Aortic Annular Sizing Using Novel Software in Three-Dimensional Transesophageal Echocardiography for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
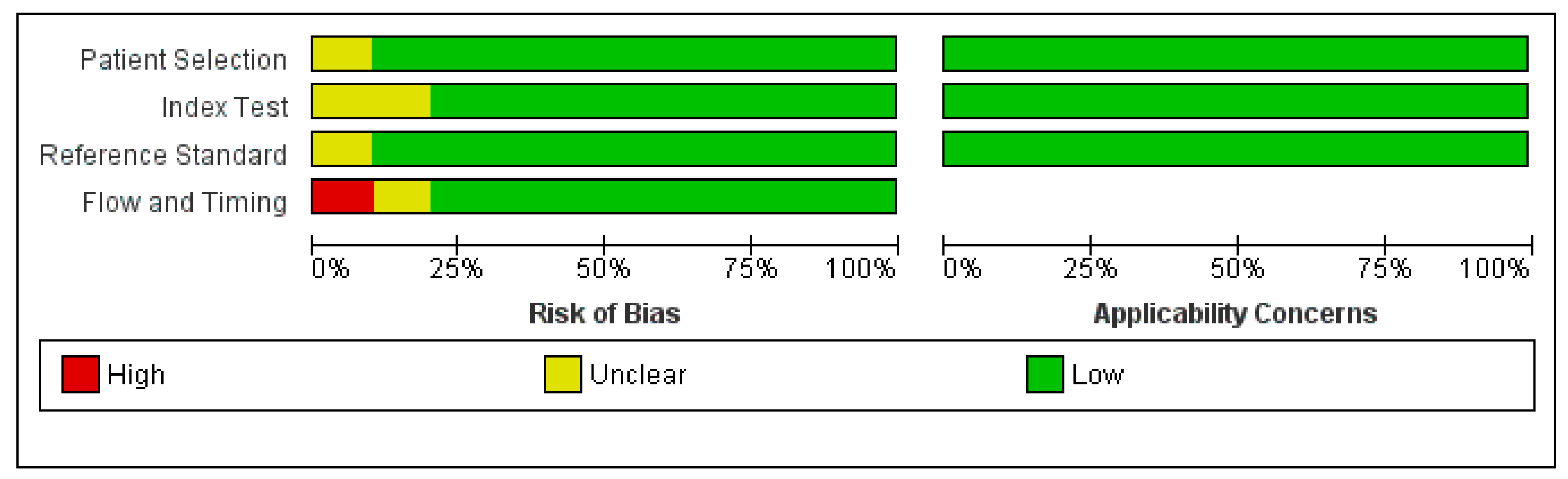
2.4. Quality Assessment
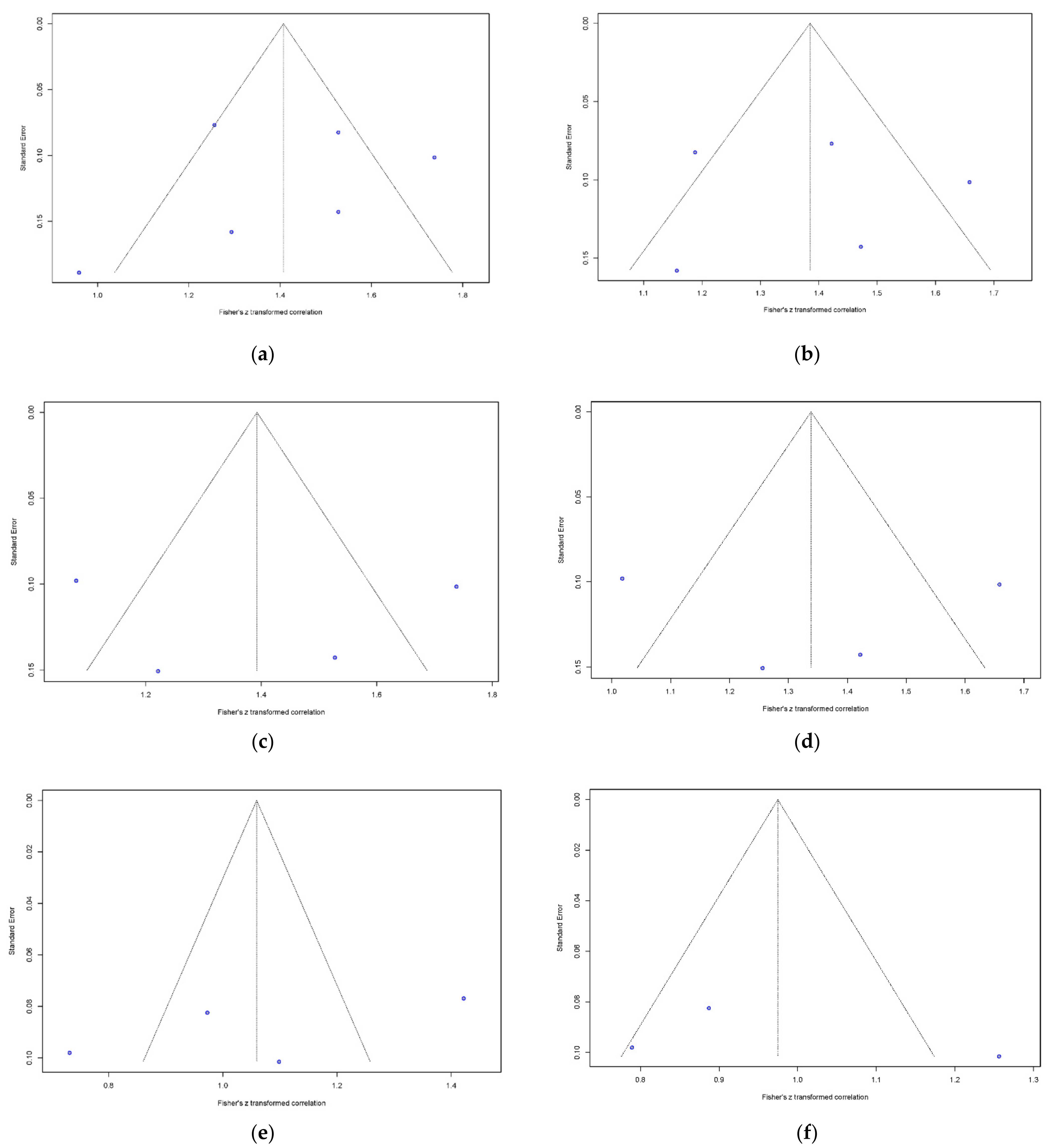
2.5. Data Synthesis and Analyses
3. Results
3.1. Study Characteristics
3.2. Quality Assessment
3.3. Correlation between 3D-TEE and MDCT for Annulus Measurements
3.4. Mean Difference between 3D-TEE and MDCT for Annulus Measurements
3.5. Meta-Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Kolte, D.; Vlahakes, G.J.; Palacios, I.F.; Sakhuja, R.; Passeri, J.J.; Inglessis, I.; Elmariah, S. Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J. Am. Coll. Cardiol. 2019, 74, 1532–1540. [Google Scholar] [CrossRef]
- Polimeni, A.; Sorrentino, S.; De Rosa, S.; Spaccarotella, C.; Mongiardo, A.; Sabatino, J.; Indolfi, C. Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients for the Treatment of Severe Aortic Stenosis. J. Clin. Med. 2020, 9, 439. [Google Scholar] [CrossRef] [Green Version]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2020. [Google Scholar] [CrossRef]
- Vahanian, A.; Alfieri, O.R.; Al-Attar, N.; Antunes, M.J.; Bax, J.; Cormier, B.; Cribier, A.; De Jaegere, P.; Fournial, G.; Kappetein, A.P.; et al. Transcatheter valve implantation for patients with aortic stenosis: A position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. J. Cardiothorac. Surg. 2008, 34, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messika-Zeitoun, D.; Serfaty, J.M.; Brochet, E.; Ducrocq, G.; Lepage, L.; Detaint, D.; Hyafil, F.; Himbert, D.; Pasi, N.; Laissy, J.P.; et al. Multimodal assessment of the aortic annulus diameter: Implications for transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2010, 55, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, G.S.; Gillam, L.D.; Hahn, R.T.; Kapadia, S.; Leipsic, J.; Lerakis, S.; Tuzcu, M.; Douglas, P.S. A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc. Imaging 2012, 5, 441–455. [Google Scholar] [CrossRef] [Green Version]
- Bax, J.J.; Delgado, V.; Hahn, R.T.; Leipsic, J.; Min, J.K.; Grayburn, P.; Sondergaard, L.; Yoon, S.H.; Windecker, S. Transcatheter Aortic Valve Replacement: Role of Multimodality Imaging in Common and Complex Clinical Scenarios. JACC Cardiovasc. Imaging 2020, 13, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Webb, J.G.; Blanke, P.; Dvir, D.; Hansson, N.C.; Nørgaard, B.L.; Thompson, C.R.; Thomas, M.; Wendler, O.; Vahanian, A.; et al. Incidence and severity of paravalvular aortic regurgitation with multidetector computed tomography nominal area oversizing or undersizing after transcatheter heart valve replacement with the Sapien 3: A comparison with the Sapien XT. JACC Cardiovasc. Interv. 2015, 8, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc. Interv. 2015, 8, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Blanke, P.; Reinöhl, J.; Schlensak, C.; Siepe, M.; Pache, G.; Euringer, W.; Geibel-Zehender, A.; Bode, C.; Langer, M.; Beyersdorf, F.; et al. Prosthesis oversizing in balloon-expandable transcatheter aortic valve implantation is associated with contained rupture of the aortic root. Circ. Cardiovasc. Interv. 2012, 5, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pibarot, P.; Magne, J.; Leipsic, J.; Côté, N.; Blanke, P.; Thourani, V.H.; Hahn, R. Imaging for Predicting and Assessing Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 149–162. [Google Scholar] [CrossRef]
