Cystic Fibrosis-Related Diabetes (CFRD): Overview of Associated Genetic Factors
Abstract
1. Introduction
1.1. Clinical Presentation and Management
1.2. Diagnosis and Screening
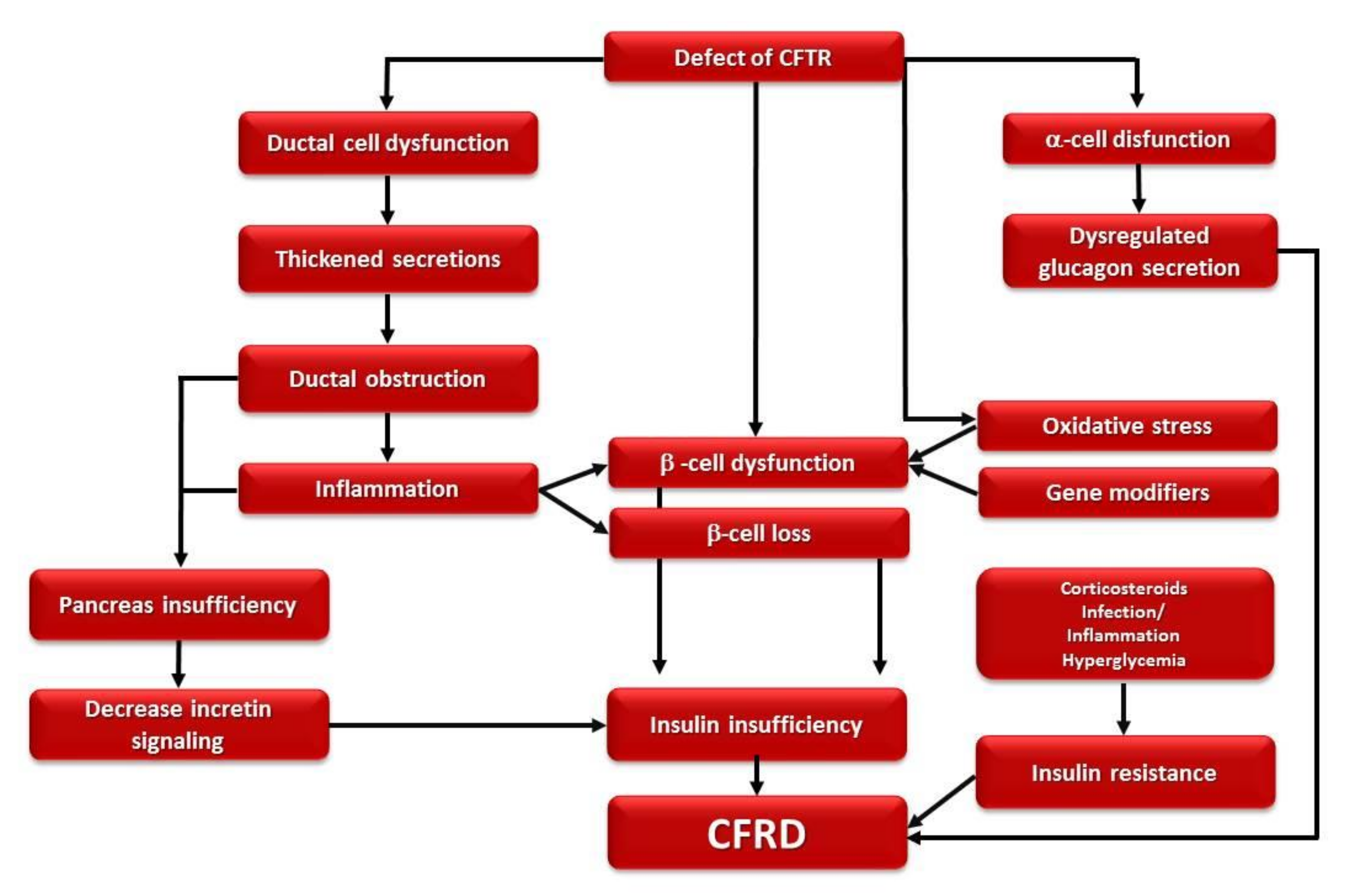
1.3. Pathophysiology
2. Method of Search
3. Genetic Factors
3.1. CFRD and CFTR Genotypes
3.2. CFRD and Modifier Genes
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Kayani, K.; Mohammed, R.; Mohiaddin, H. Cystic Fibrosis-Related Diabetes. Front. Endocrinol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Prinz, N.; Zolin, A.; Konrad, K.; Nährlich, L.; Laubner, K.; Olesen, H.V.; Bauer, M.; Jung, A.; Frischer, T.; Holl, R.W. Characteristics of Cystic Fibrosis-Related Diabetes: Data from Two Different Sources the European Cystic Fibrosis Society Patient Registry and German/Austrian Diabetes Prospective Follow-up Registry. Pediatr. Diabetes 2019, 20, 255–262. [Google Scholar] [CrossRef]
- Alves, C.; Della-Manna, T.; Albuquerque, C.T.M. Cystic Fibrosis-Related Diabetes: An Update on Pathophysiology, Diagnosis, and Treatment. J. Pediatr. Endocrinol. Metab. JPEM 2020, 33, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Briel, M.; Greger, R.; Kunzelmann, K. Cl−Transport by Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Contributes to the Inhibition of Epithelial Na+ Channels (ENaCs) in Xenopus Oocytes Co-Expressing CFTR and ENaC. J. Physiol. 1998, 508, 825–836. [Google Scholar] [CrossRef]
- Schwiebert, E.M.; Benos, D.J.; Egan, M.E.; Stutts, M.J.; Guggino, W.B. CFTR Is a Conductance Regulator as Well as a Chloride Channel. Physiol. Rev. 1999, 79, S145–S166. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, R.; Manderson Koivula, F.N.; McClenaghan, N.H.; Kelly, C. Cystic Fibrosis–Related Diabetes: Pathophysiology and Therapeutic Challenges. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 117955141985177. [Google Scholar] [CrossRef]
- Lee, M.G.; Choi, J.Y.; Luo, X.; Strickland, E.; Thomas, P.J.; Muallem, S. Cystic Fibrosis Transmembrane Conductance Regulator Regulates Luminal Cl−/HCO3− Exchange in Mouse Submandibular and Pancreatic Ducts. J. Biol. Chem. 1999, 274, 14670–14677. [Google Scholar] [CrossRef]
- Quinton, P.M. Cystic Fibrosis: Impaired Bicarbonate Secretion and Mucoviscidosis. Lancet 2008, 372, 415–417. [Google Scholar] [CrossRef]
- Choi, J.Y.; Muallem, D.; Kiselyov, K.; Lee, M.G.; Thomas, P.J.; Muallem, S. Aberrant CFTR-Dependent HCO-3 Transport in Mutations Associated with Cystic Fibrosis. Nature 2001, 410, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Barrio, R. Management of endocrine disease: Cystic Fibrosis-Related Diabetes: Novel Pathogenic Insights Opening New Therapeutic Avenues. Eur. J. Endocrinol. 2015, 172, R131–R141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cystic Fibrosis Mutation Database: Contact. Available online: http://www.genet.sickkids.on.ca/Contact.html (accessed on 11 December 2020).
