How Can We Manage Gallbladder Lesions by Transabdominal Ultrasound?
Abstract
1. Introduction
2. Tips for GB Evaluation
3. Morphological Classification of US Appearance
4. Differentiation of GB Polypoid Lesions (GPLs)
4.1. Multiplicity
4.2. Size
4.3. Surface Contour
4.4. Internal Structure
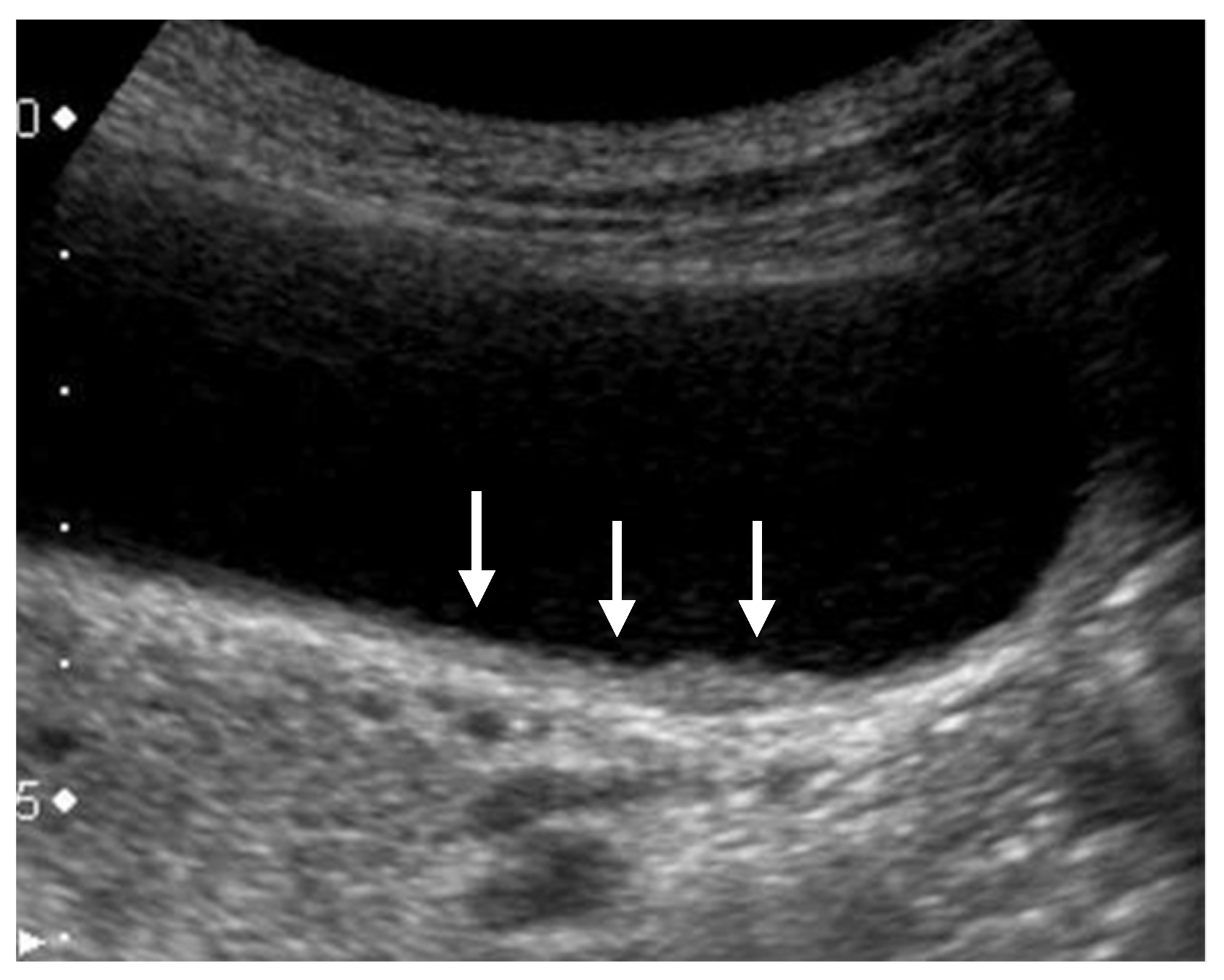
4.4.1. Hyperechoic Spots and Aggregation of Echogenic Spots (Mulberry Echo Pattern)
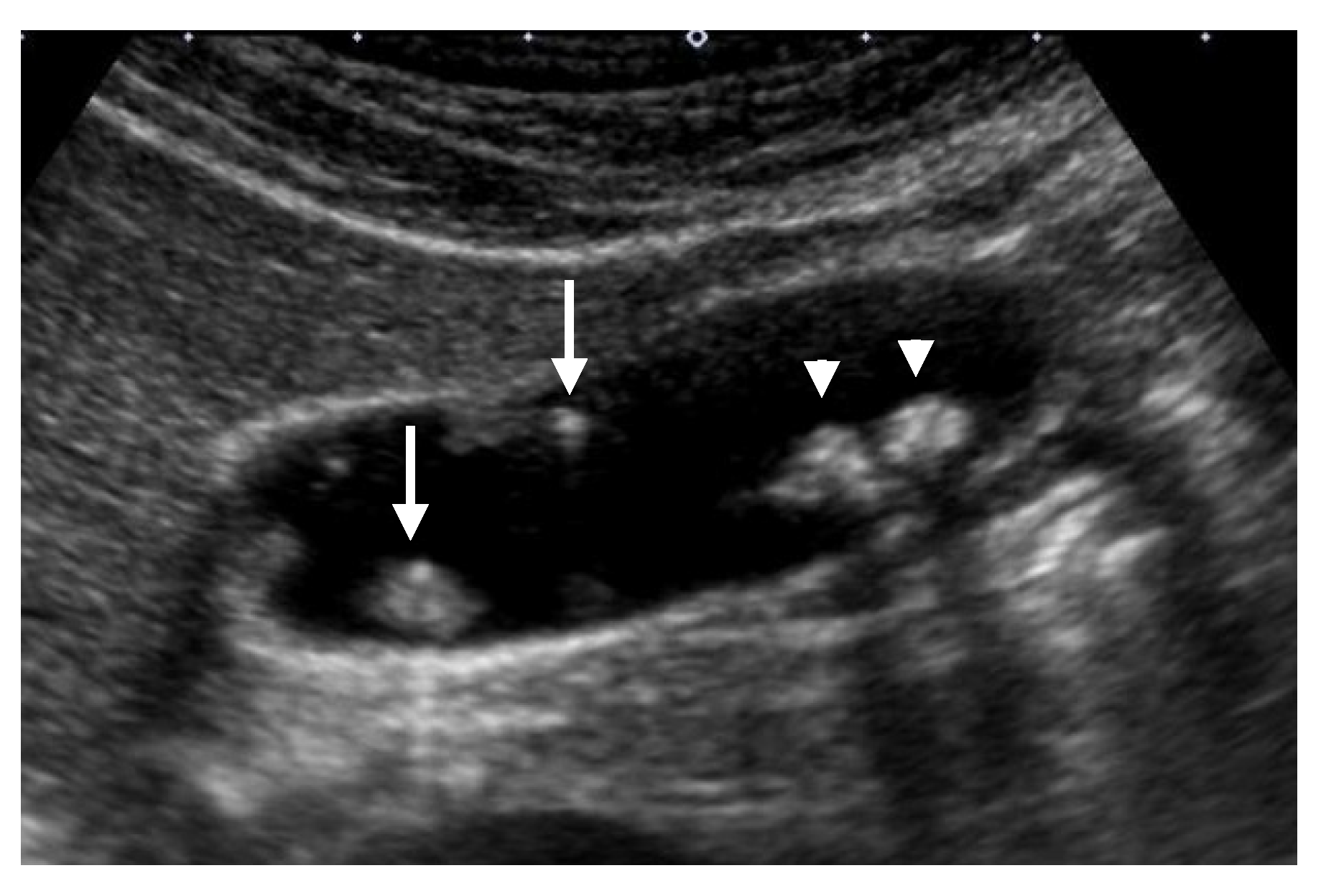
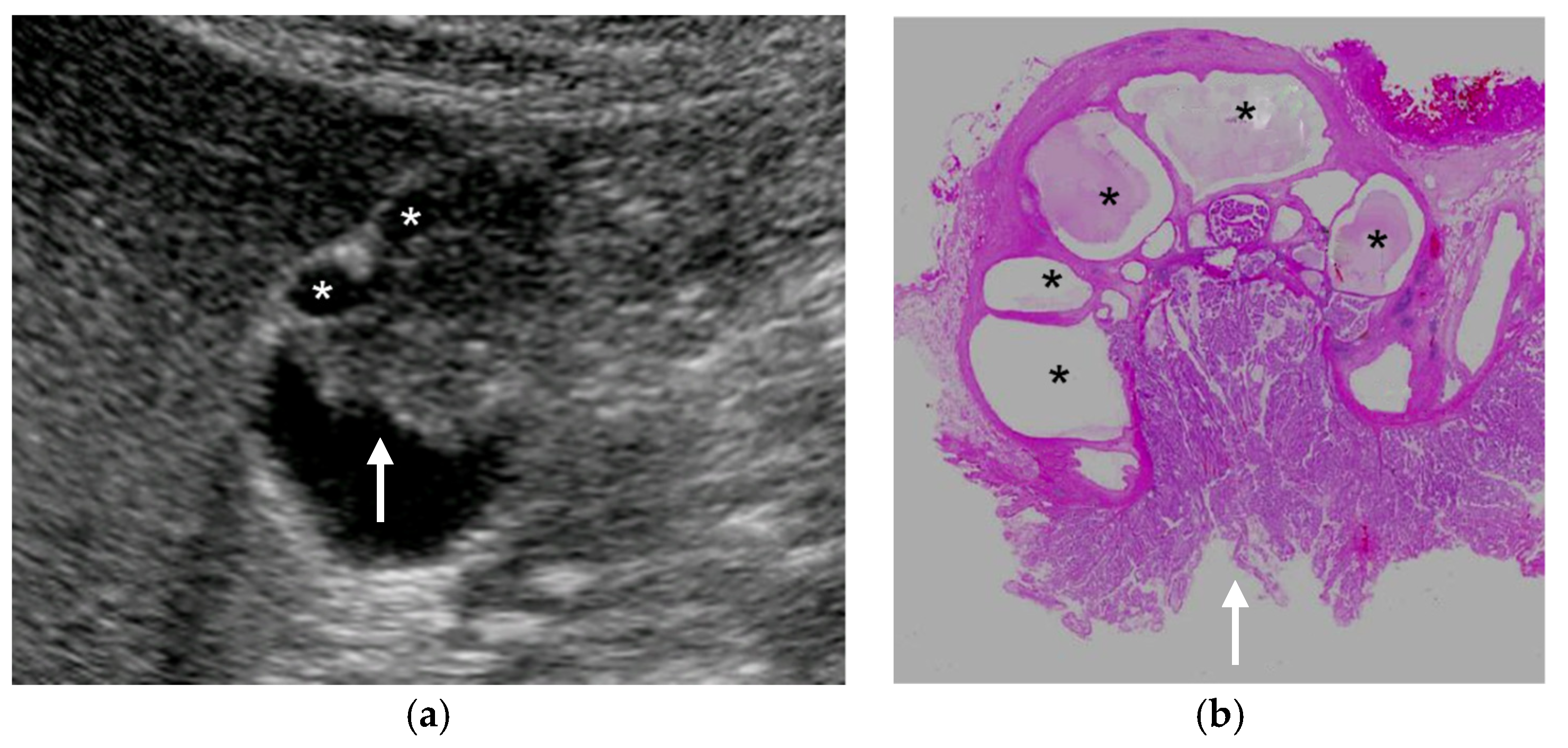
4.4.2. Cystic Structures (Anechoic Spots), Comet Tail Artifacts, Echogenic Foci
4.5. Stalk Width
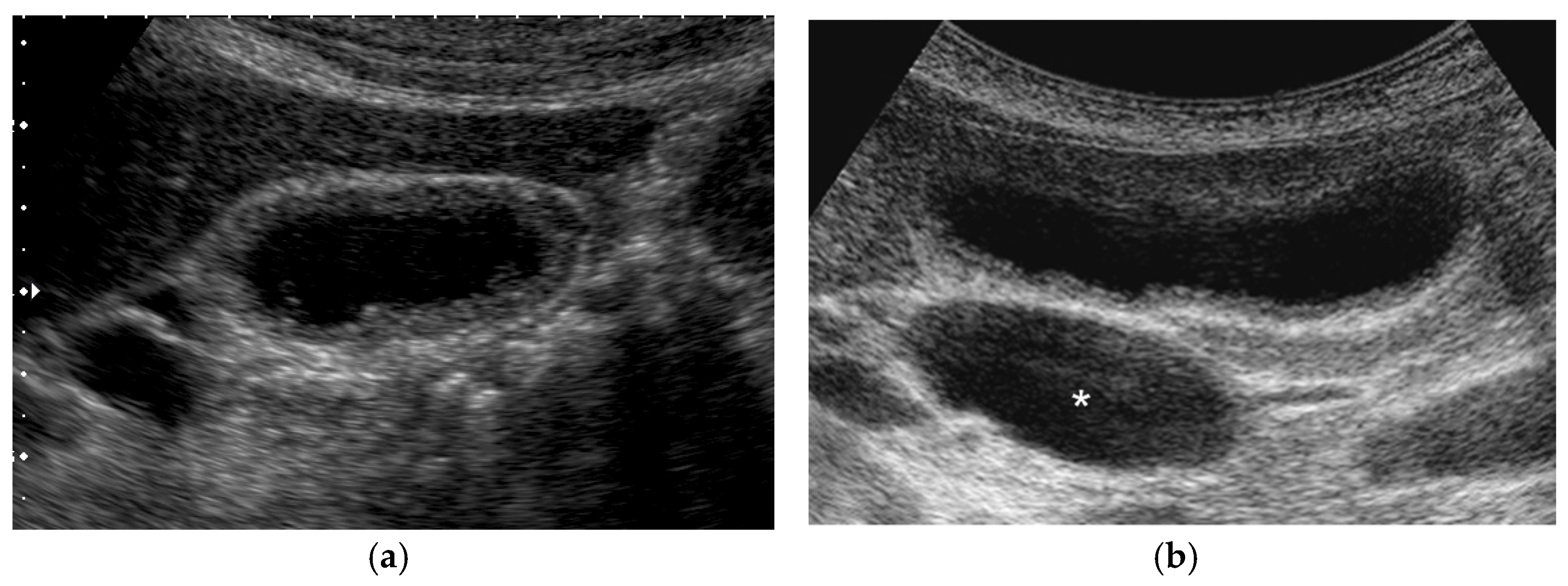
4.6. Localized Slight Thickening of Inner Hypoechoic Layer around GPLs
