Forgotten No More—The Role of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: An Echocardiographic Perspective
Abstract
:1. Introduction
2. The Echocardiographic Assessment of the Right Ventricle
3. Tricuspid Annular Plane Systolic Excursion (TAPSE)
4. Tricuspid Lateral Annular Systolic Velocity (S’ Wave)
5. Right Ventricular Myocardial Performance Index (RV MPI)
6. Right Ventricular Fractional Area Change (RV FAC)
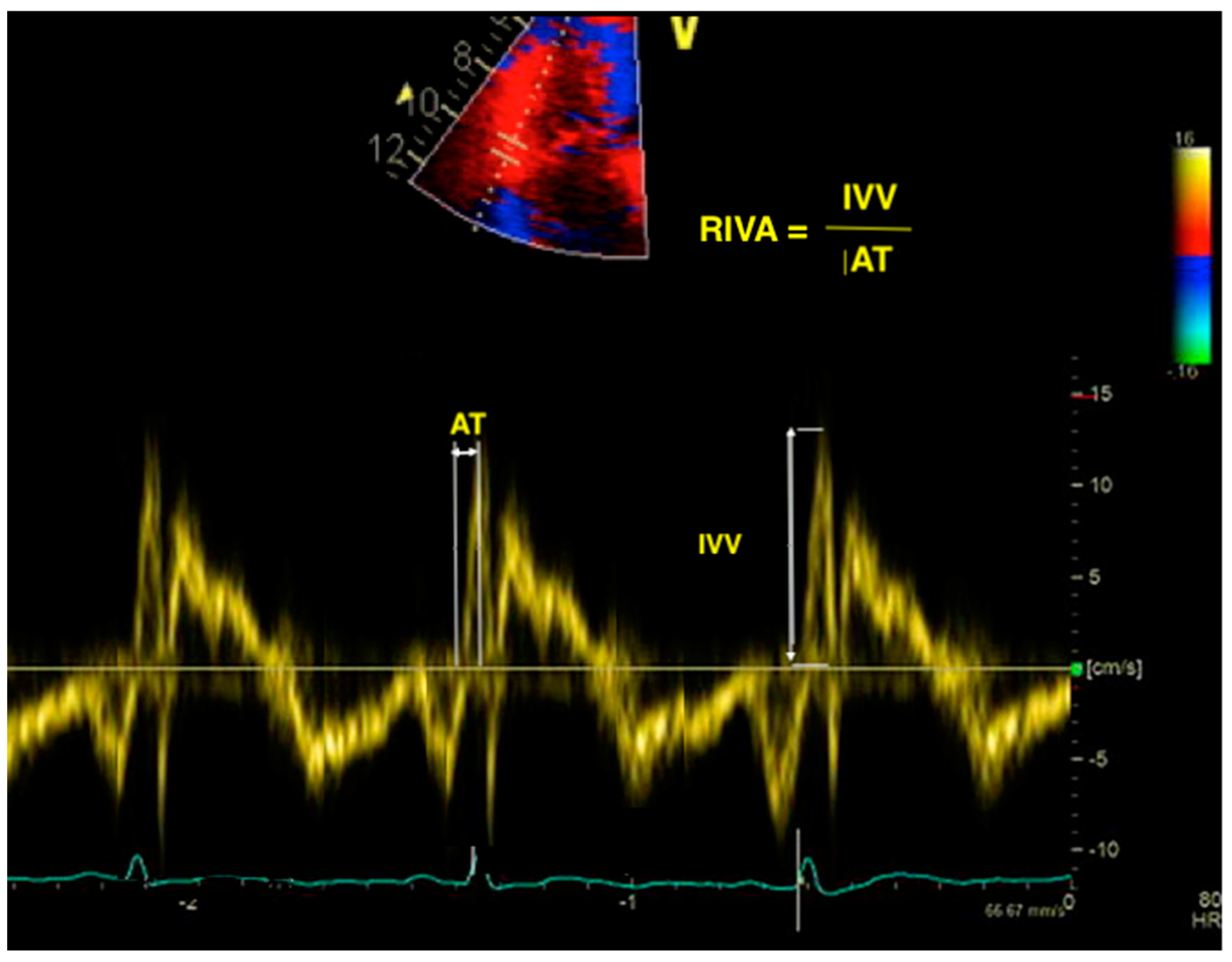
7. Right Ventricular Isovolumic Acceleration
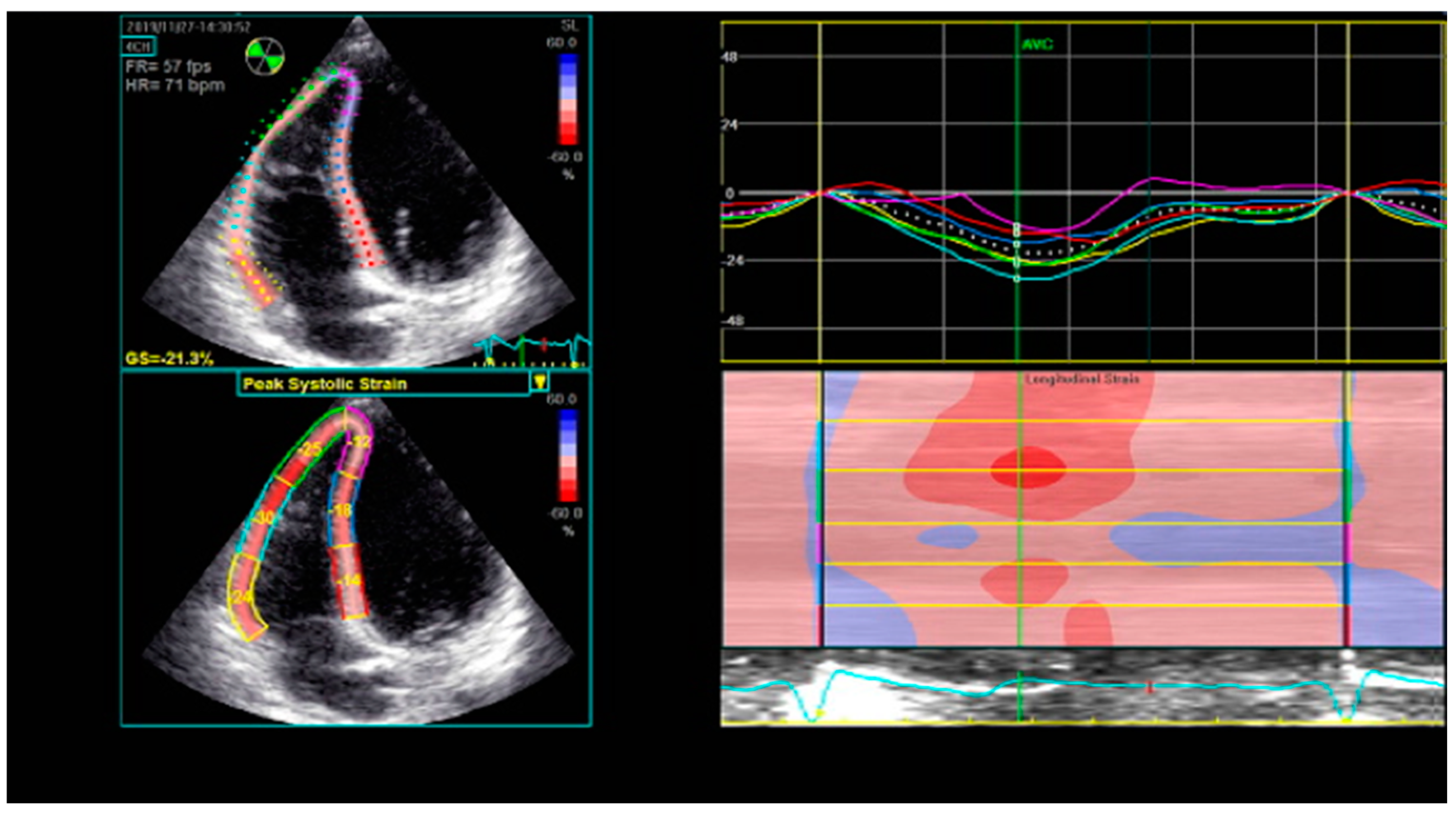
8. Right Ventricular Strain and Strain Rate Derived from Two-Dimensional Speckle-Tracking Echocardiography (2D STE)
9. Three-Dimensional Right Ventricular Ejection Fraction (3D RVEF)
10. Three-Dimensional Speckle-Tracking Echocardiography (3D STE)
11. Other Parameters of Right Ventricular Function
12. Artificial Intelligence Algorithms
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update; A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Buneo, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Meta-Analysis Global Group in Chronic Heart Failure. Predicting Survival in Heart Failure: A Risk Score Based on 39,372 Patients from 30 Studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, F.; Redington, A. The Right Ventricle: Anatomy, Physiology and Clinical Imaging. Heart 2008, 94, 1510–1515. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Sugeng, L.; Lindner, J.R. Imaging the Forgotten Chamber: Is the Devil in the Boundary? J. Am. Soc. Echocardiogr. 2010, 23, 141–143. [Google Scholar] [CrossRef]
- Fine, N.M.; Chen, L.; Bastiansen, P.M.; Frantz, R.P.; Pellikka, P.A.; Oh, J.K.; Kane, G.C. Outcome Prediction by Quantitative Right Ventricular Function Assessment in 575 subjects Evaluated for Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2013, 6, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Ghio, S.; Temporelli, P.L.; Klersy, C.; Simioniuc, A.; Girardi, B.; Scelsi, L.; Rossi, A.; Cicoira, M.; Genta, F.T.; Dini, F.L. Prognostic Relevance of a Non-Invasive Evaluation of Right Ventricular Function and Pulmonary Artery Pressure in Patients with Chronic Heart Failure. Eur. J. Heart Fail. 2013, 15, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens, L.L.; Friedberg, M.K. Imaging the Right Ventricle—Current State of the Art. Nat. Rev. Cardiol. 2010, 7, 551–563. [Google Scholar] [CrossRef]
- McLure, L.E.R.; Peacock, A.J. Cardiac Magnetic Resonance Imaging for the Assessment of the Heart and Pulmonary Circulation in Pulmonary Hypertension. Eur. Respir. J. 2009, 33, 1454–1466. [Google Scholar] [CrossRef]
- Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; Manning, W.J.; et al. ACCF/ACR/AHA/NASCI/SCMR 2010 Expert Consensus Document on Cardiovascular Magnetic Resonance: A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J. Am. Coll. Cardiol. 2010, 55, 2614–2662. [Google Scholar] [CrossRef]
- Petersen, S.E.; Khanji, M.Y.; Plein, S.; Lancellotti, P.; Bucciarelli-Ducci, C. European Association of Cardiovascular Imaging Expert Consensus Paper: A Comprehensive Review of Cardiovascular Magnetic Resonance Normal Values of Cardiac Chamber Size and Aortic Root in Adults and Recommendations for Grading Severity. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1321–1331. [Google Scholar] [CrossRef] [Green Version]
- Gulati, A.; Ismail, T.F.; Jabbour, A.; Alpendurada, F.; Guha, K.; Ismail, N.A.; Raza, S.; Khwaja, J.; Brown, T.D.H.; Morarji, K.; et al. The Prevalence and Prognostic Significance of Right Ventricular Systolic Dysfunction in Nonischemic Dilated Cardiomyopathy. Circulation 2013, 128, 1623–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikami, Y.; Jolly, U.; Heydari, B.; Peng, M.; Almehmadi, F.; Zahrani, M.; Bokhari, M.; Stirrat, J.; Lydell, C.P.; Howarth, A.G.; et al. Right Ventricular Ejection Fraction Is Incremental to Left Ventricular Ejection Fraction for the Prediction of Future Arrhythmic Events in Patients with Systolic Dysfunction. Circ. Arrhythm. Electrophysiol. 2017, 10, e004067. [Google Scholar] [CrossRef] [PubMed]