- Achenbach, S.; Delgado, V.; Hausleiter, J.; Schoenhagen, P.; Min, J.K.; Leipsic, J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 2012, 6, 366–380. [Google Scholar] [CrossRef]
- Willson, A.B.; Webb, J.G.; Freeman, M.; Wood, D.A.; Gurvitch, R.; Thompson, C.R.; Moss, R.R.; Toggweiler, S.; Binder, R.K.; Munt, B.; et al. Computed tomography-based sizing recommendations for transcatheter aortic valve replacement with balloon-expandable valves: Comparison with transesophageal echocardiography and rationale for implementation in a prospective trial. J. Cardiovasc. Comput. Tomogr. 2012, 6, 406–414. [Google Scholar] [CrossRef]
- Otto, C.M.; Kumbhani, D.J.; Alexander, K.P.; Calhoon, J.H.; Desai, M.Y.; Kaul, S.; Lee, J.C.; Ruiz, C.E.; Vassileva, C.M. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults with Aortic Stenosis: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2017, 69, 1313–1346. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Norgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2019, 13, 1–20. [Google Scholar] [CrossRef]
- Sinning, J.M.; Ghanem, A.; Steinhäuser, H.; Adenauer, V.; Hammerstingl, C.; Nickenig, G.; Werner, N. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2010, 3, 1141–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, T.; Goel, K.; Kolte, D.; Khera, S.; Villablanca, P.A.; Aronow, W.S.; Bortnick, A.E.; Slovut, D.P.; Taub, C.C.; Kizer, J.R.; et al. Association of Chronic Kidney Disease with In-Hospital Outcomes of Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 2050–2060. [Google Scholar] [CrossRef]
- Jochheim, D.; Schneider, V.S.; Schwarz, F.; Kupatt, C.; Lange, P.; Reiser, M.; Massberg, S.; Gutiérrez-Chico, J.L.; Mehilli, J.; Becker, H.C. Contrast-induced acute kidney injury after computed tomography prior to transcatheter aortic valve implantation. Clin. Radiol. 2014, 69, 1034–1038. [Google Scholar] [CrossRef]
- Dima, C.N.; Gaspar, M.; Mornos, C.; Mornos, A.; Deutsch, P.; Cioloca, H.; Cerbu, S.; Dinu, M.; Hoinoiu, B.; Luca, C.T.; et al. Three-Dimensional Transesophageal Echocardiography as an Alternative to Multidetector Computed Tomography in Aortic Annular Diameter Measurements for Transcatheter Aortic Valve Implantation. Biology 2021, 10, 132. [Google Scholar] [CrossRef]