4.7. Irregularity or Discontinuity of GB Wall Layer Structure
4.8. Blood Flow Analysis and Contrast Effect
5. Differentiation of GB Wall Thickenings (GWTs)
5.1. Layer Structure
5.1.1. Characteristics of Innermost Hyperechoic Layer (IHL)
5.1.2. Sonolucent Layer, Hypoechoic Zone, “Three-Layer” Thickening
5.1.3. Striations
5.1.4. Irregularity or Discontinuity of Layer Structure, Irregular Thickening of Outer Hyperechoic Layer
5.2. Internal Structure
5.2.1. Cystic Structures (Anechoic Spots), Comet-Tail Artifacts, Echogenic Foci
5.2.2. Hypoechoic Nodules and Bands
5.3. Shape of the GB (Symmetrical or Asymmetrical)
5.4. Cholecystolithiasis
5.5. Blood Flow Analysis
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ADM | adenomyomatosis |
| CEUS | contrast-enhanced ultrasound |
| EUS | endoscopic ultrasound |
| GB | gallbladder |
| GBC | gallbladder carcinoma |
| GPL | gallbladder polypoid lesion |
| GWBF | gallbladder wall blood flow |
| GWT | gallbladder wall thickening |
| HRUS | high-resolution ultrasound |
| IHL | innermost hyperechoic layer |
| PBM | pancreaticobiliary maljunction |
| RAS | Rokitansky–Aschoff sinuses |
| SMI | superb microvascular imaging |
| US | ultrasound |
| XGC | xanthogranulomatous cholecystitis |
References
- Manual for Abdominal Ultrasound in Cancer Screening and Health Checkups. Available online: https://www.ningen-dock.jp/wp/wp-content/uploads/2018/06/Abdominal-Ultrasound.pdf (accessed on 22 March 2021).