- Pueschner, A.; Chattranukulchai, P.; Heitner, J.F.; Shah, D.J.; Hayes, B.; Rehwald, W.; Parker, M.A.; Kim, H.W.; Judd, R.M.; Kim, R.J.; et al. The Prevalence, Correlates, and Impact on Cardiac Mortality of Right Ventricular Dysfunction in Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Doesch, C.; Dierks, D.M.; Haghi, D.; Schimpf, R.; Kuschyk, J.; Suselbeck, T.; Schoenberg, S.O.; Borgggrefe, M.; Papavassiliu, T. Right Ventricular Dysfunction, Late Gadolinium Enhancement, and Female Gender Predict Poor Outcome in Patients with Dilated Cardiomyopathy. Int. J. Cardiol. 2014, 177, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Negishi, K.; Kwon, D.H.; Popović, Z.B.; Grimm, R.A.; Marwick, T.H. Validation of Global Longitudinal Strain and Strain Rate as Reliable Markers of Right Ventricular Dysfunction: Comparison With Cardiac Magnetic Resonance and Outcome. J. Cardiovasc. Ultrasound 2014, 22, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Muraru, D.; Spadotto, V.; Cecchetto, A.; Romeo, G.; Aruta, P.; Ermacora, D.; Jenei, C.; Cucchini, U.; Iliceto, S.; Badano, L.P. New Speckle-Tracking Algorithm for Right Ventricular Volume Analysis from Three-Dimensional Echocardiographic Data Sets: Validation with Cardiac Magnetic Resonance and Comparison with the Previous Analysis Tool. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1279–1289. [Google Scholar] [CrossRef]
- Santamore, W.P.; Dell’Italia, L.J. Ventricular Interdependence: Significant Left Ventricular Contributions to Right Ventricular Systolic Function. Prog. Cardiovasc. Dis. 1998, 40, 289–308. [Google Scholar] [CrossRef]
- Buckberg, G.; Hoffman, J.I. Right Ventricular Architecture Responsible for Mechanical Performance: Unifying Role of Ventricular Septum. J. Thorac. Cardiovasc. Surg. 2014, 148, 3166–3171. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, A.; Lakatos, B.; Tokodi, M.; Merkely, B. Right Ventricular Mechanical Pattern in Health and Disease: Beyond Longitudinal Shortening. Heart Fail. Rev. 2019, 24, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, B.K.; Tokodi, M.; Assabiny, A.; Tozer, Z.; Kosztin, A.; Doronina, A.; Racz, K.; Koritsansky, K.B.; Berzsenyi, V.; Nemeth, E.; et al. Dominance of Free Wall Radial Motion in Global Right Ventricular Function of Heart Transplant Recipients. Clin. Transpl. 2018, 32, e13192. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, B.K.; Nabeshima, Y.; Tokodi, M.; Nagata, Y.; Toser, Z.; Otani, K.; Kitano, T.; Fabian, A.; Ujvari, A.; Boros, A.M.; et al. Importance of Nonlongitudinal Motion Components in Right Ventricular Function: Three-Dimensional Echocardiographic Study in Healthy Volunteers. J. Am. Soc. Echocardiogr. 2020, 33, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Sciaccaluga, C.; D’Ascenzi, F.; Mandoli, G.E.; Rizzo, L.; Sisti, N.; Carrucola, C.; Cameli, P.; Bigio, E.; Mondillo, S.; Cameli, M. Traditional and Novel Imaging of Right Ventricular Function in Patients with Heart Failure and Reduced Ejection Fraction. Curr. Heart Fail. Rep. 2020, 17, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, P.L.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Lakatos, B.; Toser, Z.; Tokodi, M.; Doronina, A.; Kosztin, A.; Muraru, D.; Badano, L.P.; Kovacs, A.; Merkely, B. Quantification of the Relative Contribution of the Different Right Ventricular Wall Motion Components to Right Ventricular Ejection Fraction: The ReVISION Method. Cardiovasc. Ultrasound 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopecna, D.; Briongos, S.; Castillo, H.; Moreno, C.; Recio, M.; Navas, P.; Lobo, J.L.; Alonso-Gomez, A.; Obieta-Fresnedo, I.; Fernández-Golfin, C.; et al. Interobserver Reliability of Echocardiography for Prognostication of Normotensive Patients with Pulmonary Embolism. Cardiovasc. Ultrasound 2014, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Smolarek, D.; Gruchala, M.; Sobiczewski, W. Echocardiographic Evaluation of Right Ventricular Systolic Function: The Traditional and Innovative Approach. Cardiol. J. 2017, 24, 563–572. [Google Scholar] [CrossRef]
