Henriksen, K.; Sondergaard, B.C.; Pastoureau, P.; Qvist, P.; Karsdal, M.A. Which Elements Are Involved in Reversible and Irreversible Cartilage Degradation in Osteoarthritis? Rheumatol. Int. 2010, 30, 435–442. [Google Scholar] [CrossRef]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for Standardized Definitions of Osteoarthritis and Risk Stratification for Clinical Trials and Clinical Use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, D.J.; Guermazi, A.; Lo, G.H.; Grainger, A.J.; Conaghan, P.G.; Boudreau, R.M.; Roemer, F.W. Evolution of Semi-Quantitative Whole Joint Assessment of Knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 2011, 19, 990–1002. [Google Scholar] [CrossRef] [Green Version]
- Kellgren, J.; Lawrence, J. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Kraan, P.M.; Berenbaum, F.; Blanco, F.J.; Cosimo, D.B.; Lafeber, F.; Hauge, E.; Higginbottom, A.; Ioan-Facsinay, A.; Loughlin, J.; Meulenbelt, I.; et al. Translation of Clinical Problems in Osteoarthritis into Pathophysiological Research Goals. RMD Open 2016, 2, e000224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman Spectroscopy to Characterize Biological Materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Samuel, A.; Sil, S.; Chaturvedi, D.; Dutta, A.; Ariese, F.; Umapathy, S. Raman and Mid-Infrared Spectroscopic Imaging: Applications and Advancements. Curr. Sci. 2015, 108, 341–356. [Google Scholar]
- Pavlou, E.; Zhang, X.; Wang, J.; Kourkoumelis, N. Raman Spectroscopy for the Assessment of Osteoarthritis. Ann. Jt. 2018, 3, 83. [Google Scholar] [CrossRef]
- Hosu, C.D.; Moisoiu, V.; Stefancu, A.; Antonescu, E.; Leopold, L.F.; Leopold, N.; Fodor, D. Raman Spectroscopy Applications in Rheumatology. Lasers Med. Sci. 2019, 34, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Albro, M.B.; Bergholt, M.S.; St-Pierre, J.P.; Vinals Guitart, A.; Zlotnick, H.M.; Evita, E.G.; Stevens, M.M. Raman Spectroscopic Imaging for Quantification of Depth-Dependent and Local Heterogeneities in Native and Engineered Cartilage. NPJ Regen. Med. 2018, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bergholt, M.S.; Serio, A.; Albro, M.B. Raman Spectroscopy: Guiding Light for the Extracellular Matrix. Front. Bioeng. Biotechnol. 2019, 7, 303. [Google Scholar] [CrossRef]
- Casal-Beiroa, P.; González, P.; Blanco, F.J.; Magalhães, J. Molecular Analysis of the Destruction of Articular Joint Tissues by Raman Spectroscopy. Expert Rev. Mol. Diagn. 2020, 20, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Casal-Beiroa, P.; Blanco, F.J.; Magalhães, J. Raman spectroscopy for cartilage damage severity, degradation and repair assessment. In Avances de la Bioingeniería Para el Envejecimiento Saludable; González, P., Ed.; Universidade de Vigo: Vigo, Spain, 2020; pp. 43–54. ISBN 978-84-8158-832-3. [Google Scholar]
- Esmonde-White, K.A.; Esmonde-White, F.W.L.; Morris, M.D.; Roessler, B.J. Fiber-Optic Raman Spectroscopy of Joint Tissues. Analyst 2011, 136, 1675–1685. [Google Scholar] [CrossRef] [Green Version]
- Richardson, W.; Wilkinson, D.; Wu, L.; Petrigliano, F.; Dunn, B.; Evseenko, D. Ensemble Multivariate Analysis to Improve Identification of Articular Cartilage Disease in Noisy Raman Spectra. J. Biophotonics 2015, 8, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sugano, N.; Takao, M.; Sakai, T.; Nishii, T.; Pezzotti, G. Raman Spectroscopy Investigation of Load-Assisted Microstructural Alterations in Human Knee Cartilage: Preliminary Study into Diagnostic Potential for Osteoarthritis. J. Mech. Behav. Biomed. Mater. 2014, 31, 77–85. [Google Scholar] [CrossRef]