- Mihara, S.; Nagano, K.; Kuroda, K.; Yoshioka, R.; Sawatari, M.; Koba, H.; Tanaka, S.; Hirao, S.; Machihara, M.; Hondou, K.; et al. Efficacy of Ultrasonic Mass Survey for Abdominal Cancer. J. Med. Syst. 1998, 22, 55–62. [Google Scholar] [CrossRef]
- Elmasry, M.; Lindop, D.; Dunne, D.F.; Malik, H.; Poston, G.J.; Fenwick, S.W. The risk of malignancy in ultrasound detected gallbladder polyps: A systematic review. Int. J. Surg. 2016, 33 Pt A, 28–35. [Google Scholar] [CrossRef]
- De Groen, P.C.; Gores, G.J.; LaRusso, N.F.; Gunderson, L.L.; Nagorney, D.M. Biliary tract cancers. N. Engl. J. Med. 1999, 341, 1368–1378. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yoshitomi, H.; Miyakawa, S.; Uesaka, K.; Unno, M.; Endo, I.; Ota, T.; Ohtsuka, M.; Kinoshita, H.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2015: The 2nd English edition. J. Hepatobiliary Pancreat. Sci. 2015, 22, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.-H.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018, 25, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Okaniwa, S. Role of conventional ultrasonography in the diagnosis of gallbladder polypoid lesions. J. Med. Ultrason. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.Y.; Baek, J.H.; Eun, H.W.; Kim, Y.J.; Han, J.K.; Choi, B.I. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. Am. J. Roentgenol. 2015, 204, W150–W159. [Google Scholar] [CrossRef]
- Joo, I.; Lee, J.Y.; Kim, J.H.; Kim, S.J.; Kim, M.A.; Han, J.K.; Choi, B.I. Differentiation of adenomyomatosis of the gallbladder from early-stage, wall-thickening-type gallbladder cancer using high-resolution ultrasound. Eur. Radiol. 2013, 23, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Okaniwa, S.; Fujita, N.; Noda, Y.; Kobayashi, G.; Kimura, K.; Yago, A.; Mochizuki, F.; Yamazaki, T.; Sawai, T. A clinico-pathological study of early gallbladder carcinoma. Jpn. J. Gastroenterol. 1996, 93, 628–633, (In Japanese with English abstract). [Google Scholar]
- Fujita, N.; Noda, Y.; Kobayashi, G.; Kimura, K.; Yago, A. Diagnosis of the depth of invasion of gallbladder carcinoma by EUS. Gastrointest. Endosc. 1999, 50, 659–663. [Google Scholar] [CrossRef]
- Iri, M.; Takehara, Y.; Matsuzawa, K.; Yasui, H.; Oka, T. Gallbladder carcinoma with ultrasonographically intact outer hypoechoic layer: A sign of a favorable outcome. J. Med. Ultrason. 2002, 29, 105–112. [Google Scholar] [CrossRef]
- Miwa, H.; Numata, K.; Sugimori, K.; Sanga, K.; Hirotani, A.; Tezuka, S.; Goda, Y.; Irie, K.; Ishii, T.; Kaneko, T.; et al. Differential diagnosis of gallbladder polypoid lesions using contrast-enhanced ultrasound. Abdom. Radiol. 2019, 44, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Kawashima, H.; Ohno, E.; Iida, T.; Nishio, R.; Suzuki, H.; Uetsuki, K.; Yashika, J.; Yamada, K.; Yoshikawa, M.; et al. Usefulness of transabdominal three-dimensional ultrasound for the evaluation of elevated gallbladder lesions. Tando 2020, 34, 145–152, (In Japanese with English abstract). [Google Scholar]
- Kubota, K.; Bandai, Y.; Noie, T.; Ishizaki, Y.; Teruya, M.; Makuuchi, M. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery 1995, 117, 481–487. [Google Scholar] [CrossRef]
- Sun, X.J.; Shi, J.S.; Han, Y.; Wang, J.S.; Ren, H. Diagnosis and treatment of polypoid lesions of the gallbladder: Report of 194 cases. Hepatobiliary Pancreat. Dis. Int. 2004, 3, 591–594. [Google Scholar]
- Bhatt, N.R.; Gillis, A.; Smoothey, C.O.; Awan, F.N.; Ridgway, P.F. Evidence based management of polyps of the gallbladder: A systematic review of the risk factors of malignancy. Surgeon 2016, 14, 278–286. [Google Scholar] [CrossRef]
- Choi, T.W.; Kim, J.H.; Park, S.J.; Ahn, S.J.; Joo, I.; Han, J.K. Risk stratification of gallbladder polyps larger than 10 mm using high-resolution ultrasonography and texture analysis. Eur. Radiol. 2018, 28, 196–205. [Google Scholar] [CrossRef]
- Wiles, R.; Varadpande, M.; Muly, S.; Webb, J. Growth rate and malignant potential of small gallbladder polyps—Systematic review of evidence. Surgeon 2014, 12, 221–226. [Google Scholar] [CrossRef]
- Kozuka, S.; Tsubone, N.; Yasui, A.; Hachisuka, K. Relation of adenoma to carcinoma in the gallbladder. Cancer 1982, 50, 2226–2234. [Google Scholar] [CrossRef]