- Dandel, M.; Hetzer, R. Echocardiographic Assessment of the Right Ventricle: Impact of the Distinctly Load Dependency of its Size, Geometry and Performance. Int. J. Cardiol. 2016, 221, 1132–1142. [Google Scholar] [CrossRef]
- Motoji, Y.; Tanaka, H.; Fukuda, Y.; Sano, H.; Ryo, K.; Sawa, T.; Miyoshi, T.; Imanishi, J.; Mochizuki, Y.; Tatsumi, K.; et al. Association of Apical Longitudinal Rotation with Right Ventricular Performance in Patients with Pulmonary Hypertension: Insights into Overestimation of Tricuspid Annular Plane Systolic Excursion. Echocardiography 2016, 33, 207–215. [Google Scholar] [CrossRef]
- Zhao, H.; Kang, Y.; Pickle, J.; Wang, J.; Han, Y. Tricuspid Annular Plane Systolic Excursion is Dependent on Right Ventricular Volume in Addition to Function. Echocardiography 2019, 36, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Recusani, F.; Klersy, C.; Sebastiani, R.; Laudisa, M.L.; Campana, C.; Gavazzi, A.; Tavazzi, L. Prognostic Usefulness of the Tricuspid Annular Plane Systolic Excursion in Patients with Congestive Heart Failure Secondary to Idiopathic or Ischemic Dilated Cardiomyopathy. Am. J. Cardiol. 2000, 85, 837–842. [Google Scholar] [CrossRef]
- Venner, C.; Selton-Suty, C.; Huttin, O.; Erpelding, M.L.; Aliot, E.; Juilliere, Y. Right Ventricular Dysfunction in Patients with Idiopathic Dilated Cardiomyopathy: Prognostic Value and Predictive Factors. Arch. Cardiovasc. Dis. 2016, 109, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, J.; Akkan, D.; Iversen, K.K.; Køber, L.; Torp-Pedersen, C.; Hassager, C. Right Ventricular Dysfunction as an Independent Predictor of Short- and Long-Term Mortality in Patients with Heart Failure. Eur. J. Heart Fail. 2007, 9, 610–616. [Google Scholar] [CrossRef]
- Dini, F.L.; Demmer, R.T.; Simioniuc, A.; Morrone, D.; Donati, F.; Guarini, G.; Orsini, E.; Caravelli, P.; Marzilli, M.; Colombo, P.C. Right Ventricular Dysfunction is Associated with Chronic Kidney Disease and Predicts Survival in Patients with Chronic Systolic Heart Failure. Eur. J. Heart Fail. 2012, 14, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Kallvikbacka-Bennett, A.; Goode, K.; Khaleva, O.; Lewinter, C.; Hobkirk, J.; Nikitin, N.P.; Dubois-Rande, J.L.; Hittinger, L.; Clark, A.L.; et al. Prevalence of, Associations With, and Prognostic Value of Tricuspid Annular Plane Systolic Excursion (TAPSE) Among Out-Patients Referred for the Evaluation of Heart Failure. J. Card. Fail. 2012, 18, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Bistola, V.; Parissis, J.T.; Paraskevaidis, I.; Panou, F.; Nikolaou, M.; Ikonomidis, I.; Flessas, N.; Filippatos, G.; Iliodromitis, E.; Kremastinos, D.T. Prognostic Value of Tissue Doppler Right Ventricular Systolic and Diastolic Function Indexes Combined with Plasma B-Type Natriuretic Peptide in Patients With Advanced Heart Failure Secondary to Ischemic or Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2010, 105, 249–254. [Google Scholar] [CrossRef] [PubMed]
- de Groote, P.; Fertin, M.; Goéminne, C.; Petyt, G.; Peyrot, S.; Foucher-Hossein, C.; Mouquet, F.; Bauters, C.; Lamblin, N. Right Ventricular Systolic Function for Risk Stratification in Patients with Stable Left Ventricular Systolic Dysfunction: Comparison of Radionuclide Angiography to EchoDoppler Parameters. Eur. Heart J. 2012, 33, 2672–2679. [Google Scholar] [CrossRef] [Green Version]
- Dokainish, H.; Sengupta, R.; Patel, R.; Lakkis, N. Usefulness of Right Ventricular Tissue Doppler Imaging to Predict Outcome in Left Ventricular Heart Failure Independent of Left Ventricular Diastolic Function. Am. J. Cardiol. 2007, 99, 961–965. [Google Scholar] [CrossRef]