- Kumar, R.; Grønhaug, K.M.; Afseth, N.K.; Isaksen, V.; de Lange Davies, C.; Drogset, J.O.; Lilledahl, M.B. Optical Investigation of Osteoarthritic Human Cartilage (ICRS Grade) by Confocal Raman Spectroscopy: A Pilot Study. Anal. Bioanal. Chem. 2015, 407, 8067–8077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunstar, A.; Leijten, J.; van Leuveren, S.; Hilderink, J.; Otto, C.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.A. Recognizing Different Tissues in Human Fetal Femur Cartilage by Label-Free Raman Microspectroscopy. J. Biomed. Opt. 2012, 17, 116012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, R.A.; Xavier, M.; Mangueira, N.M.; Santos, A.P.; Pinheiro, A.L.B.; Villaverde, A.B.; Silveira, L. Raman Spectroscopy Detection of Molecular Changes Associated with Two Experimental Models of Osteoarthritis in Rats. Lasers Med. Sci. 2014, 29, 797–804. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Klaushofer, K.; Paschalis, E.P. Raman Analysis of Proteoglycans Simultaneously in Bone and Cartilage. J. Raman Spectrosc. 2014, 45, 794–800. [Google Scholar] [CrossRef]
- Eberhardt, K.; Stiebing, C.; Matthäus, C.; Schmitt, M.; Popp, J. Advantages and Limitations of Raman Spectroscopy for Molecular Diagnostics: An Update. Expert Rev. Mol. Diagn. 2015, 15, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.S.J.; Hamed, Z.; Yeow, C.H.; Chan, C.; Huang, Z. Early Detection of Biomolecular Changes in Disrupted Porcine Cartilage Using Polarized Raman Spectroscopy. J. Biomed. Opt. 2011, 16, 017003. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Buchwald, T.; Niciejewski, K.; Kozielski, M.; Szybowicz, M.; Siatkowski, M.; Krauss, H. Identifying Compositional and Structural Changes in Spongy and Subchondral Bone from the Hip Joints of Patients with Osteoarthritis Using Raman Spectroscopy. J. Biomed. Opt. 2012, 17, 017007. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.F.; Awais, M.; Khan, A.S.; Tabassum, S.; Chaudhry, A.A.; Rehman, I.U. Raman Spectroscopy of Natural Bone and Synthetic Apatites. Appl. Spectrosc. Rev. 2013, 48, 329–355. [Google Scholar] [CrossRef]
- Levillain, A.; Boulocher, C.; Kaderli, S.; Viguier, E.; Hannouche, D.; Hoc, T.; Magoariec, H. Meniscal Biomechanical Alterations in an ACLT Rabbit Model of Early Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1186–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Singh, G.P.; Grønhaug, K.M.; Afseth, N.K.; de Lange Davies, C.; Drogset, J.O.; Lilledahl, M.B. Single Cell Confocal Raman Spectroscopy of Human Osteoarthritic Chondrocytes: A Preliminary Study. Int. J. Mol. Sci. 2015, 16, 9341–9353. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and Infrared Spectra of Carbonates of Calcite Structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Wang, D.; Hamm, L.M.; Bodnar, R.J.; Dove, P.M. Raman Spectroscopic Characterization of the Magnesium Content in Amorphous Calcium Carbonates. J. Raman Spectrosc. 2012, 43, 543–548. [Google Scholar] [CrossRef]
- Awonusi, A.; Morris, M.D.; Tecklenburg, M.M.J. Carbonate Assignment and Calibration in the Raman Spectrum of Apatite. Calcif. Tissue Int. 2007, 81, 46–52. [Google Scholar] [CrossRef]
- Fernández-Puente, P.; Mateos, J.; Fernández-Costa, C.; Oreiro, N.; Fernández-López, C.; Ruiz-Romero, C.; Blanco, F.J. Identification of a Panel of Novel Serum Osteoarthritis Biomarkers. J. Proteome Res. 2011, 10, 5095–5101. [Google Scholar] [CrossRef] [PubMed]