- Park, H.Y.; Oh, S.H.; Lee, K.H.; Lee, J.K.; LeeIs, K.T. Cholecystectomy a reasonable treatment option for simple gallbladder polyps larger than 10 mm? World J. Gastroenterol. 2015, 21, 4248–4254. [Google Scholar] [CrossRef]
- Taskin, O.C.; Basturk, O.; Reid, M.D.; Dursun, N.; Bagci, P.; Saka, B.; Balci, S.; Memis, B.; Bellolio, E.; Araya, J.C.; et al. Gallbladder polyps: Correlation of size and clinicopathologic characteristics based on updated definitions. PLoS ONE 2020, 15, e0237979. [Google Scholar] [CrossRef]
- Kimura, K. Diagnosis for pedunculated polypoid lesions of the gallbladder by endoscopic ultrasonography. Jpn. J. Gastroenterol. 1997, 94, 249–260, (In Japanese with English abstract). [Google Scholar]
- Choi, W.B.; Lee, S.K.; Kim, M.H.; Seo, D.W.; Kim, H.J.; Kim, D.I.; Park, E.T.; Yoo, K.S.; Lim, B.C.; Myung, S.J.; et al. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest. Endosc. 2000, 52, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Atomi, Y.; Kuroda, A.; Muto, T.; Wada, N. Large cholesterol polyps of the gallbladder: Diagnosis by means of US and endoscopic US. Radiology 1995, 196, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Xie, X.Y.; Atomi, Y.; Saito, M. Differential diagnosis of small polypoid lesions of the gallbladder: The value of endoscopic ultrasonography. Ann. Surg. 1999, 229, 498–504. [Google Scholar] [CrossRef]
- Sadamoto, Y.; Oda, S.; Tanaka, M.; Hanada, N.; Kubo, H.; Eguchi, T.; Nawata, H. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy 2002, 34, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Sahara, H.; Seto, K.; Saito, H.; Kiriyama, M.; Tomita, F.; Kosaka, T.; Kita, I.; Takashima, S.; Matsunou, H. Gallbladder cancer associated with cholesterosis. J. Gastroenterol. 1996, 31, 470–474. [Google Scholar] [CrossRef]
- Niizawa, M.; Itoh, M.; Mukohjima, T.; Ishida, H.; Ishioka, T.; Nakmaura, J.; Naganuma, T.; Morikawa, P.; Masamune, O. Ultrasonographic features of papillary adenoma of the gallbladder. Jpn. J. Med. Ultrason. 1991, 18, 168–173, (In Japanese with English abstract). [Google Scholar]
- Noda, Y.; Kobayashi, G.; Ito, K.; Horaguchi, J.; Iwashita, Y.; Koshida, S.; Kanno, Y.; Ogawa, T.; Masu, K.; Michikawa, Y.; et al. Pyloric-type adenoma and carcinoma in adenoma of the gallbladder, their imaging and histological findings. Tando 2015, 29, 74–84, (In Japanese with English abstract). [Google Scholar]
- Yoshimitsu, K.; Irie, K.; Aibe, H.; Tajima, T.; Nishie, A.; Asayama, Y.; Matake, K.; Yamaguchi, K.; Matsuura, S.; Honda, H. Well-differentiated adenocarcinoma of the gallbladder with intratumoral cystic components due to abundant mucin production: A mimicker of adenomyomatosis. Eur. Radiol. 2005, 15, 229–233. [Google Scholar] [CrossRef]
- Lichtenstein, J.E. Adenomyomatosis and cholesterosis: The “hyperplastic cholecystosis”. In Radiology of the Liver, Biliary Tract, and Pancreas, 1st ed.; Friedman, A.C., Dachman, A.H., Eds.; Mosby: St. Louis, MO, USA, 1994; pp. 539–553. [Google Scholar]
- Raghavendra, B.N.; Subramanyam, B.R.; Balthazar, E.J.; Horii, S.C.; Megibow, A.J.; Hilton, S. Sonography of adenomyomatosis of the gallbladder: Radiologic-pathologic correlation. Radiology 1983, 146, 747–752. [Google Scholar] [CrossRef]
- Yoshimitsu, K.; Honda, H.; Aibe, H.; Shinozaki, K.; Kuroiwa, T.; Irie, H.; Asayama, Y.; Masuda, K. Radiologic diagnosis of adenomyomatosis of the gallbladder: Comparative study among MRI, helical CT, and transabdominal US. J. Comput. Assist. Tomogr. 2001, 25, 843–850. [Google Scholar] [CrossRef]
- Tang, S.S.; Huang, L.P.; Wang, Y.; Wang, Y.J. Contrast-enhanced ultrasonography diagnosis of fundal localized type of gallbladder adenomyomatosis. BMC Gastroenterol. 2015, 15, 99. [Google Scholar] [CrossRef]
- Yuan, H.X.; Wang, W.P.; Guan, P.S.; Lin, L.W.; Wen, J.X.; Yu, Q.; Chen, X.J. Contrast-enhanced ultrasonography in differential diagnosis of focal gallbladder adenomyomatosis and gallbladder cancer. Clin. Hemorheol. Microcirc. 2018, 70, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Kawarada, Y.; Sanda, M.; Mizumoto, R.; Yatani, R. Early carcinoma of the gallbladder, noninvasive carcinoma originating in the Rokitansky-Aschoff sinus: A case report. Am. J. Gastroenterol. 1986, 81, 61–66. [Google Scholar] [PubMed]
- Kurihara, K.; Mizuseki, K.; Ninomiya, T.; Shoji, I.; Kajiwara, S. Carcinoma of the gallbladder arising in adenomyomatosis. Acta Pathol. Jpn. 1993, 43, 82–85. [Google Scholar] [PubMed]