- Ebuchi, K.; Yoshitani, K.; Kanemaru, E.; Fujii, T.; Tsukinaga, A.; Shimahara, Y.; Ohnishi, Y. Measurement of the Aortic Annulus Area and Diameter by Three-Dimensional Transesophageal Echocardiography in Transcatheter Aortic Valve Replacement. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Islas, F.; Almería, C.; García-Fernández, E.; Jiménez, P.; Nombela-Franco, L.; Olmos, C.; Marcos-Alberca, P.; Cuadrado, A.; Fernández-Ortiz, A.; Macaya, C.; et al. Usefulness of echocardiographic criteria for transcatheter aortic valve implantation without balloon predilation: A single-center experience. J. Am. Soc. Echocardiogr. 2015, 28, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Kaku, K.; Takeuchi, M.; Otani, K.; Yoshitani, H.; Tamura, M.; Abe, H.; Lin, F.C.; Otsuji, Y. Aortic root geometry in patients with aortic stenosis assessed by real-time three-dimensional transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 32–41. [Google Scholar] [CrossRef]
- Hahn, R.T.; Little, S.H.; Monaghan, M.J.; Kodali, S.K.; Williams, M.; Leon, M.B.; Gillam, L.D. Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVR. JACC Cardiovasc. Imaging 2015, 8, 261–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calleja, A.; Thavendiranathan, P.; Ionasec, R.I.; Houle, H.; Liu, S.; Voigt, I.; Sai Sudhakar, C.; Crestanello, J.; Ryan, T.; Vannan, M.A. Automated quantitative 3-dimensional modeling of the aortic valve and root by 3-dimensional transesophageal echocardiography in normals, aortic regurgitation, and aortic stenosis: Comparison to computed tomography in normals and clinical implications. Circ. Cardiovasc. Imaging 2013, 6, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Khalique, O.K.; Hamid, N.B.; White, J.M.; Bae, D.J.; Kodali, S.K.; Nazif, T.M.; Vahl, T.P.; Paradis, J.M.; George, I.; Leon, M.B.; et al. Impact of Methodologic Differences in Three-Dimensional Echocardiographic Measurements of the Aortic Annulus Compared with Computed Tomographic Angiography Before Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2017, 30, 414–421. [Google Scholar] [CrossRef]
- Queirós, S.; Morais, P.; Fehske, W.; Papachristidis, A.; Voigt, J.U.; Fonseca, J.C.; D’Hooge, J.; Vilaça, J.L. Assessment of aortic valve tract dynamics using automatic tracking of 3D transesophageal echocardiographic images. Int. J. Cardiovasc. Imaging 2019, 35, 881–895. [Google Scholar] [CrossRef]
- Zhang, M.; Wan, L.; Liu, K.; Wu, W.; Li, H.; Wang, Y.; Lu, B.; Wang, H. Aortic roots assessment by an automated three-dimensional transesophageal echocardiography: An intra-individual comparison. Int. J. Cardiovasc. Imaging 2019, 35, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Khalique, O.K.; Kodali, S.K.; Paradis, J.M.; Nazif, T.M.; Williams, M.R.; Einstein, A.J.; Pearson, G.D.; Harjai, K.; Grubb, K.; George, I.; et al. Aortic annular sizing using a novel 3-dimensional echocardiographic method: Use and comparison with cardiac computed tomography. Circ. Cardiovasc. Imaging 2014, 7, 155–163. [Google Scholar] [CrossRef] [Green Version]