- Damy, T.; Viallet, C.; Lairez, O.; Deswarte, G.; Paulino, A.; Maison, P.; Vermes, E.; Gueret, P.; Adnot, S.; Dubois-Rande, J.L.; et al. Comparison of Four Right Ventricular Systolic Echocardiographic Parameters to Predict Adverse Outcomes in Chronic Heart Failure. Eur. J. Heart Fail. 2009, 11, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Meluzin, J.; Špinarová, L.; Hude, P.; Krejčí, J.; Dušek, L.; Vítovec, J.; Panovsky, R. Combined Right Ventricular Systolic and Diastolic Dysfunction Represents a Strong Determinant of Poor Prognosis in Patients with Symptomatic Heart Failure. Int. J. Cardiol. 2005, 105, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Vizzardi, E.; D’Aloia, A.; Bordonali, T.; Bugatti, S.; Piovanelli, B.; Bonadei, I.; Quinzani, F.; Rovetta, R.; Vaccari, A.; Curnis, A.; et al. Long-Term Prognostic Value of the Right Ventricular Myocardial Performance Index Compared to Other Indexes of Right Ventricular Function in Patients with Moderate Chronic Heart Failure. Echocardiography 2012, 29, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Field, M.E.; Solomon, S.D.; Lewis, E.F.; Kramer, D.B.; Baughman, K.L.; Stevenson, L.W.; Tedrow, U.B. Right Ventricular Dysfunction and Adverse Outcome in Patients with Advanced Heart Failure. J. Card. Fail. 2006, 12, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, I.Z.; Ruisanchez, C.; Dawson, D.; Grapsa, J.; North, B.; Howard, L.S.; Pinto, F.J.; Nihoyannopoulos, P.; Gibbs, J.S.R. Right Ventricular Function in Patients with Pulmonary Hypertension; the Value of Myocardial Performance Index Measured by Tissue Doppler Imaging. Eur. J. Echocardiogr. 2010, 11, 719–724. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, A.; Knight, D.S.; Augustine, D.X.; Harkness, A.; Oxborough, D.; Pearce, K.; Ring, L.; Robinson, S.; Stout, M.; Willis, J.; et al. Echocardiographic Assessment of the Right Heart in Adults: A Practical Guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G19–G41. [Google Scholar] [CrossRef] [Green Version]
- Genovese, D.; Mor-Avi, V.; Palermo, C.; Muraru, D.; Volpato, V.; Kruse, E.; Yamat, M.; Aruta, P.; Addetia, K.; Badano, L.P.; et al. Comparison Between Four-Chamber and Right Ventricular-Focused Views for the Quantitative Evaluation of Right Ventricular Size and Function. J. Am. Soc. Echocardiogr. 2019, 32, 484–494. [Google Scholar] [CrossRef]
- Lai, W.W.; Gauvreau, K.; Rivera, E.S.; Saleeb, S.; Powell, A.J.; Geva, T. Accuracy of Guideline Recommendations for Two-Dimensional Quantification of the Right Ventricle by Echocardiography. Int. J. Cardiovasc. Imaging 2008, 24, 691–698. [Google Scholar] [CrossRef]
- Jones, N.; Burns, A.T.; Prior, D.L. Echocardiographic Assessment of the Right Ventricle—State of the Art. Heart Lung Circ. 2019, 28, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Zornoff, L.A.M.; Skali, H.; Pfeffer, M.A.; Sutton, M.S.J.; Rouleau, J.L.; Lamas, G.A.; Plappert, T.; Rouleau, J.R.; Moyé, L.A.; Lewis, S.J.; et al. Right Ventricular Dysfunction and Risk of Heart Failure and Mortality After Myocardial Infarction. J. Am. Coll. Cardiol. 2002, 39, 1450–1455. [Google Scholar] [CrossRef] [Green Version]
- Anavekar, N.S.; Skali, H.; Bourgoun, M.; Ghali, J.K.; Kober, L.; Maggioni, A.P.; McMurray, J.J.; Velazquez, E.; Califf, R.; Pfeffer, M.A.; et al. Usefulness of Right Ventricular Fractional Area Change to Predict Death, Heart Failure, and Stroke Following Myocardial Infarction (from the VALIANT ECHO Study). Am. J. Cardiol. 2008, 101, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kawata, T.; Daimon, M.; Kimura, K.; Nakao, T.; Lee, S.L.; Hirokawa, M.; Kato, T.S.; Watanabe, M.; Yatomi, Y.; Komuro, I. Echocardiographic Assessment of Right Ventricular Function in Routine Practice: Which Parameters Are Useful to Predict One-Year Outcome in Advanced Heart Failure Patients with Dilated Cardiomyopathy? J. Cardiol. 2017, 70, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Merlo, M.; Gobbo, M.; Stolfo, D.; Losurdo, P.; Ramani, F.; Barbati, G.; Pivetta, A.; Di Lenarda, A.; Anzini, M.; Gigli, M.; et al. The Prognostic Impact of the Evolution of RV Function in Idiopathic DCM. JACC Cardiovasc. Imaging 2016, 9, 1034–1042. [Google Scholar] [CrossRef]