- Katoh, T.; Nakai, T.; Hayashi, S.; Satake, T. Noninvasive carcinoma of the gallbladder arising in localized type adenomyomatosis. Am. J. Gastroenterol. 1998, 83, 670–674. [Google Scholar]
- Patel, S.; Slade, J.; Jakate, S. An Unusual Case of Noninvasive Adenocarcinoma Arising in a Localized Adenomyoma of the Gallbladder and Review of Literature. Int. J. Surg. Pathol. 2016, 24, 341–346. [Google Scholar] [CrossRef]
- Kin, T.; Maguchi, H.; Takahashi, K.; Katanuma, A.; Osanai, M.; Yane, K.; Takagi, R.; Matsumoto, K.; Matsumori, T.; Gon, K.; et al. Clinicopathological features of gallbladder cancer with adenomyomatosis. Tando 2014, 28, 633–640, (In Japanese with English abstract). [Google Scholar]
- Kurtz, A.B.; Middleton, W.D. Ultrasound; Mosby: St. Louis, MO, USA, 1996. [Google Scholar]
- Miwa, H.; Numata, K.; Sugimori, K.; Kaneko, T.; Maeda, S. Vascular evaluation using transabdominal ultrasound for gallbladder polyps. J. Med. Ultrason. 2020. [Google Scholar] [CrossRef]
- Fei, X.; Lu, W.P.; Luo, Y.K.; Xu, J.H.; Li, Y.M.; Shi, H.Y.; Jiao, Z.Y.; Li, H. Contrast-enhanced ultrasound may distinguish gallbladder adenoma from cholesterol polyps: A prospective case-control study. Abdom. Imaging 2015, 40, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, H.; Ishikawa, O.; Ohigashi, H.; Kasugai, T.; Yokoyama, S.; Yamada, T.; Doki, Y.; Murata, K.; Miyashiro, I.; Sasaki, Y.; et al. Surgical Significance of Superficial Cancer Spread in Early Gallbladder Cancer. Jpn. J. Clin. Oncol. 2005, 35, 134–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakai, T.; Ajioka, Y.; Nagino, N.; Yamaguchi, N.; Shirai, Y.; Hatakeyama, K. Morphological features of early gallbladder carcinoma. Hepatogastroenterology 2012, 59, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Fujita, N.; Noda, Y.; Kobayashi, G.; Itoh, K.; Horaguchi, J.; Takasawa, O. A case of pedunculated polypoid cancer associated with flat-type cancer of the gallbladder. Dig. Endosc. 2005, 17, 93–96. [Google Scholar] [CrossRef]
- Aibe, T.; Noguchi, T.; Ohtani, T.; Fuji, T.; Takemoto, T. A study on layers structure of the gallbladder by endoscopic ultrasonography—including normal cases and chronic cholecystitis. JSUM Proc. 1986, 48, 761–762, (In Japanese with English abstract). [Google Scholar]
- Fujita, N.; Noda, Y.; Kobayashi, G.; Kimura, K.; Yago, A.; Mochizuki, F. Analysis of the layer structure of the gallbladder by endoscopic ultrasound using the pinning method. Dig. Endosc. 1995, 4, 353–356. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, M.; Ma, B.; Ma, X. Potential role of contrast enhanced ultrasound for the differentiation of malignant and benign gallbladder lesions in East Asia: A meta-analysis and systematic review. Medicine 2018, 97, e11808. [Google Scholar] [CrossRef]
- Xie, X.H.; Xu, H.X.; Xie, X.Y.; Lu, M.D.; Kuang, N.; Xu, Z.F.; Liu, G.J.; Wang, Z.; Liang, J.Y.; Chen, L.D.; et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur. Radiol. 2010, 20, 239–248. [Google Scholar] [CrossRef]
- Hirooka, Y.; Naitoh, Y.; Goto, H.; Furukawa, T.; Ito, A.; Hayakawa, T. Differential diagnosis of gall-bladder masses using color Doppler ultrasonography. J. Gastroenterol. Hepatol. 1996, 11, 840–846. [Google Scholar] [CrossRef]
- Kin, T.; Nagai, K.; Hayashi, T.; Takahashi, K.; Katanuma, A. Efficacy of superb microvascular imaging of ultrasound for diagnosis of gallbladder lesion. J. Hepatobiliary Pancreat. Sci. 2020, 27, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Goto, H.; Hirooka, Y.; Itoh, A.; Taki, T.; Watanabe, Y.; Hayakawa, T.; Naitoh, Y. Color Doppler-guided spectral analysis of gallbladder wall flow. J. Gastroenterol. Hepatol. 1998, 13, 181–185. [Google Scholar] [CrossRef]
- Sun, L.P.; Guo, L.H.; Hu, H.X.; Liu, L.N.; Xu, J.M.; Zhang, Y.F.; Liu, C.; Bo, X.W.; Xu, X.H. Value of contrast-enhanced ultrasound in the differential diagnosis between gallbladder adenoma and gallbladder adenoma canceration. Int. J. Clin. Exp. Med. 2015, 8, 1115–1121. [Google Scholar]
- Zhang, H.P.; Bai, M.; Gu, J.Y.; He, Y.Q.; Qiao, X.H.; Du, L.F. Value of contrast-enhanced ultrasound in the differential diagnosis of gallbladder lesion. World J. Gastroenterol. 2018, 24, 744–751. [Google Scholar] [CrossRef]