- Lourido, L.; Ayoglu, B.; Fernández-Tajes, J.; Oreiro, N.; Henjes, F.; Hellström, C.; Schwenk, J.M.; Ruiz-Romero, C.; Nilsson, P.; Blanco, F.J. Discovery of Circulating Proteins Associated to Knee Radiographic Osteoarthritis. Sci. Rep. 2017, 7, 137. [Google Scholar] [CrossRef] [Green Version]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.P.; Roughley, P.J. Cartilage in Normal and Osteoarthritis Conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef]
- Bayliss, M.T.; Osborne, D.; Woodhouse, S.; Davidson, C. Sulfation of Chondroitin Sulfate in Human Articular Cartilage: The Effect of Age, Topographical Position, and Zone of Cartilage on Tissue Composition. J. Biol. Chem. 1999, 274, 15892–15900. [Google Scholar] [CrossRef] [Green Version]
- Söder, S.; Hambach, L.; Lissner, R.; Kirchner, T.; Aigner, T. Ultrastructural Localization of Type VI Collagen in Normal Adult and Osteoarthritic Human Articular Cartilage. Osteoarthr. Cartil. 2002, 10, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Gelse, K. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieppo, L.; Töyräs, J.; Saarakkala, S. Vibrational Spectroscopy of Articular Cartilage. Appl. Spectrosc. Rev. 2017, 52, 249–266. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular Cartilage and Osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar]
- Lorenzo, P.; Bayliss, M.T.; Heinegård, D. Altered Patterns and Synthesis of Extracellular Matrix Macromolecules in Early Osteoarthritis. Matrix Biol. 2004, 23, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Nam, J. The Role of Changes in Extracellular Matrix of Cartilage in the Presence of Inflammation on the Pathology of Osteoarthritis. Biomed. Res. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearle, A.D.; Warren, R.F.; Rodeo, S.A. Basic Science of Articular Cartilage and Osteoarthritis. Clin. Sports Med. 2005, 24, 1–12. [Google Scholar] [CrossRef]
- Weis, M.A.; Hudson, D.M.; Kim, L.; Scott, M.; Wu, J.-J.; Eyre, D.R. Location of 3-Hydroxyproline Residues in Collagen Types I, II, III, and V/XI Implies a Role in Fibril Supramolecular Assembly. J. Biol. Chem. 2010, 285, 2580–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.; Bazin, D.; Daudon, M.; Chatron-Colliet, A.; Hannouche, D.; Bianchi, A.; Côme, D.; So, A.; Busso, N.; Lioté, F.; et al. Revisiting Spatial Distribution and Biochemical Composition of Calcium-Containing Crystals in Human Osteoarthritic Articular Cartilage. Arthritis Res. Ther. 2013, 15, R103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Qin, W.; Xiao, B.; Wan, Q.; Tay, F.R.; Niu, L.; Jiao, K. Pathological Calcification in Osteoarthritis: An Outcome or a Disease Initiator? Biol. Rev. 2020, 9. [Google Scholar] [CrossRef]
- McCarthy, G.M.; Dunne, A. Calcium Crystal Deposition Diseases—Beyond Gout. Nat. Rev. Rheumatol. 2018, 14, 592–602. [Google Scholar] [CrossRef]
- Durcan, L.; Bolster, F.; Kavanagh, E.C.; McCarthy, G.M. The Structural Consequences of Calcium Crystal Deposition. Rheum. Dis. Clin. N. Am. 2014, 40, 311–328. [Google Scholar] [CrossRef]
- Muehleman, C.; Li, J.; Aigner, T.; Rappoport, L.; Mattson, E.; Hirschmugl, C.; Masuda, K.; Rosenthal, A.K. Association between Crystals and Cartilage Degeneration in the Ankle. J. Rheumatol. 2008, 35, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Pay, S.; Terkeltaub, R. Calcium Pyrophosphate Dihydrate and Hydroxyapatite Crystal Deposition in the Joint: New Developments Relevant to the Clinician. Curr. Rheumatol. Rep. 2003, 5, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, H.; Healey, R.M.; Terkeltaub, R.A.; Coutts, R.D.; Amiel, D. Calcification of Human Articular Knee Cartilage Is Primarily an Effect of Aging Rather than Osteoarthritis. Osteoarthr. Cartil. 