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic Fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef]
- Zielenski, J.; Tsui, L.-C. Cystic Fibrosis: Genotypic and Phenotypic Variations. Annu. Rev. Genet. 1995, 29, 777–807. [Google Scholar] [CrossRef]
- Rowntree, R.K.; Harris, A. The Phenotypic Consequences of CFTR Mutations. Ann. Hum. Genet. 2003, 67, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.C. Annual Data Report 2013 Cystic Fibrosis Foundation Patient Registry. 2013, pp. 1–96. Available online: https://www.cff.org/2013_cff_annual_data_report_to_the_center_directors.pdf (accessed on 6 March 2021).
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From CFTR Biology toward Combinatorial Pharmacotherapy: Expanded Classification of Cystic Fibrosis Mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Cystic Fibrosis Genetics: From Molecular Understanding to Clinical Application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, A.; Cernera, G.; Iacotucci, P.; Cimbalo, C.; Gelzo, M.; Comegna, M.; Di Lullo, A.M.; Tosco, A.; Carnovale, V.; Raia, V.; et al. TAS2R38 Is a Novel Modifier Gene in Patients with Cystic Fibrosis. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic Fibrosis. Lancet Lond. Engl. 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Pineau, F.; Caimmi, D.; Magalhães, M.; Fremy, E.; Mohamed, A.; Mely, L.; Leroy, S.; Murris, M.; Claustres, M.; Chiron, R.; et al. Blood Co-Expression Modules Identify Potential Modifier Genes of Diabetes and Lung Function in Cystic Fibrosis. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.; Chan, C.L.; Granados, A.; Ode, K.L.; Moran, A.; Battezzati, A. Hypoglycemia in Cystic Fibrosis: Prevalence, Impact and Treatment. J. Cyst. Fibros. 2019, 18, S19–S24. [Google Scholar] [CrossRef]
- Moran, A.; Dunitz, J.; Nathan, B.; Saeed, A.; Holme, B.; Thomas, W. Cystic Fibrosis-Related Diabetes: Current Trends in Prevalence, Incidence, and Mortality. Diabetes Care 2009, 32, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Becker, D.; Casella, S.J.; Gottlieb, P.A.; Kirkman, M.S.; Marshall, B.C.; Slovis, B. The CFRD Consensus Conference Committee Epidemiology, Pathophysiology, and Prognostic Implications of Cystic Fibrosis-Related Diabetes: A Technical Review. Diabetes Care 2010, 33, 2677–2683. [Google Scholar] [CrossRef]
- Lewis, C.; Blackman, S.M.; Nelson, A.; Oberdorfer, E.; Wells, D.; Dunitz, J.; Thomas, W.; Moran, A. Diabetes-Related Mortality in Adults with Cystic Fibrosis. Role of Genotype and Sex. Am. J. Respir. Crit. Care Med. 2015, 191, 194–200. [Google Scholar] [CrossRef]
- Ode, K.L.; Frohnert, B.; Laguna, T.; Phillips, J.; Holme, B.; Regelmann, W.; Thomas, W.; Moran, A. Oral Glucose Tolerance Testing in Children with Cystic Fibrosis. Pediatr. Diabetes 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Mozzillo, E.; Raia, V.; Fattorusso, V.; Falco, M.; Sepe, A.; De Gregorio, F.; Nugnes, R.; Valerio, G.; Franzese, A. Glucose Derangements in Very Young Children with Cystic Fibrosis and Pancreatic Insufficiency. Diabetes Care 2012, 35, e78. [Google Scholar] [CrossRef][Green Version]
- Gottlieb, P.A.; Yu, L.; Babu, S.; Wenzlau, J.; Bellin, M.; Frohnert, B.I.; Moran, A. No Relation Between Cystic Fibrosis-Related Diabetes and Type 1 Diabetes Autoimmunity. Diabetes Care 2012, 35, e57. [Google Scholar] [CrossRef]
- Moran, A.; Pillay, K.; Becker, D.; Granados, A.; Hameed, S.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Management of Cystic Fibrosis-Related Diabetes in Children and Adolescents. Pediatr. Diabetes 2018, 19 (Suppl. 27), 64–74. [Google Scholar] [CrossRef]
- Terliesner, N.; Vogel, M.; Steighardt, A.; Gausche, R.; Henn, C.; Hentschel, J.; Kapellen, T.; Klamt, S.; Gebhardt, J.; Kiess, W.; et al. Cystic-Fibrosis Related-Diabetes (CFRD) Is Preceded by and Associated with Growth Failure and Deteriorating Lung Function. J. Pediatr. Endocrinol. Metab. JPEM 2017, 30, 815–821. [Google Scholar] [CrossRef]
- Adler, A.I.; Shine, B.S.F.; Chamnan, P.; Haworth, C.S.; Bilton, D. Genetic Determinants and Epidemiology of Cystic Fibrosis-Related Diabetes: Results from a British Cohort of Children and Adults. Diabetes Care 2008, 31, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Olesen, H.V.; Drevinek, P.; Gulmans, V.A.; Hatziagorou, E.; Jung, A.; Mei-Zahav, M.; Stojnic, N.; Thomas, M.; Zolin, A.; ECFSPR Steering Group; et al. Cystic Fibrosis Related Diabetes in Europe: Prevalence, Risk Factors and Outcome. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2020, 19, 321–327. [Google Scholar] [CrossRef]
- Fattorusso, V.; Casale, A.; Raia, V.; Mozzillo, E.; Franzese, A. Long-Term Follow-Up in a Girl with Cystic Fibrosis and Diabetes Since the First Year of Life. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2017, 8, 1187–1190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moheet, A.; Moran, A. CF-Related Diabetes: Containing the Metabolic Miscreant of Cystic Fibrosis. Pediatr. Pulmonol. 2017, 52, S37–S43. [Google Scholar] [CrossRef]
- Moran, A.; Brunzell, C.; Cohen, R.C.; Katz, M.; Marshall, B.C.; Onady, G.; Robinson, K.A.; Sabadosa, K.A.; Stecenko, A.; Slovis, B.; et al. Clinical Care Guidelines for Cystic Fibrosis-Related Diabetes: A Position Statement of the American Diabetes Association and a Clinical Practice Guideline of the Cystic Fibrosis Foundation, Endorsed by the Pediatric Endocrine Society. Diabetes Care 2010, 33, 2697–2708. [Google Scholar] [CrossRef]