- García-Martín, A.; Lázaro-Rivera, C.; Fernández-Golfín, C.; Salido-Tahoces, L.; Moya-Mur, J.L.; Jiménez-Nacher, J.J.; Casas-Rojo, E.; Aquila, I.; González-Gómez, A.; Hernández-Antolín, R.; et al. Accuracy and reproducibility of novel echocardiographic three-dimensional automated software for the assessment of the aortic root in candidates for thanscatheter aortic valve replacement. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 772–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mediratta, A.; Addetia, K.; Medvedofsky, D.; Schneider, R.J.; Kruse, E.; Shah, A.P.; Nathan, S.; Paul, J.D.; Blair, J.E.; Ota, T.; et al. 3D echocardiographic analysis of aortic annulus for transcatheter aortic valve replacement using novel aortic valve quantification software: Comparison with computed tomography. Echocardiography 2017, 34, 690–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, N.; Shibayama, K.; Noguchi, M.; Makihara, Y.; Okumura, H.; Obunai, K.; Isobe, M.; Hirao, K.; Watanabe, H. Superiority of novel automated assessment of aortic annulus by intraoperative three-dimensional transesophageal echocardiography in patients with severe aortic stenosis: Comparison with conventional cross-sectional assessment. J. Cardiol. 2018, 72, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Podlesnikar, T.; Prihadi, E.A.; van Rosendael, P.J.; Vollema, E.M.; van der Kley, F.; de Weger, A.; Ajmone Marsan, N.; Naji, F.; Fras, Z.; Bax, J.J.; et al. Influence of the Quantity of Aortic Valve Calcium on the Agreement Between Automated 3-Dimensional Transesophageal Echocardiography and Multidetector Row Computed Tomography for Aortic Annulus Sizing. Am. J. Cardiol. 2018, 121, 86–93. [Google Scholar] [CrossRef]
- Prihadi, E.A.; van Rosendael, P.J.; Vollema, E.M.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Feasibility, Accuracy, and Reproducibility of Aortic Annular and Root Sizing for Transcatheter Aortic Valve Replacement Using Novel Automated Three-Dimensional Echocardiographic Software: Comparison with Multi-Detector Row Computed Tomography. J. Am. Soc. Echocardiogr. 2018, 31, 505–514.e3. [Google Scholar] [CrossRef]
- Queirós, S.; Morais, P.; Dubois, C.; Voigt, J.U.; Fehske, W.; Kuhn, A.; Achenbach, T.; Fonseca, J.C.; Vilaça, J.L.; D’Hooge, J. Validation of a Novel Software Tool for Automatic Aortic Annular Sizing in Three-Dimensional Transesophageal Echocardiographic Images. J. Am. Soc. Echocardiogr. 2018, 31, 515–525.e5. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Italia, L.; Geremia, G.; Rosa, I.; Ancona, F.; Marini, C.; Capogrosso, C.; Giglio, M.; Montorfano, M.; Latib, A.; et al. Accuracy and reproducibility of aortic annular measurements obtained from echocardiographic 3D manual and semi-automated software analyses in patients referred for transcatheter aortic valve implantation: Implication for prosthesis size selection. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Ladeiras-Lopes, R.; Guerreiro, C.; Carvalho, M.; Fontes-Carvalho, R.; Braga, P.; Sampaio, F. Accuracy of three-dimensional echocardiography in candidates for transcatheter aortic valve replacement. Int. J. Cardiovasc. Imaging 2020, 36, 291–298. [Google Scholar] [CrossRef]
