Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter for the Diagnosis of Liver Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical and Laboratory Assessment
2.3. VCTE Examination
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
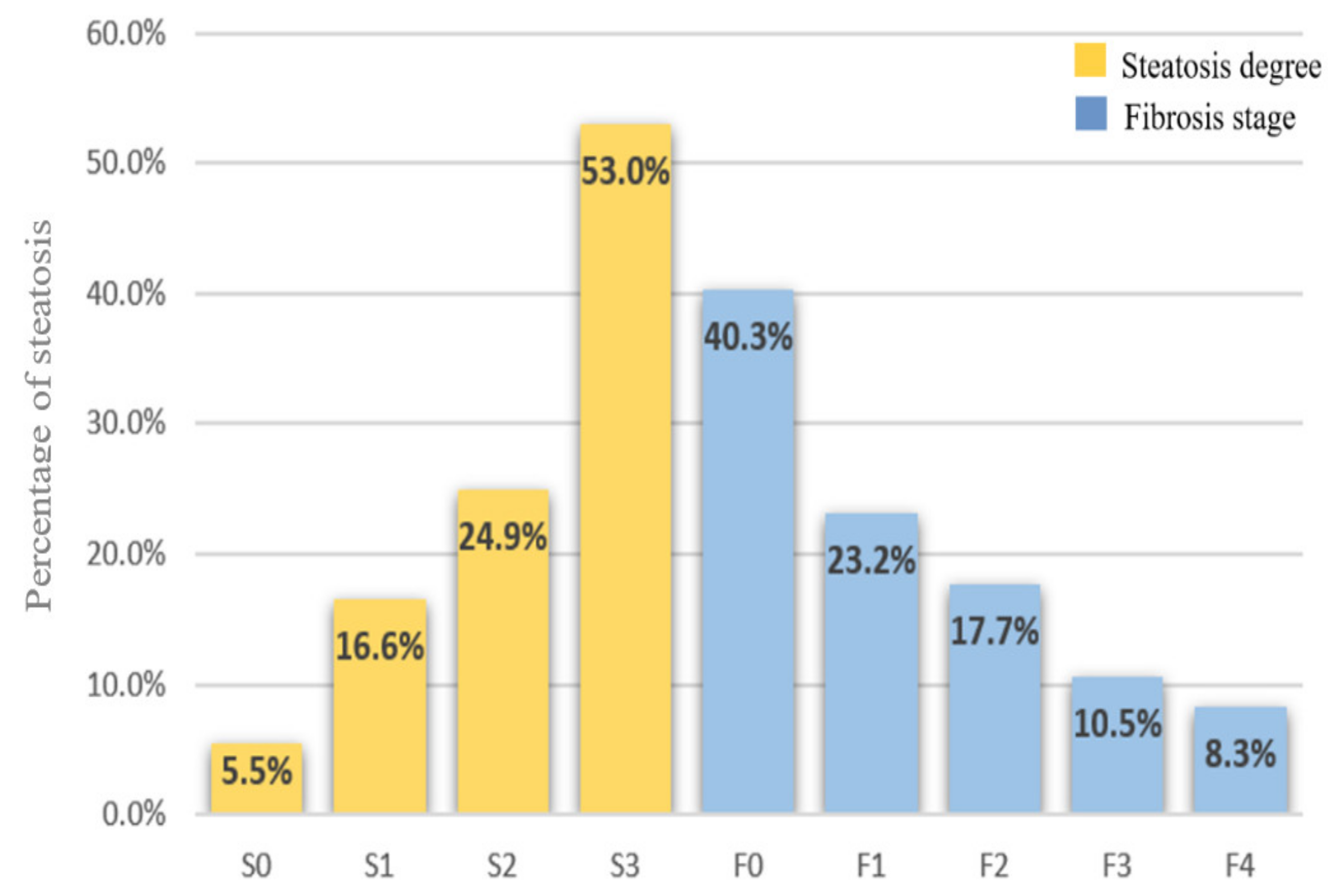
3.2. CAP and LSM Values According to Steatosis Degree and Fibrosis Stage
3.3. Factors Associated with CAP and LSM
3.4. Correlation between Surrogate Serum Fibrosis Markers, LSM, and CAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takalkar, U.V.; Nageshwar, D.R. Non-alcoholic fatty liver disease-hepatic manifestation of obesity. Austin J. Obes. Metab. Synd. 2018, 3, 1–3. [Google Scholar]
- Williamson, R.M.; Price, J.F.; Glancy, S.; Perry, E.; Nee, L.D.; Hayes, P.C.; Frier, B.M.; Van Look, L.A.F.; Johnston, G.I.; Reynolds, R.M.; et al. Prevalence of and Risk Factors for Hepatic Steatosis and Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes Care 2011, 34, 1139–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Stanciu, C.; Jurcău, M.; Zenovia, S.; Frunzuc, G.; Timofte, D. Nonalcoholic steatohepatitis. Medicine 2019, 98, e18221. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, B.; Stål, P.; Wahlin, S.; Björkström, N.K.; Hagström, H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019, 39, 1098–1108. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifan, A.; Stanciu, C. Checkmate to liver biopsy in chronic hepatitis C? World J. Gastroenterol. 2012, 18, 5514–5520. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling Variability of Liver Biopsy in Nonalcoholic Fatty Liver Disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V. The Lido Study Group Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Asociación Latinoamericana para el Estudio del Hígado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection (Updated Version). Available online: http://www.who.int/hepatitis/publications/hepatitis-cguidelines-2016/en/ (accessed on 10 February 2021).
- World Health Organization (WHO). Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Available online: http://apps.who.int/iris/bitstream/10665/154590/1/9789241549059eng.pdf?ua=1&ua=1 (accessed on 10 February 2021).
- Yoneda, M.; Fujita, K.; Inamori, M.; Nakajima, A.; Tamano, M.; Hiraishi, H. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut 2007, 56, 1330–1331. [Google Scholar] [CrossRef]