- Moran, A.; Pillay, K.; Becker, D.J.; Acerini, C.L. International Society for Pediatric and Adolescent Diabetes ISPAD Clinical Practice Consensus Guidelines 2014. Management of Cystic Fibrosis-Related Diabetes in Children and Adolescents. Pediatr. Diabetes 2014, 15 (Suppl. 20), 65–76. [Google Scholar] [CrossRef]
- Franzese, A.; Mozzillo, E.; Fattorusso, V.; Raia, V.; Valerio, G. Screening of Glucose Metabolism Derangements in Pediatric Cystic Fibrosis Patients: How, When, Why. Acta Diabetol. 2015, 52, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Minicucci, L.; Cotellessa, M.; Pittaluga, L.; Minuto, N.; d’Annunzio, G.; Avanzini, M.A.; Lorini, R. Beta-Cell Autoantibodies and Diabetes Mellitus Family History in Cystic Fibrosis. J. Pediatr. Endocrinol. Metab. JPEM 2005, 18, 755–760. [Google Scholar] [CrossRef]
- Granados, A.; Chan, C.L.; Ode, K.L.; Moheet, A.; Moran, A.; Holl, R. Cystic Fibrosis Related Diabetes: Pathophysiology, Screening and Diagnosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2019, 18 (Suppl. 2), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, A.; Mukai, K.; Johnson, D.; Burke, B. Endocrine Pancreas in Cystic Fibrosis: An Immunohistochemical Study. Hum. Pathol. 1984, 15, 278–284. [Google Scholar] [CrossRef]
- Hart, N.J.; Aramandla, R.; Poffenberger, G.; Fayolle, C.; Thames, A.H.; Bautista, A.; Spigelman, A.F.; Babon, J.A.B.; DeNicola, M.E.; Dadi, P.K.; et al. Cystic Fibrosis–Related Diabetes Is Caused by Islet Loss and Inflammation. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Kopito, L.E.; Shwachman, H.; Vawter, G.F.; Edlow, J. The Pancreas in Cystic Fibrosis: Chemical Composition and Comparative Morphology. Pediatr. Res. 1976, 10, 742–749. [Google Scholar] [CrossRef]
- Soejima, K.; Landing, B.H. Pancreatic Islets in Older Patients with Cystic Fibrosis with and without Diabetes Mellitus: Morphometric and Immunocytologic Studies. Pediatr. Pathol. 1986, 6, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Bogdani, M.; Blackman, S.M.; Ridaura, C.; Bellocq, J.-P.; Powers, A.C.; Aguilar-Bryan, L. Structural Abnormalities in Islets from Very Young Children with Cystic Fibrosis May Contribute to Cystic Fibrosis-Related Diabetes. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, S.M.P.; Dattani, M.T.; Hindmarsh, P.C. Cystic Fibrosis-Related Diabetes in Childhood. Horm. Res. Paediatr. 2010, 73, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.; Esguerra, J.L.; Wendt, A.; Flodström-Tullberg, M.; Eliasson, L. CFTR and Anoctamin 1 (ANO1) Contribute to CAMP Amplified Exocytosis and Insulin Secretion in Human and Murine Pancreatic Beta-Cells. BMC Med. 2014, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Boom, A.; Lybaert, P.; Pollet, J.-F.; Jacobs, P.; Jijakli, H.; Golstein, P.E.; Sener, A.; Malaisse, W.J.; Beauwens, R. Expression and Localization of Cystic Fibrosis Transmembrane Conductance Regulator in the Rat Endocrine Pancreas. Endocrine 2007, 32, 197–205. [Google Scholar] [CrossRef]
- Olivier, A.K.; Yi, Y.; Sun, X.; Sui, H.; Liang, B.; Hu, S.; Xie, W.; Fisher, J.T.; Keiser, N.W.; Lei, D.; et al. Abnormal Endocrine Pancreas Function at Birth in Cystic Fibrosis Ferrets. J. Clin. Investig. 2012, 122, 3755–3768. [Google Scholar] [CrossRef]
- Best, L. Glucose-Induced Electrical Activity in Rat Pancreatic β-Cells: Dependence on Intracellular Chloride Concentration. J. Physiol. 2005, 568, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Koivula, F.N.M.; McClenaghan, N.H.; Harper, A.G.S.; Kelly, C. Islet-Intrinsic Effects of CFTR Mutation. Diabetologia 2016, 59, 1350–1355. [Google Scholar] [CrossRef]
- Guo, J.H.; Chen, H.; Ruan, Y.C.; Zhang, X.L.; Zhang, X.H.; Fok, K.L.; Tsang, L.L.; Yu, M.K.; Huang, W.Q.; Sun, X.; et al. Glucose-Induced Electrical Activities and Insulin Secretion in Pancreatic Islet β-Cells Are Modulated by CFTR. Nat. Commun. 2014, 5, 4420. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Laguna, T.; Leschyshyn, J.; Regelmann, W.; Dunitz, J.; Billings, J.; Moran, A. Insulin Secretion Improves in Cystic Fibrosis Following Ivacaftor Correction of CFTR: A Small Pilot Study. Pediatr. Diabetes 2013, 14, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; De Leon, D.D.; Sheikh, S.; Camburn, D.; Kubrak, C.; Peleckis, A.J.; Stefanovski, D.; Hadjiliadis, D.; Rickels, M.R.; Rubenstein, R.C. Islet Hormone and Incretin Secretion in Cystic Fibrosis after Four Months of Ivacaftor Therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 342–351. [Google Scholar] [CrossRef]
- Huang, W.Q.; Guo, J.H.; Zhang, X.H.; Yu, M.K.; Chung, Y.W.; Ruan, Y.C.; Chan, H.C. Glucose-Sensitive CFTR Suppresses Glucagon Secretion by Potentiating KATP Channels in Pancreatic Islet α Cells. Endocrinology 2017, 158, 3188–3199. [Google Scholar] [CrossRef]
- Hull, R.L.; Gibson, R.L.; McNamara, S.; Deutsch, G.H.; Fligner, C.L.; Frevert, C.W.; Ramsey, B.W.; Sanda, S. Islet Interleukin-1β Immunoreactivity Is an Early Feature of Cystic Fibrosis That May Contribute to β-Cell Failure. Diabetes Care 2018, 41, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Böni-Schnetzler, M.; Ellingsgaard, H.; Halban, P.A.; Ehses, J.A. Cytokine Production by Islets in Health and Diabetes: Cellular Origin, Regulation and Function. Trends Endocrinol. Metab. TEM 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Rottner, M.; Tual-Chalot, S.; Mostefai, H.A.; Andriantsitohaina, R.; Freyssinet, J.-M.; Martínez, M.C. Increased Oxidative Stress Induces Apoptosis in Human Cystic Fibrosis Cells. PLoS ONE 2011, 6, e24880. [Google Scholar] [CrossRef]