- Sciatti, E.; Vizzardi, E.; Bonadei, I.; Curnis, A.; D’Aloia, A.; Metra, M. Prognostic Value of RV Isovolumic Acceleration and Tissue Strain in Moderate HFrEF. Eur. J. Clin. Investig. 2015, 45, 1052–1059. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Longobardo, L.; Suma, V.; Jain, R.; Carerj, S.; Zito, C.; Zwicke, D.L.; Khandheria, B.K. Role of Two-Dimensional Speckle-Tracking Echocardiography Strain in the Assessment of Right Ventricular Systolic Function and Comparison with Conventional Parameters. J. Am. Soc. Echocardiogr. 2017, 30, e6. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of Left Atrial, Right Ventricular, and Right Atrial Deformation Imaging Using Two-Dimensional Speckle-Tracking Echocardiography: A Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Giusca, S.; Dambrauskaite, V.; Scheurwegs, C.; D’Hooge, J.; Claus, P.; Herbots, L.; Magro, M.; Rademakers, F.; Meyns, B.; Delcroix, M.; et al. Deformation Imaging Describes Right Ventricular Function Better than Longitudinal Displacement of the Tricuspid Ring. Heart 2010, 96, 281–288. [Google Scholar] [CrossRef]
- Hernandez-Suarez, D.F.; Lopez-Candales, A. Strain Imaging Echocardiography: What Imaging Cardiologists Should Know. Curr. Cardiol. Rev. 2017, 12, 118–129. [Google Scholar]
- Focardi, M.; Cameli, M.; Carbone, S.F.; Massoni, A.; De Vito, R.; Lisi, M.; Mondillo, S. Traditional and Innovative Echocardiographic Parameters for the Analysis of Right Ventricular Performance in Comparison with Cardiac Magnetic Resonance. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 47–52. [Google Scholar] [CrossRef]
- Grapsa, J.; Tan, T.C.; Dawson, D.; Nihoyannopoulos, P. Right Ventricular Strain is a More Sensitive Marker of Right Ventricular Dysfunction Than Right Ventricular Ejection Fraction in a Cohort of Patients with Idiopathic Pulmonary Arterial Hypertension. Circulation 2014, 130, A16108. [Google Scholar]
- Morris, D.A.; Krisper, M.; Nakatani, S.; Köhncke, C.; Otsuji, Y.; Belyavskiy, E.; Krishnan, A.K.R.; Kropf, M.; Osmanoglou, E.; Boldt, L.H.; et al. Normal Range and Usefulness of Right Ventricular Systolic Strain to Detect Subtle Right Ventricular Systolic Abnormalities in Patients with Heart Failure: A Multicenter Study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Lisi, M.; Cameli, M.; Righini, F.M.; Malandrino, A.; Tacchini, D.; Focardi, M.; Tsioulpas, C.; Bernazzali, S.; Tanganelli, P.; Maccherini, M.; et al. RV Longitudinal Deformation Correlates with Myocardial Fibrosis in Patients with End-Stage Heart Failure. JACC Cardiovasc. Imaging 2015, 8, 514–522. [Google Scholar] [CrossRef] [Green Version]
- García-Martín, A.; Moya-Mur, J.L.; Carbonell-Dan Román, S.A.; García-Lledó, A.; Navas-Tejedor, P.; Muriel, A.; Rodríguez-Muñoz, D.; Casas-Rojo, E.; Jiménez-Nacher, J.J.; Fernández-Golfín, C.; et al. Four Chamber Right Ventricular Longituindal Strain Versus Right Free Wall Longitudinal Strain. Prognostic Value in Patients with Left Heart Disease. Cardiol. J. 2016, 23, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Motoki, H.; Borowski, A.G.; Shrestha, K.; Hu, B.; Kusunose, K.; Troughton, R.W.; Tang, W.; Klein, A.L. Right Ventricular Global Longitudinal Strain Provides Prognostic Value Incremental to Left Ventricular Ejection Fraction in Patients with Heart Failure. J. Am. Soc. Echocardiogr. 2014, 27, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Lauciello, R.; Zuchi, C.; Mengoni, A.; Bardelli, G.; Alunni, G.; Gronda, E.G.; Ambrosio, G. Superior Prognostic Value of Right Ventricular Free Wall Compared to Global Longitudinal Strain in Patients with Heart Failure. J. Am. Soc. Echocardiogr. 2019, 32, 836–844. [Google Scholar] [CrossRef]