- Yoneda, M.; Mawatari, H.; Fujita, K.; Endo, H.; Iida, H.; Nozaki, Y.; Yonemitsu, K.; Higurashi, T.; Takahashi, H.; Kobayashi, N.; et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Liver Dis. 2008, 40, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.-M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- De Lédinghen, V.; Wong, G.L.-H.; Vergniol, J.; Chan, H.L.-Y.; Hiriart, J.-B.; Chan, A.W.-H.; Chermak, F.; Choi, P.C.-L.; Foucher, J.; Chan, C.K.-M.; et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016, 31, 848–855. [Google Scholar] [CrossRef]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled Attenuation Parameter (CAP): A Novel VCTE™ Guided Ultrasonic Attenuation Measurement for the Evaluation of Hepatic Steatosis: Preliminary Study and Validation in a Cohort of Patients with Chronic Liver Disease from Various Causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasso, M.; Audière, S.; Kemgang, A.; Gaouar, F.; Corpechot, C.; Chazouillères, O.; Fournier, C.; Golsztejn, O.; Prince, S.; Menu, Y.; et al. Liver Steatosis Assessed by Controlled Attenuation Parameter (CAP) Measured with the XL Probe of the FibroScan: A Pilot Study Assessing Diagnostic Accuracy. Ultrasound Med. Biol. 2016, 42, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Castéra, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; de Lédinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Vergniol, J.; Wong, G.L.-H.; Foucher, J.; Chan, H.L.-Y.; Le Bail, B.; Choi, P.C.-L.; Kowo, M.; Chan, A.W.-H.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef]
- Sawaf, B.; Ali, A.H.; Jaafar, R.F.; Kanso, M.; Mukherji, D.; Khalife, M.J.; Faraj, W. Spectrum of liver diseases in patients referred for Fibroscan: A single center experience in the Middle East. Ann. Med. Surg. 2020, 57, 166–170. [Google Scholar] [CrossRef]
- Kwok, R.; Choi, K.C.; Wong, G.L.-H.; Zhang, Y.; Chan, H.L.-Y.; Luk, A.O.-Y.; Shu, S.S.-T.; Chan, A.W.-H.; Yeung, M.-W.; Chan, J.C.-N.; et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: A prospective cohort study. Gut 2015, 65, 1359–1368. [Google Scholar] [CrossRef]
- Jarvis, H.; Craig, D.; Barker, R.; Spiers, G.; Stow, D.; Anstee, Q.M.; Hanratty, B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020, 17, e1003100. [Google Scholar] [CrossRef] [PubMed]
- Oeda, S.; Takahashi, H.; Imajo, K.; Seko, Y.; Ogawa, Y.; Moriguchi, M.; Yoneda, M.; Anzai, K.; Aishima, S.; Kage, M.; et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: A multicenter prospective study. J. Gastroenterol. 2019, 55, 428–440. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Vuppalanchi, R.; Van Natta, M.L.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D.; et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 156–163.e2. [Google Scholar] [CrossRef]