- Poitout, V.; Robertson, R.P. Glucolipotoxicity: Fuel Excess and Beta-Cell Dysfunction. Endocr. Rev. 2008, 29, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Ntimbane, T.; Krishnamoorthy, P.; Huot, C.; Legault, L.; Jacob, S.V.; Brunet, S.; Levy, E.; Guéraud, F.; Lands, L.C.; Comte, B. Oxidative Stress and Cystic Fibrosis-Related Diabetes: A Pilot Study in Children. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2008, 7, 373–384. [Google Scholar] [CrossRef]
- Ntimbane, T.; Comte, B.; Mailhot, G.; Berthiaume, Y.; Poitout, V.; Prentki, M.; Rabasa-Lhoret, R.; Levy, E. Cystic Fibrosis-Related Diabetes: From CFTR Dysfunction to Oxidative Stress. Clin. Biochem. Rev. 2009, 30, 153–177. [Google Scholar] [PubMed]
- Ntimbane, T.; Mailhot, G.; Spahis, S.; Rabasa-Lhoret, R.; Kleme, M.-L.; Melloul, D.; Brochiero, E.; Berthiaume, Y.; Levy, E. CFTR Silencing in Pancreatic β-Cells Reveals a Functional Impact on Glucose-Stimulated Insulin Secretion and Oxidative Stress Response. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E200–E212. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Rayner, C.K.; Horowitz, M. Incretins. Handb. Exp. Pharmacol. 2016, 233, 137–171. [Google Scholar] [CrossRef]
- Kuo, P.; Stevens, J.E.; Russo, A.; Maddox, A.; Wishart, J.M.; Jones, K.L.; Greville, H.; Hetzel, D.; Chapman, I.; Horowitz, M.; et al. Gastric Emptying, Incretin Hormone Secretion, and Postprandial Glycemia in Cystic Fibrosis--Effects of Pancreatic Enzyme Supplementation. J. Clin. Endocrinol. Metab. 2011, 96, E851–E855. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; De Luca, F.; Arrigo, T.; Di Benedetto, A.; Sferlazzas, C.; Gigante, A.; Rigoli, L.; Magazzù, G. First-Phase Insulin Response to Intravenous Glucose in Cystic Fibrosis Patients with Different Degrees of Glucose Tolerance. J. Pediatr. Endocrinol. 1994, 7, 13–17. [Google Scholar] [CrossRef]
- Couce, M.; O’Brien, T.D.; Moran, A.; Roche, P.C.; Butler, P.C. Diabetes Mellitus in Cystic Fibrosis Is Characterized by Islet Amyloidosis. J. Clin. Endocrinol. Metab. 1996, 81, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.L.; Geddes, D.M.; Gyi, K.M.; Baker, E.H. Clinical Importance of Cystic Fibrosis-Related Diabetes. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2004, 3, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Janson, J.; Ashley, R.H.; Harrison, D.; McIntyre, S.; Butler, P.C. The Mechanism of Islet Amyloid Polypeptide Toxicity Is Membrane Disruption by Intermediate-Sized Toxic Amyloid Particles. Diabetes 1999, 48, 491–498. [Google Scholar] [CrossRef]
- Yung, B.; Noormohamed, F.H.; Kemp, M.; Hooper, J.; Lant, A.F.; Hodson, M.E. Cystic Fibrosis-Related Diabetes: The Role of Peripheral Insulin Resistance and Beta-Cell Dysfunction. Diabet. Med. J. Br. Diabet. Assoc. 2002, 19, 221–226. [Google Scholar] [CrossRef]
- Mohan, K.; Miller, H.; Dyce, P.; Grainger, R.; Hughes, R.; Vora, J.; Ledson, M.; Walshaw, M. Mechanisms of Glucose Intolerance in Cystic Fibrosis. Diabet. Med. J. Br. Diabet. Assoc. 2009, 26, 582–588. [Google Scholar] [CrossRef]
- Ahmad, T.; Nelson, R.; Taylor, R. Insulin Sensitivity and Metabolic Clearance Rate of Insulin in Cystic Fibrosis. Metabolism. 1994, 43, 163–167. [Google Scholar] [CrossRef]
- Moran, A.; Pyzdrowski, K.L.; Weinreb, J.; Kahn, B.B.; Smith, S.A.; Adams, K.S.; Seaquist, E.R. Insulin Sensitivity in Cystic Fibrosis. Diabetes 1994, 43, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Hardin, D.S.; Leblanc, A.; Marshall, G.; Seilheimer, D.K. Mechanisms of Insulin Resistance in Cystic Fibrosis. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1022–E1028. [Google Scholar] [CrossRef]
- Boudreau, V.; Coriati, A.; Hammana, I.; Ziai, S.; Desjardins, K.; Berthiaume, Y.; Rabasa-Lhoret, R. Variation of Glucose Tolerance in Adult Patients with Cystic Fibrosis: What Is the Potential Contribution of Insulin Sensitivity? J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2016, 15, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Spaggiari, C.; Ziveri, M.A.; Rossi, M.; Volta, C.; Viani, I.; Grzincich, G.L.; Sartori, C.; Zanzucchi, M.; Raia, V.; et al. Insulin Production and Resistance in Cystic Fibrosis: Effect of Age, Disease Activity, and Genotype. J. Endocrinol. Investig. 2012, 35, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.; Ode, K.L. Hypoglycaemia in Patients with Cystic Fibrosis- Harbinger of Poor Outcomes or Innocent Bystander? J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2018, 17, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Battezzati, A.; Battezzati, P.M.; Costantini, D.; Seia, M.; Zazzeron, L.; Russo, M.C.; Daccò, V.; Bertoli, S.; Crosignani, A.; Colombo, C. Spontaneous Hypoglycemia in Patients with Cystic Fibrosis. Eur. J. Endocrinol. 2007, 156, 369–376. [Google Scholar] [CrossRef]
- Haliloglu, B.; Gokdemir, Y.; Atay, Z.; Abali, S.; Guran, T.; Karakoc, F.; Ersu, R.; Karadag, B.; Turan, S.; Bereket, A. Hypoglycemia Is Common in Children with Cystic Fibrosis and Seen Predominantly in Females. Pediatr. Diabetes 2017, 18, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Armaghanian, N.; Hetherington, J.; Parameswaran, V.; Chua, E.L.; Markovic, T.P.; Brand-Miller, J.; Steinbeck, K. Hypoglycemia in Cystic Fibrosis during an Extended Oral Glucose Tolerance Test. Pediatr. Pulmonol. 2020, 55, 3391–3399. [Google Scholar] [CrossRef]
- Edlund, A.; Pedersen, M.G.; Lindqvist, A.; Wierup, N.; Flodström-Tullberg, M.; Eliasson, L. CFTR Is Involved in the Regulation of Glucagon Secretion in Human and Rodent Alpha Cells. Sci. Rep. 2017, 7, 90. [Google Scholar] [CrossRef]