- Kim, P.N.; Ha, H.K.; Kim, Y.H.; Lee, M.G.; Kim, M.H.; Auh, Y.H. US findings of Xanthogranulomatous cholecystitis. Clin. Radiol. 1998, 53, 290–292. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, J.H.; Joo, I.; Lee, J.Y.; Han, J.K.; Choi, B.I. Xanthogranulomatous cholecystitis: Diagnostic performance of US, CT, and MRI for differentiation from gallbladder carcinoma. Abdom. Imaging 2015, 40, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, H.; Zhao, E.; Cui, N.; Li, Z. Differential Diagnosis and Treatment Options for Xanthogranulomatous Cholecystitis. Med. Princ. Pr. 2013, 22, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Teefey, S.A.; Baron, R.L.; Bigler, S.A. Sonography of the Gallbladder: Significance of Striated (Layered) Thickening of the Gallbladder Wall. Am. J. Roentgenol. 1991, 156, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Wibbenmeyer, L.A.; Sharafuddin, M.J.; Wolverson, M.K.; Heiberg, E.V.; Wade, T.P.; Shields, J.B. Sonographic diagnosis of unsuspected gallbladder cancer: Imaging findings in comparison with benign gallbladder conditions. Am. J. Roentgenol. 1995, 165, 1169–1174. [Google Scholar] [CrossRef]
- Xu, J.M.; Guo, L.H.; Xu, H.X.; Zheng, S.G.; Liu, L.N.; Sun, L.P.; Lu, M.D.; Xie, X.Y.; Wang, W.P.; Hu, B.; et al. Differential diagnosis of gallbladder wall thickening: The usefulness of contrast-enhanced ultrasound. Ultrasound Med. Biol. 2014, 40, 2794–2804. [Google Scholar] [CrossRef]
- Tsuchida, A.; Itoi, T.; Endo, M.; Kitamura, K.; Mukaide, M.; Itokawa, F.; Ozawa, T.; Aoki, T. Pathological features and surgical outcome of pancreaticobiliary maljunction without dilatation of the extrahepatic bile duct. Oncol. Rep. 2004, 11, 269–276. [Google Scholar] [CrossRef]
- Tanno, S.; Obara, T.; Fujii, T.; Mizukami, Y.; Shudo, R.; Nishino, N.; Ura, H.; Klein-Szanto, A.J.; Kohgo, Y. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gallbladder in patients with anomalous pancreaticobiliary ductal union. Cancer 1998, 83, 267–275. [Google Scholar] [CrossRef]
- Takuma, K.; Kamisawa, T.; Tabata, T.; Hara, S.; Kuruma, S.; Inaba, Y.; Kurata, M.; Honda, G.; Tsuruta, K.; Horiguchi, S.; et al. Importance of early diagnosis of pancreaticobiliary maljunction without biliary dilatation. World J. Gastroenterol. 2012, 18, 3409–3414. [Google Scholar] [CrossRef]
- Marchal, G.J.; Casaer, M.; Baert, A.L.; Goddeeris, P.G.; Kerremans, R.; Fevery, J. Gallbladder wall sonolucency in acute cholecystitis. Radiology 1979, 133, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Päivänsalo, M.; Siniluoto, T.; Myllylä, V.; Kairaluoma, M.I.; Kallioinen, M. Ultrasound in acute and chronic cholecystitis. Rofo 1987, 147, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Cohan, R.H.; Mahony, B.S.; Bowie, J.D.; Cooper, C.; Baker, M.E.; Illescas, F.F. Striated intramural gallbladder lucencies on US studies: Predictors of acute cholecystitis. Radiology 1987, 164, 31–35. [Google Scholar] [CrossRef]
- Suk, K.T.; Kim, C.H.; Baik, S.K.; Kim, M.K.; Park, D.H.; Kim, K.H.; Kim, J.W.; Kim, H.S.; Kwon, S.O.; Lee, D.K.; et al. Gallbladder wall thickening in patients with acute hepatitis. J. Clin. Ultrasound 2009, 37, 144–148. [Google Scholar] [CrossRef]
- Nabatame, N.; Shirai, Y.; Nishimura, A.; Yokoyama, N.; Wakai, T.; Hatakeyama, K. High risk of gallbladder carcinoma in elderly patients with segmental adenomyomatosis of the gallbladder. J. Exp. Clin. Cancer Res. 2004, 23, 593–598. [Google Scholar]
- Ootani, T.; Shirai, Y.; Tsukada, K.; Muto, T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer 1992, 69, 2647–2652. [Google Scholar] [CrossRef]
- Parra, J.A.; Acinas, O.; Bueno, J.; Güezmes, A.; Fernández, M.A.; Fariñas, M.C. Xanthogranulomatous cholecystitis: Clinical, sonographic, and CT findings in 26 patients. Am. J. Roentgenol. 2000, 174, 979–983. [Google Scholar] [CrossRef]