- Tapper, E.B.; Challies, T.; Nasser, I.; Afdhal, N.H.; Lai, M. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients with Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2016, 111, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Mansour, A.M.F.; Bayoumy, E.M.; ElGhandour, A.M.; El-Talkawy, M.D.; Badr, S.M.; Ahmed, A.E.-M. Assessment of hepatic fibrosis and steatosis by vibration-controlled transient elastography and controlled attenuation parameter versus non-invasive assessment scores in patients with non-alcoholic fatty liver disease. Egypt Liver J. 2020, 10, 33. [Google Scholar] [CrossRef]
- Fallatah, H.I.; Akbar, H.O.; Fallatah, A.M. Fibroscan Compared to FIB-4, APRI, and AST/ALT Ratio for Assessment of Liver Fibrosis in Saudi Patients with Nonalcoholic Fatty Liver Disease. Zahedan J. Res. Med Sci. 2016, 16, e38346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, N.A.; Kolgelier, S.; Ozcimen, S.; Gungor, G.; Sumer, S.; Demir, L.S.; Inkaya, A.C.; Ural, O. Evaluation of the Relation Between Hepatic Fibrosis and Basic Laboratory Parameters in Patients With Chronic Hepatitis B Fibrosis and Basic Laboratory Parameters. Zahedan J. Res. Med Sci. 2014, 14, e16975. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, C.M.F.; Aller, R.; García, M.L.G.; Ampuero, J.; Gomez, J.; Burgos-Santamaría, D.; Rosales, J.M.; Aspichueta, P.; Buque, X.; Latorre, M.; et al. Higher levels of serum uric acid influences hepatic damage in patients with non-alcoholic fatty liver disease (NAFLD). Rev. Española Enferm. Dig. 2019, 111, 264–269. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.-J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress: Potential Role in Fructose-Dependent and- Independent Fatty Liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [Green Version]
- Jaruvongvanich, V.; Ahuja, W.; Wirunsawanya, K.; Wijarnpreecha, K.; Ungprasert, P. Hyperuricemia is associated with nonalcoholic fatty liver disease activity score in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1031–1035. [Google Scholar] [CrossRef] [PubMed]


| Overall Cohort | No Steatosis | Any Steatosis (≥S1) | p–Value | |
|---|---|---|---|---|
| Patients characteristics | n = 181 | n, (%) = 10 (5.5) | n, (%) = 171 (94.5) | |
| Gender (female), n (%) | 96 (53) | 4 (40) | 92 (54) | 0.184 |