- Radike, K.; Molz, K.; Holl, R.W.; Poeter, B.; Hebestreit, H.; Ballmann, M. Prognostic Relevance of Hypoglycemia Following an Oral Glucose Challenge for Cystic Fibrosis-Related Diabetes. Diabetes Care 2011, 34, e43. [Google Scholar] [CrossRef]
- Ahmed, N.; Corey, M.; Forstner, G.; Zielenski, J.; Tsui, L.-C.; Ellis, L.; Tullis, E.; Durie, P. Molecular Consequences of Cystic Fibrosis Transmembrane Regulator (CFTR) Gene Mutations in the Exocrine Pancreas. Gut 2003, 52, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Lanng, S.; Thorsteinsson, B.; Erichsen, G.; Nerup, J.; Koch, C. Glucose Tolerance in Cystic Fibrosis. Arch. Dis. Child. 1991, 66, 612–616. [Google Scholar] [CrossRef][Green Version]
- Marshall, B.C.; Butler, S.M.; Stoddard, M.; Moran, A.M.; Liou, T.G.; Morgan, W.J. Epidemiology of Cystic Fibrosis-Related Diabetes. J. Pediatr. 2005, 146, 681–687. [Google Scholar] [CrossRef]
- Vanscoy, L.L.; Blackman, S.M.; Collaco, J.M.; Bowers, A.; Lai, T.; Naughton, K.; Algire, M.; McWilliams, R.; Beck, S.; Hoover-Fong, J.; et al. Heritability of Lung Disease Severity in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2007, 175, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Minicucci, L.; Lorini, R.; Giannattasio, A.; Colombo, C.; Iapichino, L.; Reali, M.F.; Padoan, R.; Calevo, M.G.; Casciaro, R.; De Alessandri, A.; et al. Liver Disease as Risk Factor for Cystic Fibrosis-Related Diabetes Development. Acta Paediatr. Oslo Nor. 1992 2007, 96, 736–739. [Google Scholar] [CrossRef]
- Blackman, S.M.; Hsu, S.; Vanscoy, L.L.; Collaco, J.M.; Ritter, S.E.; Naughton, K.; Cutting, G.R. Genetic Modifiers Play a Substantial Role in Diabetes Complicating Cystic Fibrosis. J. Clin. Endocrinol. Metab. 2009, 94, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Lanng, S.; Thorsteinsson, B.; Pociot, F.; Marshall, M.O.; Madsen, H.O.; Schwartz, M.; Nerup, J.; Koch, C. Diabetes Mellitus in Cystic Fibrosis: Genetic and Immunological Markers. Acta Paediatr. Oslo Nor. 1992 1993, 82, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Rainisio, M.; Madessani, U.; Harms, H.K.; Hodson, M.E.; Mastella, G.; McKenzie, S.G.; Navarro, J.; Strandvik, B. Investigators of the European Epidemiologic Registry of Cystic Fibrosis Presence of Cystic Fibrosis-Related Diabetes Mellitus Is Tightly Linked to Poor Lung Function in Patients with Cystic Fibrosis: Data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr. Pulmonol. 2001, 32, 343–350. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Mathiesen, E.R.; Eriksen, V.; Skov, M.; Nielsen, K.G.; Johannesen, J.; Pressler, T. The Development of Diabetes among Danish Cystic Fibrosis Patients over the Last Two Decades. Pediatr. Diabetes 2015, 16, 219–226. [Google Scholar] [CrossRef]
- Koch, C.; Cuppens, H.; Rainisio, M.; Madessani, U.; Harms, H.; Hodson, M.; Mastella, G.; Navarro, J.; Strandvik, B.; McKenzie, S.; et al. European Epidemiologic Registry of Cystic Fibrosis (ERCF): Comparison of Major Disease Manifestations between Patients with Different Classes of Mutations. Pediatr. Pulmonol. 2001, 31, 1–12. [Google Scholar] [CrossRef]
- Preumont, V.; Hermans, M.P.; Lebecque, P.; Buysschaert, M. Glucose Homeostasis and Genotype-Phenotype Interplay in Cystic Fibrosis Patients with CFTR Gene DeltaF508 Mutation. Diabetes Care 2007, 30, 1187–1192. [Google Scholar] [CrossRef]
- De Boeck, K.; Zolin, A.; Cuppens, H.; Olesen, H.V.; Viviani, L. The Relative Frequency of CFTR Mutation Classes in European Patients with Cystic Fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2014, 13, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Ikpa, P.T.; Bijvelds, M.J.C.; De Jonge, H.R. Cystic Fibrosis: Toward Personalized Therapies. Int. J. Biochem. Cell Biol. 2014, 52, 192–200. [Google Scholar] [CrossRef]
- Bell, S.C.; De Boeck, K.; Amaral, M.D. New Pharmacological Approaches for Cystic Fibrosis: Promises, Progress, Pitfalls. Pharmacol. Ther. 2015, 145, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.A.; Doe, S.J. A New Era in the Treatment of Cystic Fibrosis. Clin. Med. Lond. Engl. 2014, 14, 76–78. [Google Scholar] [CrossRef]
- Norris, A.W. Is Cystic Fibrosis-Related Diabetes Reversible? New Data on CFTR Potentiation and Insulin Secretion. Am. J. Respir. Crit. Care Med. 2019, 199, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.G.; Zhong, P.; Zheng, W.; Beekman, J.M. Pharmacological Analysis of CFTR Variants of Cystic Fibrosis Using Stem Cell-Derived Organoids. Drug Discov. Today 2019, 24, 2126–2138. [Google Scholar] [CrossRef]
- Amato, F.; Scudieri, P.; Musante, I.; Tomati, V.; Caci, E.; Comegna, M.; Maietta, S.; Manzoni, F.; Di Lullo, A.M.; De Wachter, E.; et al. Two CFTR Mutations within Codon 970 Differently Impact on the Chloride Channel Functionality. Hum. Mutat. 2019, 40, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Blackman, S.M.; Hsu, S.; Ritter, S.E.; Naughton, K.M.; Wright, F.A.; Drumm, M.L.; Knowles, M.R.; Cutting, G.R. A Susceptibility Gene for Type 2 Diabetes Confers Substantial Risk for Diabetes Complicating Cystic Fibrosis. Diabetologia 2009, 52, 1858–1865. [Google Scholar] [CrossRef]
- Shu, L.; Sauter, N.S.; Schulthess, F.T.; Matveyenko, A.V.; Oberholzer, J.; Maedler, K. Statement of Retraction. Transcription Factor 7-Like 2 Regulates β-Cell Survival and Function in Human Pancreatic Islets. Diabetes 2017, 66, 1729–1730. [Google Scholar] [CrossRef]