- Thalappillil, R.; Datta, P.; Datta, S.; Zhan, Y.; Wells, S.; Mahmood, F.; Cobey, F.C. Artificial Intelligence for the Measurement of the Aortic Valve Annulus. J. Cardiothorac. Vasc. Anesth. 2020, 34, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, N.; de Jaegere, P.; Schultz, C.; Becker, A.E.; Serruys, P.W.; Anderson, R.H. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ. Cardiovasc. Interv. 2008, 1, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Leipsic, J.; Gurvitch, R.; LaBounty, T.M.; Min, J.K.; Wood, D.; Johnson, M.; Ajlan, A.M.; Wijesinghe, N.; Webb, J.G. Multidetector Computed Tomography in Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Imaging 2011, 4, 416–429. [Google Scholar] [CrossRef] [Green Version]
- Barbanti, M.; Yang, T.H.; Rodès Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013, 128, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamorano, J.L.; Gonçalves, A.; Lang, R. Imaging to select and guide transcatheter aortic valve implantation. Eur. Heart J. 2014, 35, 1578–1587. [Google Scholar] [CrossRef]
- Ruile, P.; Blanke, P.; Krauss, T.; Dorfs, S.; Jung, B.; Jander, N.; Leipsic, J.; Langer, M.; Neumann, F.-J.; Pache, G. Pre-procedural assessment of aortic annulus dimensions for transcatheter aortic valve replacement: Comparison of a non-contrast 3D MRA protocol with contrast-enhanced cardiac dual-source CT angiography. Eur. Heart J. Cardiovasc. Imaging 2015, 17, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Binder, R.K.; Webb, J.G.; Willson, A.B.; Urena, M.; Hansson, N.C.; Norgaard, B.L.; Pibarot, P.; Barbanti, M.; Larose, E.; Freeman, M.; et al. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: A prospective, multicenter, controlled trial. J. Am. Coll. Cardiol. 2013, 62, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Pontone, G.; Andreini, D.; Bartorelli, A.L.; Bertella, E.; Mushtaq, S.; Gripari, P.; Loguercio, M.; Cortinovis, S.; Baggiano, A.; Conte, E.; et al. Comparison of accuracy of aortic root annulus assessment with cardiac magnetic resonance versus echocardiography and multidetector computed tomography in patients referred for transcatheter aortic valve implantation. Am. J. Cardiol. 2013, 112, 1790–1799. [Google Scholar] [CrossRef]
- Rogers, T.; Waksman, R. Role of CMR in TAVR. JACC Cardiovasc. Imaging 2016, 9, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Mach, M.; Hasan, W.; Andreas, M.; Winkler, B.; Weiss, G.; Adlbrecht, C.; Delle-Karth, G.; Grabenwöger, M. Evaluating the Association between Contrast Medium Dosage and Acute Kidney Injury in Transcatheter Aortic Valve Replacement Using Different Predictive Models. J. Clin. Med. 2020, 9, 3476. [Google Scholar] [CrossRef]
- Vaquerizo, B.; Spaziano, M.; Alali, J.; Mylote, D.; Theriault-Lauzier, P.; Alfagih, R.; Martucci, G.; Buithieu, J.; Piazza, N. Three-dimensional echocardiography vs. computed tomography for transcatheter aortic valve replacement sizing. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, A.M.; Medranda, G.A.; Kakouros, N.; Patel, J.; Kahan, J.; Gubernikoff, G.; Ray, B.; Paruchuri, V.; DeLeon, J.; Marzo, K.; et al. Is intra-procedure three-dimensional transesophageal echocardiogram an alternative to preprocedure multidetector computed tomography for the measurement of the aortic annulus in patients undergoing transcatheter aortic valve replacement? Echocardiography 2017, 34, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altiok, E.; Koos, R.; Schröder, J.; Brehmer, K.; Hamada, S.; Becker, M.; Mahnken, A.H.; Almalla, M.; Dohmen, G.; Autschbach, R.; et al. Comparison of two-dimensional and three-dimensional imaging techniques for measurement of aortic annulus diameters before transcatheter aortic valve implantation. Heart 2011, 97, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Wystub, N.; Bäz, L.; Möbius-Winkler, S.; Pörner, T.C.; Goebel, B.; Hamadanchi, A.; Doenst, T.; Grimm, J.; Lehmkuhl, L.; Teichgräber, U.; et al. Aortic annulus measurement with computed tomography angiography reduces aortic regurgitation after transfemoral aortic valve replacement compared to 3-D echocardiography: A single-centre experience. Clin. Res. Cardiol. 2019, 108, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Elkaryoni, A.; Nanda, N.C.; Baweja, P.; Arisha, M.J.; Zamir, H.; Elgebaly, A.; Altibi, A.M.; Sharma, R. Three-dimensional transesophageal echocardiography is an attractive alternative to cardiac multi-detector computed tomography for aortic annular sizing: Systematic review and meta-analysis. Echocardiography 2018, 35, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.Q.; Hameed, I.; Salemi, A.; Rahouma, M.; Khan, F.M.; Wijeysundera, H.C.; Angiolillo, D.J.; Shore-Lesserson, L.; Biondi-Zoccai, G.; Girardi, L.N.; et al. Three-Dimensional Echocardiography for Transcatheter Aortic Valve Replacement Sizing: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e013463. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Kashif, M.; Fontana, G.; Furugen, A.; Shiota, T.; Friede, G.; Makhija, R.; Doctor, N.; Leon, M.B.; Makkar, R.R. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J. Am. Coll. Cardiol. 2012, 59, 1275–1286. [Google Scholar] [CrossRef] [Green Version]
- Nolan, M.T.; Thavendiranathan, P. Automated Quantification in Echocardiography. JACC Cardiovasc. Imaging 2019, 12, 1073–1092. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kocyigit, D.; Grimm, R.; Griffin, B.P.; Cheng, F. Applications of artificial intelligence in multimodality cardiovascular imaging: A state-of-the-art review. Prog. Cardiovasc. Dis. 2020, 63, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Billick, K.; Horton, K.; Jankowski, M.; Knoll, P.; Marshall, J.E.; Paloma, A.; Palma, R.; Adams, D.B. Artificial Intelligence and Echocardiography: A Primer for Cardiac Sonographers. J. Am. Soc. Echocardiogr. 2020, 33, 1061–1066. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]





| Study/Year | Total No. of Patients | Study Design | Study Period | Hospital/Institution | Country |
|---|---|---|---|---|---|
| Thalappillil/2020 [46] | 47 | Retrospective | 1 January 2017 to 1 February 2019 | Tufts Medical Center | United States |