| Age, y | 57.62 ± 11.8 | 51.08 ± 12.74 | 59.19 ± 11.05 | <0.001 |
| BMI (kg/m2) | 29.48 ± 4.85 | 25.76 ± 4.30 | 30.37 ± 4.56 | <0.001 |
| BMI ≥ 30 kg/m2, n (%) | 87 (48) | 2 (20) | 86 (50.3) | <0.001 |
| Diabetes, n (%) | 39 (21.5) | 1 (10) | 38 (25.9) | <0.001 |
| Hypertension, n (%) | 54 (29.8) | 2 (20) | 52 (30.4) | <0.001 |
| Platelet count (G/L) | 251.67 ± 81.18 | 256.42 ± 131.39 | 250.53 ± 64.23 | 0.798 |
| ALT (IU/L) | 40.2 ± 41.29 | 29.75 ± 19.34 | 42.72 ± 44.71 | 0.136 |
| AST (IU/L) | 31.77 ± 22.61 | 25.21 ± 11.15 | 33.35 ± 23.37 | 0.079 |
| GGT (IU/L) | 58.89 ± 67.91 | 41.64 ± 31.07 | 57.33 ± 68.03 | 0.237 |
| ALP (IU/L) | 80.70 ± 36.92 | 76.67 ± 30.65 | 79.37 ± 35.01 | 0.709 |
| Total bilirubin (mg/dL) | 0.70 ± 0.38 | 0.75 ± 0.39 | 0.69 ± 0.38 | 0.465 |
| Albumin (g/dL) | 4.56 ± 0.38 | 4.53 ± 0.44 | 4.57 ± 0.36 | 0.559 |
| Creatinine (mg/dL) | 0.83 ± 0.13 | 0.814 ± 0.13 | 0.834 ± 0.13 | 0.505 |
| Urea (mg/dL) | 36.56 ± 10.81 | 34.40 ± 37.08 | 37.08 ± 11.22 | 0.188 |
| Fasting glucose (mg/dL) | 111.37 ± 43.77 | 96.37 ± 18.86 | 114.96 ± 47.19 | 0.024 |
| Ferritin (ng/mL) | 146.12 ± 114.98 | 111.54 ± 81 | 154.41 ± 120.47 | 0.047 |
| CRP (mg/dL) | 0.62 ± 2.27 | 1.38 ± 5.06 | 0.437 ± 0.482 | 0.048 |
| Total cholesterol (mg/dL) | 211.68 ± 55.04 | 191.61 ± 67.19 | 216.52 ± 50.85 | 0.031 |
| Triglycerides (mg/dL) | 148 ± 98.78 | 124.03 ± 113.49 | 153.78 ± 94.53 | 0.153 |
| LDL-c (mg/dL) | 125.34 ± 47.02 | 104.5 ± 45.59 | 130.37 ± 46.15 | 0.009 |
| HDL-c (mg/dL) | 45.06 ± 13.90 | 51.5 ± 13.47 | 43.5 ± 13.6 | 0.006 |
| Serum uric acid (mg/dL) | 5.21 ± 1.63 | 4.25 ±1.7 | 5.44 ± 1.53 | <0.001 |
| Alpha-fetoprotein (ng/mL) | 3.84 ± 1.73 | 3.73 ± 1.13 | 3.87 ± 1.85 | 0.703 |
| APRI | 0.38 ± 0.31 | 0.34 ± 0.22 | 0.39 ± 0.33 | 0.288 |
| FIB-4 | 1.36 ± 0.96 | 1.18 ± 0.33 | 1.41 ± 0.92 | 0.027 |
| NFS | −1.61 ± 1.61 | −2.37 ± 1.96 | −1.44 ± 1.48 | 0.007 |
| LSM (kPa) | 6.1 (4.8–8.3) | 5 (4.27–6.35) | 6.3 (5.1–8.85) | 0.049 |
| CAP dB/m | 293 (245.5–339) | 212(178.75–226.5) | 312 (273.5–344) | <0.001 |
| CAP | LSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate | Univariate Multivariate | |||||
| β | p | β | P | β | P | β | P | |
| Gender | 0.010 | 0.889 | 0.106 | 0.155 | ||||
| Age, y | 0.167 | 0.025 | 0.144 | 0.053 | 0.046 | 0.537 | ||
| BMI (kg/m2) | 0.542 | <0.001 | 0.339 | <0.001 | 0.244 | 0.002 | 0.094 | 0.299 |
| Diabetes | 0.233 | <0.001 | 0.020 | 0.790 | 0.141 | 0.058 | ||
| Hypertension | 0.382 | <0.001 | 0.132 | 0.084 | 0.231 | 0.002 | 0.093 | 0.257 |