- Guendouz, S.; Rappeneau, S.; Nahum, J.; Dubois-Randé, J.L.; Gueret, P.; Monin, J.L.; Lim, P.; Adnot, S.; Hittinger, L.; Damy, T. Prognostic Significance and Normal Values of 2D Strain to Assess Right Ventricular Systolic Function in Chronic Heart Failure. Circ. J. 2012, 76, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Coiro, S.; Riccini, C.; Mengoni, A.; D’Antonio, A.; Ambrosio, G. Prognostic Value of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction. Superiority of Longitudinal Strain Over Tricuspid Annular Plane Systolic Excursion. Circ. Cardiovasc. Imaging 2018, 11, e006894. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Jung, I.H.; Park, J.H.; Kim, G.S.; Lee, H.Y.; Byun, Y.S.; Kim, B.O.; Rhee, K.J. The Prognostic Value of 2D Strain in Assessment of the Right Ventricle in Patients with Dilated Cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Righini, F.M.; Lisi, M.; Bennati, E.; Navarri, R.; Lunghetti, S.; Padeletti, M.; Cameli, P.; Tsioulpas, C.; Bernazzali, S.; et al. Comparison of Right Versus Left Ventricular Strain Analysis as a Predictor of Outcome in Patients with Systolic Heart Failure Referred for Heart Transplantation. Am. J. Cardiol. 2013, 112, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Citarelli, G.; Antoncecchi, V.; Romito, R.; Monitillo, F.; Leone, M.; Puzzovivo, A.; Lattarulo, M.S.; Rizzo, C.; Caldarola, P.; et al. Right Ventricular Longitudinal Strain Measures Independently Predict Chronic Heart Failure Mortality. Echocardiography 2016, 33, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Houard, L.; Benaets, M.-B.; Ravenstein, C.D.M.D.; Rousseau, M.F.; Ahn, S.A.; Amzulescu, M.-S.; Roy, C.; Slimani, A.; Vancraeynest, D.; Pasquet, A.; et al. Additional Prognostic Value of 2D Right Ventricular Speckle-Tracking Strain for Prediction of Survival in Heart Failure and Reduced Ejection Fraction. A Comparative Study with Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2019, 12, 2373–2385. [Google Scholar] [CrossRef]
- Leibundgut, G.; Rohner, A.; Grize, L.; Bernheim, A.; Kessel-Schaefer, A.; Bremerich, J.; Zellweger, M.; Buser, P.; Handke, M. Dynamic Assessment of Right Ventricular Volumes and Function by Real-Time Three-Dimensional Echocardiography: A Comparison Study with Magnetic Resonance Imaging in 100 Adult Patients. J. Am. Soc. Echocardiogr. 2010, 23, 116–126. [Google Scholar] [CrossRef]
- Shimada, Y.J.; Shiota, M.; Siegel, R.J.; Shiota, T. Accuracy of Right Ventricular Volumes and Function Determined by Three-Dimensional Echocardiography in Comparison with Magnetic Resonance Imaging: A Meta-Analysis Study. J. Am. Soc. Echocardiogr. 2010, 23, 943–953. [Google Scholar] [CrossRef]
- Sugeng, L.; Mor-Avi, V.; Weinert, L.; Niel, J.; Ebner, C.; Steringer-Mascherbauer, R.; Bartolles, R.; Baumann, R.; Schummers, G.; Lang, R.M.; et al. Multimodality Comparison of Quantitative Volumetric Analysis of the Right Ventricle. JACC Cardiovasc. Imaging 2010, 3, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Nochioka, K.; Roca, G.Q.; Claggett, B.; Biering-Sørensen, T.; Matsushita, K.; Hung, C.-L.; Solomon, S.D.; Kitzman, D.; Shah, A.M. Right Ventricular Function, Right Ventricular-Pulmonary Artery Coupling, and Heart Failure Risk in 4 US Communities. The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2018, 3, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Magunia, H.; Dietrich, C.; Langer, H.F.; Schibilsky, D.; Schlensak, C.; Rosenberger, P.; Nowak-Machen, M. 3D Echocardiography Derived Right Ventricular Function is Associated with Right Ventricular Failure and Mid-Term Survival After Left Ventricular Assist Device Implantation. Int. J. Cardiol. 2018, 272, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, C.; Critsinelis, A.C.; Kawabori, M.; Sugiura, T.; Loor, G.; Civitello, A.B.; Morgan, J.A. Frequency and Consequences of Right-Sided Heart Failure After Continuous-Flow left Ventricular Assist Device Implantation. Am. J. Cardiol. 2018, 121, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Wu, V.C.-C.; Kado, Y.; Otani, K.; Lin, F.-C.; Otsuji, Y.; Negishi, K.; Takeuchi, M. Prognostic Value of Right Ventricular Ejection Fraction Assessed by Transthoracic 3D Echocardiography. Circ. Cardiovasc. Imaging 2017, 10, e005384. [Google Scholar] [CrossRef] [Green Version]