- Da Silva Xavier, G.; Loder, M.K.; McDonald, A.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; Barg, S.; Rutter, G.A. TCF7L2 Regulates Late Events in Insulin Secretion from Pancreatic Islet Beta-Cells. Diabetes 2009, 58, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Park, S.-Y.; Su, J.; Bailey, K.; Ottosson-Laakso, E.; Shcherbina, L.; Oskolkov, N.; Zhang, E.; Thevenin, T.; Fadista, J.; et al. TCF7L2 Is a Master Regulator of Insulin Production and Processing. Hum. Mol. Genet. 2014, 23, 6419–6431. [Google Scholar] [CrossRef] [PubMed]
- Jin, T. Current Understanding on Role of the Wnt Signaling Pathway Effector TCF7L2 in Glucose Homeostasis. Endocr. Rev. 2016, 37, 254–277. [Google Scholar] [CrossRef]
- Lyssenko, V.; Lupi, R.; Marchetti, P.; Del Guerra, S.; Orho-Melander, M.; Almgren, P.; Sjögren, M.; Ling, C.; Eriksson, K.-F.; Lethagen, A.-L.; et al. Mechanisms by Which Common Variants in the TCF7L2 Gene Increase Risk of Type 2 Diabetes. J. Clin. Investig. 2007, 117, 2155–2163. [Google Scholar] [CrossRef]
- Shah, M.; Varghese, R.T.; Miles, J.M.; Piccinini, F.; Dalla Man, C.; Cobelli, C.; Bailey, K.R.; Rizza, R.A.; Vella, A. TCF7L2 Genotype and α-Cell Function in Humans Without Diabetes. Diabetes 2016, 65, 371–380. [Google Scholar] [CrossRef]
- Redondo, M.J.; Geyer, S.; Steck, A.K.; Sosenko, J.; Anderson, M.; Antinozzi, P.; Michels, A.; Wentworth, J.; Xu, P.; Pugliese, A.; et al. TCF7L2 Genetic Variants Contribute to Phenotypic Heterogeneity of Type 1 Diabetes. Diabetes Care 2018, 41, 311–317. [Google Scholar] [CrossRef]
- Xia, Q.; Chesi, A.; Manduchi, E.; Johnston, B.T.; Lu, S.; Leonard, M.E.; Parlin, U.W.; Rappaport, E.F.; Huang, P.; Wells, A.D.; et al. The Type 2 Diabetes Presumed Causal Variant within TCF7L2 Resides in an Element That Controls the Expression of ACSL5. Diabetologia 2016, 59, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Van de Bunt, M. An Alternative Effector Gene at the Type 2 Diabetes-Associated TCF7L2 Locus? Diabetologia 2016, 59, 2292–2294. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, K.J.; Ferreira, T.; Lee, Y.; Raimondo, A.; Mägi, R.; Reschen, M.E.; Mahajan, A.; Locke, A.; Rayner, N.W.; Robertson, N.; et al. Genetic Fine Mapping and Genomic Annotation Defines Causal Mechanisms at Type 2 Diabetes Susceptibility Loci. Nat. Genet. 2015, 47, 1415–1425. [Google Scholar] [CrossRef]
- Gaulton, K.J.; Nammo, T.; Pasquali, L.; Simon, J.M.; Giresi, P.G.; Fogarty, M.P.; Panhuis, T.M.; Mieczkowski, P.; Secchi, A.; Bosco, D.; et al. A Map of Open Chromatin in Human Pancreatic Islets. Nat. Genet. 2010, 42, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Blackman, S.M.; Commander, C.W.; Watson, C.; Arcara, K.M.; Strug, L.J.; Stonebraker, J.R.; Wright, F.A.; Rommens, J.M.; Sun, L.; Pace, R.G.; et al. Genetic Modifiers of Cystic Fibrosis-Related Diabetes. Diabetes 2013, 62, 3627–3635. [Google Scholar] [CrossRef]
- Wei, F.-Y.; Suzuki, T.; Watanabe, S.; Kimura, S.; Kaitsuka, T.; Fujimura, A.; Matsui, H.; Atta, M.; Michiue, H.; Fontecave, M.; et al. Deficit of TRNA(Lys) Modification by Cdkal1 Causes the Development of Type 2 Diabetes in Mice. J. Clin. Investig. 2011, 121, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Laybutt, D.R.; Preston, A.M.; Akerfeldt, M.C.; Kench, J.G.; Busch, A.K.; Biankin, A.V.; Biden, T.J. Endoplasmic Reticulum Stress Contributes to Beta Cell Apoptosis in Type 2 Diabetes. Diabetologia 2007, 50, 752–763. [Google Scholar] [CrossRef]
- Kong, Y.; Sharma, R.B.; Ly, S.; Stamateris, R.E.; Jesdale, W.M.; Alonso, L.C. CDKN2A/B T2D Genome-Wide Association Study Risk SNPs Impact Locus Gene Expression and Proliferation in Human Islets. Diabetes 2018, 67, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Sharma, R.B.; Nwosu, B.U.; Alonso, L.C. Islet Biology, the CDKN2A/B Locus and Type 2 Diabetes Risk. Diabetologia 2016, 59, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.; Kolte, A.M.; Hansen, T.V.O.; Nielsen, F.C. IGF2 MRNA-Binding Protein 2: Biological Function and Putative Role in Type 2 Diabetes. J. Mol. Endocrinol. 2009, 43, 187–195. [Google Scholar] [CrossRef]
- Groenewoud, M.J.; Dekker, J.M.; Fritsche, A.; Reiling, E.; Nijpels, G.; Heine, R.J.; Maassen, J.A.; Machicao, F.; Schäfer, S.A.; Häring, H.U.; et al. Variants of CDKAL1 and IGF2BP2 Affect First-Phase Insulin Secretion during Hyperglycaemic Clamps. Diabetologia 2008, 51, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Balázs, A.; Mall, M.A. Role of the SLC26A9 Chloride Channel as Disease Modifier and Potential Therapeutic Target in Cystic Fibrosis. Front. Pharmacol. 2018, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.V.N.; Ribeiro, J.D.; Bertuzzo, C.S.; Marson, F.A.L. Interaction among Variants in the SLC Gene Family (SLC6A14, SLC26A9, SLC11A1, and SLC9A3) and CFTR Mutations with Clinical Markers of Cystic Fibrosis. Pediatr. Pulmonol. 2018, 53, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.-T.N.; Aksit, M.A.; Vecchio-Pagan, B.; Shelton, C.A.; Osorio, D.L.; Anzmann, A.F.; Goff, L.A.; Whitcomb, D.C.; Blackman, S.M.; Cutting, G.R. Increased Expression of Anion Transporter SLC26A9 Delays Diabetes Onset in Cystic Fibrosis. J. Clin. Investig. 2020, 130, 272–286. [Google Scholar] [CrossRef]
- Long, J.Z.; Svensson, K.J.; Bateman, L.A.; Lin, H.; Kamenecka, T.; Lokurkar, I.A.; Lou, J.; Rao, R.R.; Chang, M.R.; Jedrychowski, M.P.; et al. The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria. Cell 2016, 166, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Aksit, M.A.; Pace, R.G.; Vecchio-Pagán, B.; Ling, H.; Rommens, J.M.; Boelle, P.-Y.; Guillot, L.; Raraigh, K.S.; Pugh, E.; Zhang, P.; et al. Genetic Modifiers of Cystic Fibrosis-Related Diabetes Have Extensive Overlap With Type 2 Diabetes and Related Traits. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Mosoian, A.; Teixeira, A.; Burns, C.S.; Sander, L.E.; Gusella, G.L.; He, C.; Blander, J.M.; Klotman, P.; Klotman, M.E. Prothymosin-Alpha Inhibits HIV-1 via Toll-like Receptor 4-Mediated Type I Interferon Induction. Proc. Natl. Acad. Sci. USA 2010, 107, 10178–10183. [Google Scholar] [CrossRef]