| Maia/2020 [45] | 107 | Retrospective | December 2015 to January 2017 | Centro Hospitalar de Gaia/Espinho | Portugal |
| Stella/2019 [44] | 175 | Retrospective | October 2014 to August 2016 | San Raffaele University Hospital | Italy |
| Queiros/2018 [43] | 101 | Retrospective | August 2014 to September 2017 | St. Vinzenz-Hospital (Cologne, Germany) | Germany |
| Prihadi/2018 [42] | 150 | Retrospective | NA | The Leiden University Medical Center | The Netherlands |
| Podlesnikar/2018 [41] | 83 | Retrospective | July 2015 to March 2017 | The Leiden University Medical Center | The Netherlands |
| Kato/2018 [40] | 43 | Retrospective | January to October 2016 | The Tokyo Bay Urayasu-Ichikawa Medical Center | Japan |
| Mediratta/2017 [39] | 52 | Retrospective | NA | University of Chicago Medical Center | United States |
| Garcia-Martin/2016 [38] | 31 | Retrospective | March 2012 to March 2014 | Ramo’n y Cajal University Hospital | Spain |
| Khalique/2014 [37] | 100 | Retrospective | November 2011 to January 2013 | Columbia University Medical Center/New York Presbyterian Hospital | United States |
| Study/Year | Sex (M/F) | Age (Mean ± SD) | BMI (kg/m2) (Mean ± SD) | BSA(m2) (Mean ± SD) | Atrial fibrillation (%) | Hypertension (%) | Diabetes Mellitus (%) | AVA (cm2) (Mean ± SD) | Mean Transaortic Gradient (mmHg) (Mean ± SD) | LVEF (%) (Mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Thalappillil/2020 [46] | 23/24 | 80.9 ± 7 | NA | NA | NA | NA | NA | 0.74 ± 0.23 | 32 ± 12 | NA |
| Maia/2020 [45] | 47/60 | 81 ± 5.9 | 26.98 ± 4.45 | 1.74 ± 0.18 | 37.5 | 77.5 | 48.3 | NA | 49.16 ± 15.23 | NA |
| Stella/2019 [44] | 77/98 | 81.3 ± 6.3 | 25.2 ± 4.8 | NA | 30.9% | NA | NA | 0.77 ± 0.3 | 48.4 ± 13.9 | 56.4 ± 11.3 |
| Queiros/2018 [43] | 39/62 | 83.0 ± 5.1 | NA | NA | 36.6 | 91.1 | 29.7 | 0.72 ± 0.19 | 47.4 ± 17.4 | 54.5 ± 14.6 |
| Prihadi/2018 [42] | 74/76 | 80.7 ± 7.2 | 26.7 ± 5.5 | NA | NA | NA | NA | 0.8 ± 0.3 | 43.5 ± 19.6 | 50.0 ± 11.8 |
| Podlesnikar/2018 [41] | 39/44 | 81.7 ± 6.7 | 27.0 ± 4.5 | 1.84 ± 0.23 | NA | NA | NA | 0.7 ± 0.2 | 44 ± 16 | 57.7 ± 21.5 |
| Kato/2018 [40] | 16/27 | 83.9 ± 4.9 | NA | 1.45 ± 0.18 | NA | 81 | 9 | 0.58 ± 0.12 | 47.0 ± 16.8 | NA |
| Mediratta/2017 [39] | 28/24 | 81 ± 8 | NA | 1.9 ± 0.3 | NA | NA | NA | 0.8 ± 0.2 | 40 ± 13 | 57 ± 16 |
| Garcia-Martin/2016 [38] | 10/21 | 81.6 ± 17 | NA | NA | NA | 31 | 7 | 0.7 ± 0.2 | 46.3 ± 16 | 58.2 ± 11 |
| Khalique/2014 [37] | 45/55 | 87.8 ± 8.3 | NA | NA | NA | NA | NA | 0.67 ± 0.17 | NA | NA |
| Study/Year | 3D-TEE | MDCT | Measurement Phase | Software Used for 3D-TEE Annulus Sizing | Sex (M/F) | Time for the AA Analysis (s) (Mean ± SD) | ||
|---|---|---|---|---|---|---|---|---|
| 3D-TEE Technique | Vendor | MDCT Technique | Vendor | |||||
| Thalappillil/2020 [46] | Automated | Acuson SC2000 (Siemens) | Manual | 128-slice/ 64-slice Dual-Source CT (Siemens) | Systole | eSie Valves AI | 23/24 | NA |
| Maia/2020 [45] | Automated | Acuson SC2000 (Siemens) | Manual | 64-detector row CT(Siemens) | Systole | eSie Valves | 47/60 | NA |