- Surkova, E.; Muraru, D.; Genovese, D.; Aruta, P.; Palermo, C.; Badano, L.P. Relative Prognostic Importance of Left and Right Ventricular Ejection Fraction in Patients with Cardiac Diseases. J. Am. Soc. Echocardiogr. 2019, 32, 1407–1415.e3. [Google Scholar] [CrossRef]
- Atsumi, A.; Ishizu, T.; Kameda, Y.; Yamamoto, M.; Harimura, Y.; Machino-Ohtsuka, T.; Kawamura, R.; Enomoto, M.; Seo, Y.; Aonuma, K. Application of 3-Dimensional Speckle Tracking Imaging to the Assessment of Right Ventricular Regional Deformation. Circ. J. 2013, 77, 1760–1768. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C.F.; Dobson, G.; Dawson, D.; Charalampopoulos, A.; Grapsa, J.; Nihoyannopoulos, P. Three-Dimensional Speckle Tracking of the Right Ventricle—Toward Optimal Quantification of Right Ventricular Dysfunction in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2014, 64, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Tello, K.; Dalmer, A.; Axmann, J.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Sommer, N.; Wilhelm, J.; et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ. Heart Fail. 2019, 12, e005512. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure with Preserved Ejection Fraction. Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid Annular Plane Systolic Excursion and Pulmonary Arterial Systolic Pressure Relationship in Heart Failure: An Index of Right Ventricular Contractile Function and Prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Naeije, R.; Arena, R.; Corrà, U.; Ghio, S.; Forfia, P.; Rossi, A.; Cahalin, L.P.; Bandera, F.; Temporelli, P. Echocardiography of Right Ventriculoarterial Coupling Combined with Cardiopulmonary Exercise Testing to Predict Outcome in Heart Failure. Chest 2015, 148, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Guazzi, M.; Scardovi, A.B.; Klersy, C.; Clemenza, F.; Carluccio, E.; Temporelli, P.L.; Rossi, A.; Faggiano, P.; Traversi, E.; et al. Different Correlates but Similar Prognostic Implications for Right Ventricular Dysfunction in Heart Failure Patients with Reduced or Preserved Ejection Fraction. Eur. J. Heart Fail. 2017, 19, 873–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, L.; Lam, C.S.; Gong, L.; Chan, S.P.; Sim, D.; Yeo, D.; Jaufeerally, F.; Leong, K.T.G.; Ong, H.Y.; Ng, T.P.; et al. Right Ventricular Dysfunction in Left-Sided Heart Failure with Preserved Versus Reduced Ejection Fraction. Eur. J. Heart Fail. 2017, 19, 1664–1671. [Google Scholar] [CrossRef] [Green Version]
- Iacoviello, M.; Monitillo, F.; Citarelli, G.; Leone, M.; Grande, D.; Antoncecchi, V.; Rizzo, C.; Terlizzese, P.; Romito, R.; Caldarola, P.; et al. Right Ventriculo-Arterial Coupling Assessed by Two-Dimensional Strain: A New Parameter of Right Ventricular Function Independently Associated with Prognosis in Chronic Heart Failure Patients. Int. J. Cardiol. 2017, 241, 318–321. [Google Scholar] [CrossRef]
- Lindqvist, P.; Henein, M.; Kazzam, E. Right Ventricular Outflow Tract Fractional Shortening: An Applicable Measure of Right Ventricular Systolic Function. Eur. J. Echocardiogr. 2003, 4, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Deveci, B.; Baser, K.; Gul, M.; Sen, F.; Kafes, H.; Avci, S.; Temizer, O.; Ozeke, O.; Tufekcioglu, O.; Golbasi, Z. Right Ventricular Outflow Tract Function in Chronic Heart Failure. Indian Heart J. 2016, 68, S10–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allam, L.E.; Onsy, A.M.; Ghalib, H.A. Right Ventricular Outflow Tract Systolic Excursion and Fractional Shortening: Can These Echocardiographic Parameters be Used for the Assessment of Right Ventricular Function? J. Cardiovasc. Echogr. 