2007, 15, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Halverson, P.B.; McCarty, D.J. Patterns of Radiographic Abnormalities Associated with Basic Calcium Phosphate and Calcium Pyrophosphate Dihydrate Crystal Deposition in the Knee. Ann. Rheum. Dis. 1986, 45, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Fuerst, M.; Bertrand, J.; Lammers, L.; Dreier, R.; Echtermeyer, F.; Nitschke, Y.; Rutsch, F.; Schäfer, F.K.W.; Niggemeyer, O.; Steinhagen, J.; et al. Calcification of Articular Cartilage in Human Osteoarthritis. Arthritis Rheum. 2009, 60, 2694–2703. [Google Scholar] [CrossRef] [PubMed]
- Valdés, R.; Stefanov, S.; Chiussi, S.; López-Alvarez, M.; González, P. Pilot Research on the Evaluation and Detection of Head and Neck Squamous Cell Carcinoma by Raman Spectroscopy. J. Raman Spectrosc. 2014, 45, 550–557. [Google Scholar] [CrossRef]
- Ding, H.; Dupont, A.W.; Singhal, S.; Scott, L.D.; Guha, S.; Younes, M.; Ye, Y.; Bi, X. Effect of Physiological Factors on the Biochemical Properties of Colon Tissue—An in Vivo Raman Spectroscopy Study. J. Raman Spectrosc. 2017, 48, 902–909. [Google Scholar] [CrossRef]
- Rocha, B.; Ruiz-Romero, C.; Blanco, F.J. Mass Spectrometry Imaging: A Novel Technology in Rheumatology. Nat. Rev. Rheumatol. 2017, 13, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Cillero-Pastor, B.; Eijkel, G.; Kiss, A.; Blanco, F.J.; Heeren, R.M.A. Time-of-Flight Secondary Ion Mass Spectrometry-Based Molecular Distribution Distinguishing Healthy and Osteoarthritic Human Cartilage. Anal. Chem. 2012, 84, 8909–8916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarin, J.K.; Te Moller, N.C.R.; Mancini, I.A.D.; Brommer, H.; Visser, J.; Malda, J.; van Weeren, P.R.; Afara, I.O.; Töyräs, J. Arthroscopic near Infrared Spectroscopy Enables Simultaneous Quantitative Evaluation of Articular Cartilage and Subchondral Bone in Vivo. Sci. Rep. 2018, 8, 13409. [Google Scholar] [CrossRef] [PubMed]
- Cordero, E.; Latka, I.; Matthäus, C.; Schie, I.W.; Popp, J. In-Vivo Raman Spectroscopy: From Basics to Applications. J. Biomed. Opt. 2018, 23, 071210. [Google Scholar] [CrossRef]
- Aigner, T.; McKenna, L. Molecular Pathology and Pathobiology of Osteoarthritic Cartilage. Cell. Mol. Life Sci. 2002, 59, 5–18. [Google Scholar] [CrossRef]
- Hollander, A.P.; Heathfield, T.F.; Webber, C.; Iwata, Y.; Bourne, R.; Rorabeck, C.; Poole, A.R. Increased Damage to Type II Collagen in Osteoarthritic Articular Cartilage Detected by a New Immunoassay. J. Clin. Investig. 1994, 93, 1722–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and Its Modifications-Crucial Aspects with Concern to Its Processing and Analysis. Macromol. Mater. Eng. 2017, 302, 1600460. [Google Scholar] [CrossRef]




| Demographics | |||
| Sex | Female (F) | n = 27 | 57.4% |
| Male (M) | n = 20 | 42.6% | |
| Age | Range | 42–94 years old | |
| Mean ± SD | 72 ± 12 years old | ||
| Diagnostic | |||
| Radiological grade (K-L) | Healthy (K-L 0) | n = 6 | |
| OA (K-L I–IV) | n = 41 | ||
| Optical Biomarker | Molecular Component or Associated Event | Acronym | Definition |
|---|---|---|---|
| A960 cm−1/A1004 cm−1 | Mineralization—Phosphate Groups | MGP | Phosphate groups present at hydroxyapatite |
| A960 cm−1/A920 cm−1 | Phosphate Hydroxyapatite/Collagen | HA/Col | Bone to collagen |
| A1039–42 cm−1/A1004 cm−1 | Total GAGs | TGAGs | Total glycosaminoglycans |
| A1050 cm−1/A1004 cm−1 | CPPD | CPPD | Calcium pyrophosphate dihydrate deposits |
| A1063 cm−1/A1004 cm−1 | Sulphated GAGs | SGAGs | Sulphated glycosaminoglycans (OSO3− groups) |
| A1063 cm−1/A960 cm−1 | Sulphated GAGs/Phosphate | SGAGs/HA | Cartilage to bone |