| Platelet (G/L) | −0.008 | 0.913 | −0.258 | <0.001 | −0.168 | 0.033 | ||
| ALT (IU/L) | 0.170 | 0.041 | 0.132 | 0.361 | −0.013 | 0.874 | ||
| AST (IU/L) | 0.172 | 0.039 | 0.115 | 0.889 | 0.038 | 0.650 | ||
| GGT (IU/L) | 0.124 | 0.137 | 0.197 | 0.018 | 0.024 | 0.760 | ||
| ALP (IU/L) | 0.069 | 0.413 | 0.170 | 0.042 | 0.077 | 0.325 | ||
| TB (mg/dL) | −0.086 | 0.304 | −0.044 | 0.600 | ||||
| Albumin (g/dL) | 0.079 | 0.345 | −0.295 | <0.001 | −0.276 | <0.001 | ||
| Creatinine (mg/dL) | 0.044 | 0.600 | 0.057 | 0.494 | ||||
| Urea (mg/dL) | 0.150 | 0.044 | −0.005 | 0.938 | 0.095 | 0.204 | ||
| Fasting plasma glucose (mg/dL) | 0.160 | 0.031 | 0.173 | 0.046 | 0.045 | 0.551 | ||
| Ferritin (ng/mL) | 0.092 | 0.218 | 0.015 | 0.845 | ||||
| CRP (mg/dL) | −0.112 | 0.182 | −0.014 | 0.867 | ||||
| TC (mg/dL) | 0.177 | 0.034 | 0.048 | 0.525 | −0.102 | 0.225 | ||
| Triglycerides (mg/dL) | 0.267 | 0.001 | 0.192 | 0.043 | 0.008 | 0.924 | ||
| LDL-c (mg/dL) | 0.370 | <0.001 | 0.155 | 0.008 | 0.127 | 0.131 | ||
| HDL-c (mg/dL) | −0.254 | 0.002 | −0.008 | 0.913 | −0.118 | 0.157 | ||
| Serum uric acid (mg/dL) | 0.482 | <0.001 | 0.244 | 0.003 | 0.339 | <0.001 | 0.192 | 0.028 |
| AFP (ng/mL) | 0.075 | 0.369 | 0.211 | 0.011 | 0.161 | 0.033 | ||
| LSM (kPa) | 0.226 | 0.002 | 0.038 | 0.591 | ||||
| CAP (dB/m) | 0.226 | 0.002 | 0.060 | 0.540 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenovia, S.; Stanciu, C.; Sfarti, C.; Singeap, A.-M.; Cojocariu, C.; Girleanu, I.; Dimache, M.; Chiriac, S.; Muzica, C.M.; Nastasa, R.; et al. Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter for the Diagnosis of Liver Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Diagnostics 2021, 11, 787. https://doi.org/10.3390/diagnostics11050787
Zenovia S, Stanciu C, Sfarti C, Singeap A-M, Cojocariu C, Girleanu I, Dimache M, Chiriac S, Muzica CM, Nastasa R, et al. Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter for the Diagnosis of Liver Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Diagnostics. 2021; 11(5):787. https://doi.org/10.3390/diagnostics11050787
Chicago/Turabian StyleZenovia, Sebastian, Carol Stanciu, Catalin Sfarti, Ana-Maria Singeap, Camelia Cojocariu, Irina Girleanu, Mihaela Dimache, Stefan Chiriac, Cristina Maria Muzica, Robert Nastasa, and et al. 2021. "Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter for the Diagnosis of Liver Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease" Diagnostics 11, no. 5: 787. https://doi.org/10.3390/diagnostics11050787