2017, 27, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Tsuruda, T.; Watanabe, Y.; Onitsuka, H.; Furukawa, K.; Ideguchi, T.; Kawagoe, J.; Ishikawa, T.; Kato, J.; Takenaga, M.; et al. Reduced Fractional Shortening of Right Ventricular Outflow Tract is Associated with Adverse Outcomes in Patients with Left Ventricular Dysfunction. Cardiovasc. Ultrasound 2013, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Alsharqi, M.; Woodward, W.J.; Mumith, J.A.; Markham, D.C.; Upton, R.; Leeson, P. Artificial intelligence and echocardiography. Echo Res. Pract. 2018, 5, R115–R125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genovese, D.; Rashedi, N.; Weinert, L.; Narang, A.; Addetia, K.; Patel, A.R.; Prater, D.; Gonçalves, A.; Mor-Avi, V.; Lang, R.M. Machine learning-based three-dimensional echocardiographic quantification of right ventricular size and function: Validation against cardiac magnetic resonance. J. Am. Soc. Echocardiogr. 2019, 32, 969–977. [Google Scholar] [CrossRef] [PubMed]


| Study | Publication Year | Number of Patients | Study Type | Parameter | Proposed Cutoff |
|---|---|---|---|---|---|
| Dokainish et al. [41] | 2007 | 107 | Prospective | S’ wave | 9 cm/s |
| Damy et al. [42] | 2009 | 136 | Prospective | S’ wave | 9.5 cm/s |
| De Groote et al. [40] | 2012 | 527 | Prospective | S’ wave | 9.7 cm/s |
| Vizzardi et al. [44] | 2012 | 95 | Prospective | RV MPI | 0.38 |
| Dini et al. [37] | 2012 | 373 | Prospective | TAPSE | 14 mm |
| Damy et al. [38] | 2012 | 1547 | Prospective | TAPSE | 15.9 mm |
| Guazzi et al. [86] | 2013 | 293 | Prospective | TAPSE/PASP | 0.36 |
| Yamaguchi et al. [94] | 2013 | 81 | Prospective | RVOT-FS | 20% |
| Motoki et al. [66] | 2014 | 171 | Retrospective | Global RV strain | −14.8% |
| Sciatti et al. [55] | 2015 | 60 | Prospective | RIVA | 1.5 m/s2 |
| Garcia-Martin et al. [65] | 2016 | 103 | Prospective | Global RV strain | −17.3% |
| Iacoviello et al. [72] | 2016 | 332 | Prospective | Global RV strain, RVFW strain | −14%, −20.6% |
| Venner et al. [35] | 2016 | 136 | Retrospective | TAPSE | 15 mm |
| Merlo et al. [54] | 2016 | 512 | Retrospective | FAC | 35% |
| Kawata et al. [53] | 2017 | 68 | Retrospective | FAC | 26.7% |
| Ghio et al. [88] | 2017 | 1663 | Retrospective | TAPSE/PASP | 0.36 |
| Bosch et al. [89] | 2017 | 438 | Prospective | TAPSE/PASP, global RV strain/PASP | 0.48, −0.56 |
| Iacoviello et al. [90] | 2017 | 315 | Prospective | RV strain/PASP, RVFW strain/PASP | −0.36, −0.66 |
| Nagata et al. [80] | 2017 | 446 | Prospective | 3D RVEF | 35% for cardiac death, 41% for MACE |
| Seo et al. [70] | 2019 | 143 | Prospective | RVFW strain | −16.5% |
| Houard et al. [73] | 2019 | 266 | Prospective | Global RV strain | −19% |
| Carluccio et al. [67] | 2019 | 288 | Prospective | Global RV strain, RVFW strain | −14.6%, −15.3% |
| Surkova et al. [81] | 2019 | 394 | Prospective | 3D RVEF | 45% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijiiac, A.; Onciul, S.; Guzu, C.; Scarlatescu, A.; Petre, I.; Zamfir, D.; Onut, R.; Deaconu, S.; Dorobantu, M. Forgotten No More—The Role of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: An Echocardiographic Perspective. Diagnostics 2021, 11, 548. https://doi.org/10.3390/diagnostics11030548
Vijiiac A, Onciul S, Guzu C, Scarlatescu A, Petre I, Zamfir D, Onut R, Deaconu S, Dorobantu M. Forgotten No More—The Role of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: An Echocardiographic Perspective. Diagnostics. 2021; 11(3):548. https://doi.org/10.3390/diagnostics11030548
Chicago/Turabian StyleVijiiac, Aura, Sebastian Onciul, Claudia Guzu, Alina Scarlatescu, Ioana Petre, Diana Zamfir, Roxana Onut, Silvia Deaconu, and Maria Dorobantu. 2021. "Forgotten No More—The Role of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: An Echocardiographic Perspective" Diagnostics 11, no. 3: 548. https://doi.org/10.3390/diagnostics11030548
APA StyleVijiiac, A., Onciul, S., Guzu, C., Scarlatescu, A., Petre, I., Zamfir, D., Onut, R., Deaconu, S., & Dorobantu, M. (2021). Forgotten No More—The Role of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: An Echocardiographic Perspective. Diagnostics, 11(3), 548. https://doi.org/10.3390/diagnostics11030548