- Goodman, Z.D.; Ishak, K.G. Xanthogranulomatous cholecystitis. Am. J. Surg. Pathol. 1981, 5, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, B.; Cao, Q.; Zhang, Q.; Qiu, Y.; Yang, D.; Yu, L.; Wang, W.P. Incidentally detected focal fundal gallbladder wall thickening: Differentiation contrast enhanced ultrasound features with high-resolution linear transducers. Clin. Hemorheol. Microcirc. 2020, 74, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Shirai, Y.; Hatakeyama, K. Segmental adenomyomatosis of the gallbladder predisposes to cholecystolithiasis. J. Hepatobiliary Pancreat. Surg. 2004, 11, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Schiller, V.L.; Turner, R.R.; Sarti, D.A. Color Doppler imaging of the gallbladder wall in acute cholecystitis: Sonographic–pathologic correlation. Abdom. Imaging 1996, 21, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Hirooka, Y.; Itoh, A.; Hashimoto, S.; Itoh, I.; Hara, K.; Kanamori, A.; Ohmiya, N.; Niwa, Y.; Goto, H. Use of color Doppler ultrasonography in the diagnosis of anomalous connection in pancreatobiliary disease. World J. Gastroenterol. 2005, 11, 1018–1022. [Google Scholar] [CrossRef] [PubMed]











| US Findings | cholesterol Polyp | ADM | Adenoma | Carcinoma |
|---|---|---|---|---|
| US appearance | pedunculated | sessile | pedunculated > sessile | sessile > pedunculated |
| Multiplicity | multiple > solitary | solitary | solitary | solitary |
| Surface contour | smooth or granular | smooth or granular | nodular or lobulated | nodular or lobulated |
| Internal structure | hyperechoic spots, aggregation of echogenic spots | cystic structures (round and smooth surface) | cystic structures (multilobulated and irregular surface) | cystic structures (multilobulated and irregular surface) |
| Localized slight thickening of inner hypoechoic layer | absent | absent | absent | occasionally |
| GB wall layer structure | intact outer hyperechoic layer | intact outer hyperechoic layer | intact outer hyperechoic layer | irregular or disrupted outer hyperechoic layer in advanced lesions |
| Shape of color signal pattern | absent or regular | absent or regular | irregular | irregular |
| Caliber change of color signal pattern | absent | absent | present | present |
| GB wall blood flow (GWBF) | lower than 30 cm/s | lower than 30 cm/s | not available | higher than 30 cm/s |
| Enhanced pattern in the venous phase (late phase) | homogeneous | homogeneous | homogeneous | heterogeneous |
| Diffuse GWT | Focal GWT | ||
|---|---|---|---|
| Gallbladder | Inflammation | Acute cholecystitis | |
| Chronic cholecystitis | Chronic cholecystitis | ||
| Xanthogranulomatous cholecystitis | Xanthogranulomatous cholecystitis | ||
| Hyperplasia | Adenomyomatosis (Diffuse, Segmental) | Adenomyomatosis (Focal) | |
| Hyperplasia associated with pancreaticobiliary maljuncton | |||
| Neoplasia | Gallbladder carcinoma | Gallbladder carcinoma | |
| Lymphoma | |||
| Pseudothickening | Postprandial state | Debris, Sludge | |
| Other organs | Inflammation | Pancreatitis | |
| Peritonitis | |||
| Liver disorders | Acute hepatitis | ||
| Cirrhosis | |||
| Systemic diseases | Heart failure | ||
| Renal failure | |||
| Hypoalbuminemia | |||
| Sepsis |
| US Findings | Acute Cholecystitis | XGC | ADM | Carcinoma |
|---|---|---|---|---|
| US appearance | diffuse | focal or diffuse | focal or diffuse | focal > diffuse |
| Innermost Hyperechoic Layer (IHL) | recognized continuously | recognized continuously | recognized continuously | presence of focal or diffuse discontinuity or irregularity |
| Layer structure | preserved, sonolucent layer, striations | irregular or disrupted in some cases | preserved | irregular or disrupted in advanced lesions |
| Internal structure | No distinctive findings | hypoechoic nodules and bands | cystic structures (round and smooth surface, aligned in a linear fashion) | cystic structures (multilobulated and irregular surface) |
| Cholecystolithiasis | common | common | relatively common | relatively common |
| GB wall blood flow (GWBF) | lower than 30 cm/s (affected by disease activity) | not available | lower than 30 cm/s | higher than 30 cm/s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okaniwa, S. How Can We Manage Gallbladder Lesions by Transabdominal Ultrasound? Diagnostics 2021, 11, 784. https://doi.org/10.3390/diagnostics11050784
Okaniwa S. How Can We Manage Gallbladder Lesions by Transabdominal Ultrasound? Diagnostics. 2021; 11(5):784. https://doi.org/10.3390/diagnostics11050784
Chicago/Turabian StyleOkaniwa, Shinji. 2021. "How Can We Manage Gallbladder Lesions by Transabdominal Ultrasound?" Diagnostics 11, no. 5: 784. https://doi.org/10.3390/diagnostics11050784
APA StyleOkaniwa, S. (2021). How Can We Manage Gallbladder Lesions by Transabdominal Ultrasound? Diagnostics, 11(5), 784. https://doi.org/10.3390/diagnostics11050784






