Incidence and Clinical Implications of Autoimmune Thyroiditis in the Development of Acne in Young Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical and Paraclinical Investigations
2.3. Statistical Analysis
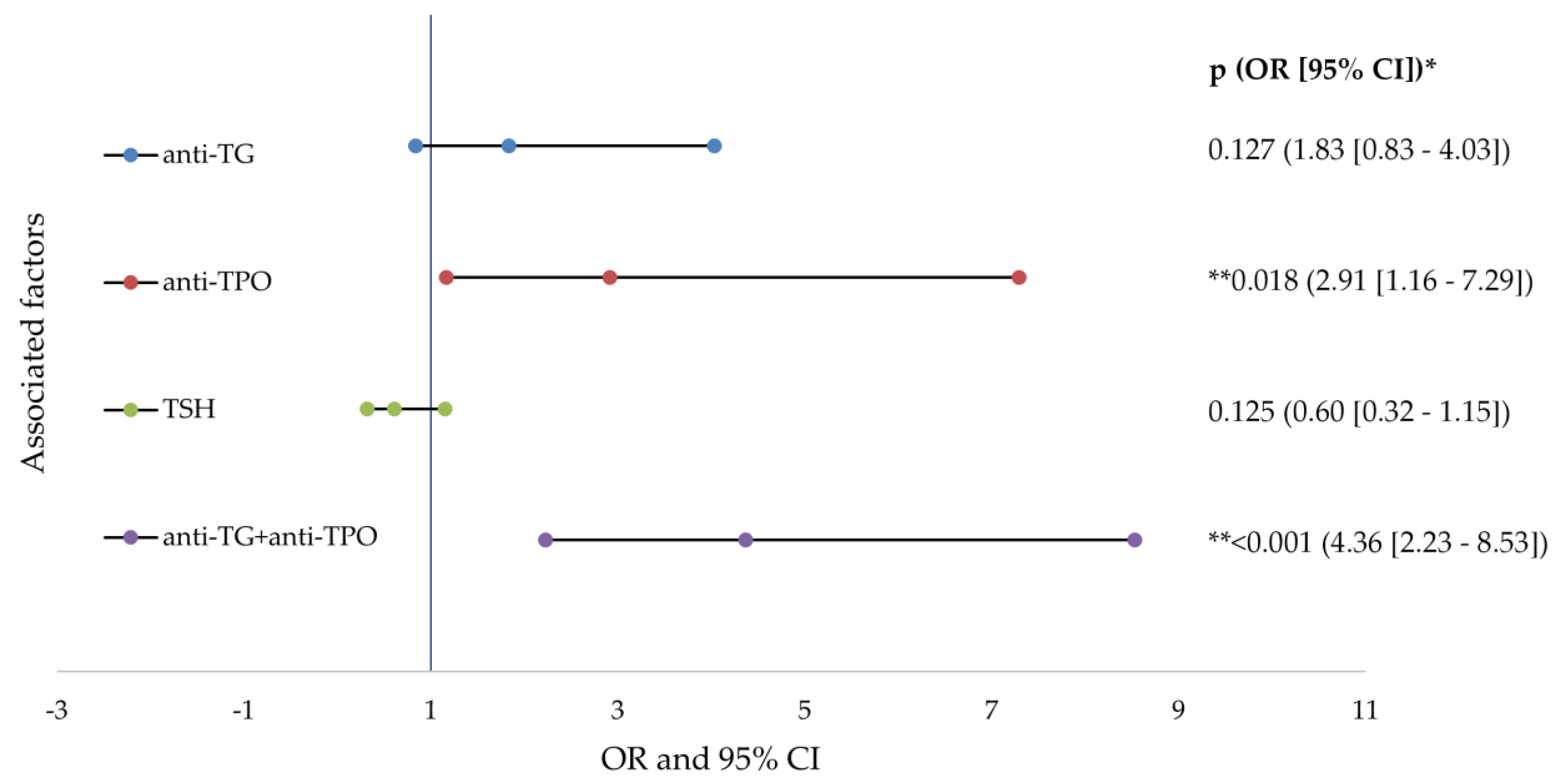
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asai, Y.; Baibergenova, A.; Dutil, M.; Humphrey, S.; Hull, P.R.; Lynde, C.; Poulin, Y.; Shear, N.H.; Tan, J.; Toole, J.; et al. Management of acne: Canadian clinical practice guideline. CMAJ 2016, 188, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.D.; Umari, T.; Dunnick, C.A.; Dellavalle, R.P. The epidemiology of acne vulgaris in late adolescence. Adolesc. Health Med. Ther. 2016, 7, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.C.; Maglione, J.; Hillebrand, G.G.; Miyamoto, K.; Kimball, A.B. Acne vulgaris in women: Prevalence across the life span. J. Womens Health 2012, 21, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Cheng, C.; Hillebrand, G.; Miyamoto, K.; Kimball, A. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J. Eur. Acad. Derm. Venereol. 2010, 25, 1054–1060. [Google Scholar] [CrossRef]
- Toyoda, M.; Morohashi, M. Pathogenesis of acne. Med. Electron Microsc. 2001, 34, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Vinkel, C.; Thomsen, S.F. Autoinflammatory syndromes associated with hidradenitis suppurativa and/or acne. Int. J. Dermatol. 2017, 56, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Vergou, T.; Mantzou, E.; Tseke, P.; Moustou, A.E.; Katsambas, A.; Alevizaki, M.; Antoniou, C. Association of thyroid autoimmunity with acne in adult women. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.S.; Chung, H.K. Recent Advances in Autoimmune Thyroid Diseases. Endocrinol. Metab. 2016, 31, 379–385. [Google Scholar] [CrossRef]
- Swain, M.; Swain, T.; Mohanty, B.K. Autoimmune thyroid disorders—An update. Indian J. Clin. Biochem. 2005, 20, 9–17. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Tsekouras, K.C.; Evangelopoulos, A.D.; Kotsiris, D.A.; Tzortzinis, A.A. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell. J. Nucl. Med. 2015, 18, 222–227. [Google Scholar]
- Bliddal, S.; Nielsen, C.H.; Feldt-Rasmussen, U. Recent advances in understanding autoimmune thyroid disease: The tallest tree in the forest of polyautoimmunity. F1000Research 2017, 6, 1776. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, J.; Schweiger, B. Isotretinoin as a Possible Environmental Trigger to Autoimmunity in Genetically Susceptible Patients. Case Report. Pediatr. 2017, 2017, 4207656. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kumar, K.; Brisc, C.; Rus, M.; Nistor-Cseppento, C.; Bustea, C.; Aron, R.; Pantis, C.; Zengin, G.; Sehgal, A.; et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed. Pharmacother. 2021, 133, 110959. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.S.; Krowchuk, D.P.; Leyden, J.J.; Lucky, A.W.; Shalita, A.R.; Siegfried, E.C.; Thiboutot, D.M.; Van Voorhees, A.S.; Beutner, K.A.; Sieck, C.K.; et al. Guidelines of care for acne vulgaris management. J. Am. Acad. Dermatol. 2007, 56, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Purdy, S.; de Berker, D. Acne vulgaris. BMJ Clin. Evid. 2011, 2011, 1714. [Google Scholar]
- Nuzzo, V.; Tauchmanova, L.; Colasanti, P.; Zuccoli, A.; Colao, A. Idiopathic chronic urticaria and thyroid autoimmunity: Experience of a single center. Derm. Endocrinol. 2011, 3, 255–258. [Google Scholar] [CrossRef][Green Version]