- Su, Y.-C.; Ou, H.-Y.; Wu, H.-T.; Wu, P.; Chen, Y.-C.; Su, B.-H.; Shiau, A.-L.; Chang, C.-J.; Wu, C.-L. Prothymosin-α Overexpression Contributes to the Development of Insulin Resistance. J. Clin. Endocrinol. Metab. 2015, 100, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Hodson, D.J.; Mitchell, R.K.; Marselli, L.; Pullen, T.J.; Gimeno Brias, S.; Semplici, F.; Everett, K.L.; Cooper, D.M.F.; Bugliani, M.; Marchetti, P.; et al. ADCY5 Couples Glucose to Insulin Secretion in Human Islets. Diabetes 2014, 63, 3009–3021. [Google Scholar] [CrossRef]
- Imamura, M.; Maeda, S.; Yamauchi, T.; Hara, K.; Yasuda, K.; Morizono, T.; Takahashi, A.; Horikoshi, M.; Nakamura, M.; Fujita, H.; et al. A Single-Nucleotide Polymorphism in ANK1 Is Associated with Susceptibility to Type 2 Diabetes in Japanese Populations. Hum. Mol. Genet. 2012, 21, 3042–3049. [Google Scholar] [CrossRef]
- Tikhmyanova, N.; Little, J.L.; Golemis, E.A. Cas Proteins in Normal and Pathological Cell Growth Control. Cell. Mol. Life Sci. CMLS 2010, 67, 1025–1048. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Chang, W.-C.; Wang, J.-M. Biological Roles of CCAAT/Enhancer-Binding Protein Delta during Inflammation. J. Biomed. Sci. 2015, 22, 1–8. [Google Scholar] [CrossRef]
- Cai, J.; Abramovici, H.; Gee, S.H.; Topham, M.K. Diacylglycerol Kinases as Sources of Phosphatidic Acid. Biochim. Biophys. Acta 2009, 1791, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.; Garrett-Sinha, L.A. Transcription Factor Ets-1 in Cytokine and Chemokine Gene Regulation. Cytokine 2010, 51, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; ZeRuth, G.; Lichti-Kaiser, K.; Vasanth, S.; Yin, Z.; Kim, Y.-S.; Jetten, A.M. Gli-Similar (Glis) Krüppel-like Zinc Finger Proteins: Insights into Their Physiological Functions and Critical Roles in Neonatal Diabetes and Cystic Renal Disease. Histol. Histopathol. 2010, 25, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.H.; Stoica, G.E.; Riegel, A.T.; Wellstein, A. Recruitment of Insulin Receptor Substrate-1 and Activation of NF-KappaB Essential for Midkine Growth Signaling through Anaplastic Lymphoma Kinase. Oncogene 2007, 26, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Hanspal, M.; Smockova, Y.; Uong, Q. Molecular Identification and Functional Characterization of a Novel Protein That Mediates the Attachment of Erythroblasts to Macrophages. Blood 1998, 92, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Grozdanov, P.N.; Roy, S.; Kittur, N.; Meier, U.T. SHQ1 Is Required Prior to NAF1 for Assembly of H/ACA Small Nucleolar and Telomerase RNPs. RNA N. Y. 2009, 15, 1188–1197. [Google Scholar] [CrossRef]
- Sharari, S.; Abou-Alloul, M.; Hussain, K.; Ahmad Khan, F. Fanconi-Bickel Syndrome: A Review of the Mechanisms That Lead to Dysglycaemia. Int. J. Mol. Sci. 2020, 21, 6286. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Lu, X.-Z.; Wang, H.; Yang, X.-X.; Geng, H.-Y.; Gong, G.; Zhan, Y.-Y.; Kim, H.J.; Yang, Z.-J. Solute Carrier Family 30 Member 8 Gene 807C/T Polymorphism and Type 2 Diabetes Mellitus in the Chinese Population: A Meta-Analysis Including 6,942 Subjects. Front. Endocrinol. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Johansen, H.K.; Carmi, P.; Høiby, N.; Cohen, I.R. Autoantibodies to Pancreatic Hsp60 Precede the Development of Glucose Intolerance in Patients with Cystic Fibrosis. J. Autoimmun. 2001, 17, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Derbel, S.; Doumaguet, C.; Hubert, D.; Mosnier-Pudar, H.; Grabar, S.; Chelly, J.; Bienvenu, T. Calpain 10 and Development of Diabetes Mellitus in Cystic Fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2006, 5, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.-W.; Bhatt, J.M.; Denvir, L.; Randell, T.; Sachdev, P. Monogenic Diabetes Mellitus in Cystic Fibrosis. Arch. Dis. Child. 2019, 104, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Hangül, M.; Erdoğan, M.; Hatipoğlu, N.; Köse, M. Maturity-Onset Diabetes of the Young: Different Diabetes in an Infant with Cystic Fibrosis. Pediatr. Pulmonol. 2020, 55, E5–E7. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Passanisi, S.; Lucanto, M.C.; Costa, S.; Pajno, G.B.; Lombardo, F. GCK-MODY in a Child with Cystic Fibrosis: The Doubt of the Treatment Plan. J. Pediatr. Endocrinol. Metab. 2020, 33, 1359–1362. [Google Scholar] [CrossRef]
- Tinto, N.; Zagari, A.; Capuano, M.; De Simone, A.; Capobianco, V.; Daniele, G.; Giugliano, M.; Spadaro, R.; Franzese, A.; Sacchetti, L. Glucokinase Gene Mutations: Structural and Genotype-Phenotype Analyses in MODY Children from South Italy. PLoS ONE 2008, 3, e1870. [Google Scholar] [CrossRef] [PubMed]
- Capuano, M.; Garcia-Herrero, C.M.; Tinto, N.; Carluccio, C.; Capobianco, V.; Coto, I.; Cola, A.; Iafusco, D.; Franzese, A.; Zagari, A.; et al. Glucokinase (GCK) Mutations and Their Characterization in MODY2 Children of Southern Italy. PLoS ONE 2012, 7, e38906. [Google Scholar] [CrossRef]