| Stella/2019 [44] | Semiautomated | GE Vivid E9 (GE Healthcare) | Manual | 64-slice CT (GE Healthcare) | Systole | 4D Auto AVQ | 77/98 | 50 ± 7 |
| Queiros/2018 [43] | Semiautomated | GE Vivid E9/E95 (GE Healthcare) | Manual | Multidetector 64-channel scanner (GE Healthcare) | Systole | Speqle3D | 39/62 | 33.9 ± 9.5 |
| Prihadi/2018 [42] | Automated | iE33 and EPIQ7 (Philips Medical Systems) | Manual | 64–detector row /320–detector row CT (Toshiba Medical Systems) | Systole | Aortic Valve Navigator [AVN] | 74/76 | 4.8 ± 1.2 (min) |
| Podlesnikar/2018 [41] | Automated | GE Vivid E9/E95 (GE-Vingmed) | Manual | 320-slice MDCT scanner (Toshiba Medical Systems) | Systole | 4D Auto AVQ | 39/44 | NA |
| Kato/2018 [40] | Automated | Acuson SC2000 (Siemens) | Manual | 320-slice MDCT scanner (Toshiba Medical Systems) | Systole | eSieValves | 16/27 | 30.1 ± 5.79 |
| Mediratta/2017 [39] | Semiautomated | iE33 (Philips Medical Systems) | Manual | 256-slice scanner (Philips Medical Systems) | Systole | Mitral Valve Quantification [MVQ] | 28/24 | NA |
| Garcia-Martin/2016 [38] | Automated | iE33 (Philips Medical Systems) | Manual | 64-slice MDCT (Philips Medical Systems) | Systole | eSieValves | 10/21 | NA |
| Khalique/2014 [37] | Semiautomated | iE33 (Philips Medical Systems) | Manual | 320-slice MDCT scanner (Toshiba Medical Systems) | Systole | Mitral Valve Quantification [MVQ] | 45/55 | NA |
| Variables | No. of Studies | Estimate | Standard Error | 95% CI (p Value) |
|---|---|---|---|---|
| Male | 6 | 2.510 | 1.315 | −0.068, 5.088 (p = 0.056) |
| Age | 6 | 0.046 | 0.036 | −0.025, 0.118 (p = 0.205) |
| Left ventricular rejection fraction | 4 | −0.041 | 0.031 | −0.102, 0.019 (p = 0.18) |
| Aortic valve area | 6 | 0.404 | 1.420 | −2.379, 3.187 (p = 0.776) |
| Mean transaortic gradient | 5 | −0.044 | 0.024 | −0.090, 0.003 (p = 0.065) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mork, C.; Wei, M.; Jiang, W.; Ren, J.; Ran, H. Aortic Annular Sizing Using Novel Software in Three-Dimensional Transesophageal Echocardiography for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 751. https://doi.org/10.3390/diagnostics11050751
Mork C, Wei M, Jiang W, Ren J, Ran H. Aortic Annular Sizing Using Novel Software in Three-Dimensional Transesophageal Echocardiography for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Diagnostics. 2021; 11(5):751. https://doi.org/10.3390/diagnostics11050751
Chicago/Turabian StyleMork, Chanrith, Minjie Wei, Weixi Jiang, Jianli Ren, and Haitao Ran. 2021. "Aortic Annular Sizing Using Novel Software in Three-Dimensional Transesophageal Echocardiography for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis" Diagnostics 11, no. 5: 751. https://doi.org/10.3390/diagnostics11050751
APA StyleMork, C., Wei, M., Jiang, W., Ren, J., & Ran, H. (2021). Aortic Annular Sizing Using Novel Software in Three-Dimensional Transesophageal Echocardiography for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Diagnostics, 11(5), 751. https://doi.org/10.3390/diagnostics11050751