| A1070 cm−1/A1004 cm−1 | Mineralization—Carbonate Groups | MGC | Mineralization grade-carbonated hydroxyapatite |
| A1245 cm−1/A1270 cm−1 | Defective/Functional Collagen | ColD/F | Collagen randomness—as the relative amount of collagen random coil (defective Col) to an α-helix structure (functional Col) |
| A1375 cm−1/A1004 cm−1 | Proteoglycans | PGs | Proteoglycans |
| A1450 cm−1/A1668 cm−1 | Indirect Lipid Index | IL | Relative amount of unspecific lipids and proteins to the total protein content |
| Raman Shift (cm−1) | Assigned Bond/ Molecule | Component | References |
|---|---|---|---|
| 850–880 856–858 875–880 | C–C stretching Pro Hyp | Collagen | [16,23] |
| 920–928 | C–C stretching Pro | Collagen | [15,16] |
| 932–941 932–938 937–941 | Symmetric stretching: C–C protein backbone C–O–C α 1–4 glycosidic bond | Collagen GAGs | [16,19,23,24] |
| 954–962 | PO43−, symmetric stretching | Phosphate hydroxyapatite (HA) | [15,19,23,25,26] |
| 1004 | Aromatic ring stretching phenylalanine (Phe) | Proteins | [15,16,19] |
| 1039–1042 | C–O–C stretching pyranose ring | GAGs | [23] |
| 1047–1055 | P–O–P symmetric stretching | CPPD | [27] |
| 1060–1064 | O–SO3− symmetric stretching | Sulphated GAGs, PGs | [15,19,21,23,28] |
| 1070–1090 1070–1073 1080–1082 1090 | CO32−, asymmetric stretching Type-B carbonate Amorphous carbonate CaCO3 | Carbonate Carbonated hydroxyapatite Amorphous carbonate Calcium carbonate deposits | [15,21,24,25,29] |
| 1230–1280 1245 1270 | C–N stretching amide III: random coil α-helix structure | Collagen: Defective Functional | [16,17,18,21,23] |
| 1375–1380 | CH3 symmetric stretching | GAGs, PGs | [21] |
| 1441–1460 | CH2 deformation/scissoring | Protein and lipids | [15,16] |
| 1630–1690 1645–1655 1660–1670 1665–1675 | C=O stretching amide I: α-helix structure random coil β-sheet structure | Collagen and other proteins | [15,16,19] |
| 1550–1600 | N–H and C–N deformation amide II | Proteins | [24] |
| SGAGs | TGAGs | PGs | ColD/F | IL | CPPD | MGP | MGC | SGAGs/HA | HA/Col | ||
| rho | −0.632 | −0.324 | −0.532 | 0.529 | 0.427 | 0.191 | 0.321 | 0.293 | −0.426 | 0.446 | |
| p | <0.001 | 0.025 | <0.001 | <0.001 | 0.002 | 0.219 | 0.260 | 0.043 | 0.005 | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casal-Beiroa, P.; Balboa-Barreiro, V.; Oreiro, N.; Pértega-Díaz, S.; Blanco, F.J.; Magalhães, J. Optical Biomarkers for the Diagnosis of Osteoarthritis through Raman Spectroscopy: Radiological and Biochemical Validation Using Ex Vivo Human Cartilage Samples. Diagnostics 2021, 11, 546. https://doi.org/10.3390/diagnostics11030546
Casal-Beiroa P, Balboa-Barreiro V, Oreiro N, Pértega-Díaz S, Blanco FJ, Magalhães J. Optical Biomarkers for the Diagnosis of Osteoarthritis through Raman Spectroscopy: Radiological and Biochemical Validation Using Ex Vivo Human Cartilage Samples. Diagnostics. 2021; 11(3):546. https://doi.org/10.3390/diagnostics11030546
Chicago/Turabian StyleCasal-Beiroa, Paula, Vanesa Balboa-Barreiro, Natividad Oreiro, Sonia Pértega-Díaz, Francisco J. Blanco, and Joana Magalhães. 2021. "Optical Biomarkers for the Diagnosis of Osteoarthritis through Raman Spectroscopy: Radiological and Biochemical Validation Using Ex Vivo Human Cartilage Samples" Diagnostics 11, no. 3: 546. https://doi.org/10.3390/diagnostics11030546
APA StyleCasal-Beiroa, P., Balboa-Barreiro, V., Oreiro, N., Pértega-Díaz, S., Blanco, F. J., & Magalhães, J. (2021). Optical Biomarkers for the Diagnosis of Osteoarthritis through Raman Spectroscopy: Radiological and Biochemical Validation Using Ex Vivo Human Cartilage Samples. Diagnostics, 11(3), 546. https://doi.org/10.3390/diagnostics11030546