- Delvecchio, M.; Mozzillo, E.; Salzano, G.; Iafusco, D.; Frontino, G.; Patera, P.I.; Rabbone, I.; Cherubini, V.; Grasso, V.; Tinto, N.; et al. Monogenic Diabetes Accounts for 6.3% of Cases Referred to 15 Italian Pediatric Diabetes Centers During 2007 to 2012. J. Clin. Endocrinol. Metab. 2017, 102, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, M.; Salzano, G.; Bonura, C.; Cauvin, V.; Cherubini, V.; d’Annunzio, G.; Franzese, A.; Giglio, S.; Grasso, V.; Graziani, V.; et al. Can HbA1c Combined with Fasting Plasma Glucose Help to Assess Priority for GCK-MODY vs HNF1A-MODY Genetic Testing? Acta Diabetol. 2018, 55, 981–983. [Google Scholar] [CrossRef] [PubMed]
| Type 1 Diabetes | Type 2 Diabetes | CFRD | |
|---|---|---|---|
| Prevalence in CF | 0.2% | 11% | 35% |
| Onset | Acute | Insidious | Insidious |
| Age of onset | Children, adolescents | Adults | 18–24 years |
| Weight | Normal | Obesity/overweight | Normal/underweight |
| Autoimmune pathogenesis | Yes | No | No |
| Insulin deficiency | Almost total | Partial | Severe but not total |
| Insulin sensitivity | Normal/slightly decreased | Severely decreased | Variably decreased (>acute phases) |
| Ketones | Yes | Rare | Rare |
| Therapy | Insulin | Diet Oral hypoglycemic agents Insulin | Insulin |
| Microvascular complications | Yes | Yes | Yes |
| Macrovascular complications | Yes | Yes | No |
| Metabolic syndrome | No | Yes | No |
| Cause of death | Cardiovascular | Cardiovascular | Pulmonary |
| Fasting | 2 h Glucose | Notes | |||
|---|---|---|---|---|---|
| mg/dL | mmol/L | mg/dL | mmol/L | ||
| Normal glucose tolerance (NGT) | <126 | <7 | <140 | <7.8 | All glucose levels <200 mg/dL (11.1 mmol/L) |
| Indeterminate glycemia (INDET) | <126 | <7 | <140 | <7.8 | Mid-OGTT glucose ≥200 mg/dL (11.1 mmol/L) |
| Impaired glucose tolerance (IGT) | <126 | <7 | 140–199 | 7.8–11 | |
| CFRD with fasting hyperglycemia | ≥126 | ≥7 | ≥200 | ≥11.1 | |
| CFRD without fasting hyperglycemia | <126 | <7 | ≥200 | ≥11.1 | |
| Symbols | Gene | Locations | Protein (https://www.genecards.org/ (Accessed on 3 November 2020) |
|---|---|---|---|
| ADCY5 | Adenylate cyclase 5 | Chr 3 | Component of the membrane-bound adenylyl cyclase enzymes. Regulates the increase in free cytosolic Ca2+ in response to increased blood glucose levels, contributing to insulin secretion [124]. |
| ANK1 | Ankyrin 1 | Chr 8 | Ankyrins play key roles in cell motility, activation, proliferation, contact, and maintenance of specialized membrane domains [125]. |
| BCAR1 | BCAR1 scaffold Protein, Cas family member | Chr 16 | Component of the Crk-associated substrate (Cas) family of scaffold proteins, involved in several cellular pathways, including cell motility, apoptosis, and cell-cycle control [126]. |
| CEBPB | CCAAT enhancer-binding protein beta | Chr 20 | Transcription factor that regulate genes involved in immune and inflammatory responses [127]. |
| DGKB | Diacylglycerol kinase beta | Chr 7 | Diacylglycerol kinases metabolize 1,2,diacylglycerol to produce phosphatidic acid, important for cellular processes [128]. |
| ETS1 | ETS proto-oncogene 1, transcription factor | Chr 11 | Component of the ETS family of transcription factors that controls the expression of cytokine and chemokine genes, the differentiation, survival, and proliferation of lymphoid cells, and angiogenesis [129]. |
| GLIS3 | GLIS family zinc finger 3 | Chr 9 | Component of the GLI-similar zinc finger protein family involved in the development of pancreatic beta-cells, thyroid, eye, liver, and kidney [130]. |
| LTK | Leukocyte receptor tyrosine kinase | Chr 15 | Component of the ros/insulin receptor family of tyrosine kinases, very important for cell growth and differentiation [131]. |
| MAEA | Macrophage erythroblast attacher, E3 ubiquitin ligase | Chr 4 | Protein that mediates the attachment of erythroblasts to macrophages. It is required for normal cell proliferation and may contribute to nuclear architecture and cell division events [132]. |
| SHQ1 | SHQ1, H/ACA ribonucleoprotein assembly factor | Chr 3 | Protein that assists in the assembly of H/ACA-box ribonucleoproteins involved in the processing of ribosomal RNAs, modification of spliceosomal small nuclear RNAs, and stabilization of telomerase [133]. |
| SLC2A2 | Solute carrier family 2 member 2 | Chr 3 | Integral plasma membrane glycoprotein important for the bidirectional glucose transfer across the plasma membrane of hepatocytes, beta-cells, intestine, and kidney epithelium [134]. |
| SLC30A8 | Solute carrier family 30 member 8 | Chr 8 | Zinc efflux transporter expressed at a high level only in the pancreas, involved in insulin maturation and/or in insulin secretion [135]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iafusco, F.; Maione, G.; Rosanio, F.M.; Mozzillo, E.; Franzese, A.; Tinto, N. Cystic Fibrosis-Related Diabetes (CFRD): Overview of Associated Genetic Factors. Diagnostics 2021, 11, 572. https://doi.org/10.3390/diagnostics11030572
Iafusco F, Maione G, Rosanio FM, Mozzillo E, Franzese A, Tinto N. Cystic Fibrosis-Related Diabetes (CFRD): Overview of Associated Genetic Factors. Diagnostics. 2021; 11(3):572. https://doi.org/10.3390/diagnostics11030572
Chicago/Turabian StyleIafusco, Fernanda, Giovanna Maione, Francesco Maria Rosanio, Enza Mozzillo, Adriana Franzese, and Nadia Tinto. 2021. "Cystic Fibrosis-Related Diabetes (CFRD): Overview of Associated Genetic Factors" Diagnostics 11, no. 3: 572. https://doi.org/10.3390/diagnostics11030572
APA StyleIafusco, F., Maione, G., Rosanio, F. M., Mozzillo, E., Franzese, A., & Tinto, N. (2021). Cystic Fibrosis-Related Diabetes (CFRD): Overview of Associated Genetic Factors. Diagnostics, 11(3), 572. https://doi.org/10.3390/diagnostics11030572