- Stewart, T.J.; Bazergy, C. Thyroid autoimmunity in female post-adolescent acne: A case-control study. Derm. Endocrinol. 2017, 9, e1405198. [Google Scholar] [CrossRef][Green Version]
- Safran, M.; Paul, T.L.; Roti, E.; Braverman, L.E. Environmental factors affecting autoimmune thyroid disease. Endocrinol. Metab. Clin. N. Am. 1987, 16, 327–342. [Google Scholar] [CrossRef]
- Saboori, A.M.; Rose, N.R.; Bresler, H.S.; Vladut-Talor, M.; Burek, C.L. Iodination of human thyroglobulin (Tg) alters its immunoreactivity. I. Iodination alters multiple epitopes of human Tg. Clin. Exp. Immunol. 1998, 113, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M. Hashimoto’s Thyroiditis. In Thyroid Diseases: Pathogenesis, Diagnosis, and Treatment; Vitti, P., Hegedüs, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 205–247. [Google Scholar] [CrossRef]
- McLeod, D.S.A.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef]
- Amouzegar, A.; Gharibzadeh, S.; Kazemian, E.; Mehran, L.; Tohidi, M.; Azizi, F. The Prevalence, Incidence and Natural Course of Positive Antithyroperoxidase Antibodies in a Population-Based Study: Tehran Thyroid Study. PLoS ONE 2017, 12, e0169283. [Google Scholar] [CrossRef]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Pedersen, I.B.; Knudsen, N.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Laurberg, P. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin. Endocrinol. 2003, 58, 36–42. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J.; Tunbrldge, W.M.G.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Evans, J.G.; Hasan, D.M.; Rodgers, H.; Tunbridge, W.M.; et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Bjoro, T.; Holmen, J.; Kruger, O.; Midthjell, K.; Hunstad, K.; Schreiner, T.; Sandnes, L.; Brochmann, H. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT). Eur. J. Endocrinol. 2000, 143, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.; Jorgensen, T.; Rasmussen, S.; Christiansen, E.; Perrild, H. The prevalence of thyroid dysfunction in a population with borderline iodine deficiency. Clin. Endocrinol. 1999, 51, 361–367. [Google Scholar] [CrossRef]
- Hoogendoorn, E.H.; Hermus, A.R.; de Vegt, F.; Ross, H.A.; Verbeek, A.L.; Kiemeney, L.A.; Swinkels, D.W.; Sweep, F.C.; Heijer, M.D. Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: Influences of age and sex. Clin. Chem. 2006, 52, 104–111. [Google Scholar] [CrossRef]
- Heydarian, P.; Azizi, F. Thyroid dysfunction and autoantibodies 10 years after implementation of universal salt iodization: Tehran Thyroid Study. Iran. J. Endocrinol. Metab. 2002, 4, 229–241. [Google Scholar]
- Li, Y.; Teng, D.; Shan, Z.; Teng, X.; Guan, H.; Yu, X.; Fan, C.; Chong, W.; Yang, F.; Dai, H.; et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J. Clin. Endocrinol. Metab. 2008, 93, 1751–1757. [Google Scholar] [CrossRef]
- Rajan, V. Raised incidence of autoimmune thyroiditis among females in 2nd, 3rd and 4th decades: A randomized study. Int. Surg. J. 2019, 6, 1074–1077. [Google Scholar]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [CrossRef]
- Whitacre, C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001, 2, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Chailurkit, L.-O.; Aekplakorn, W.; Ongphiphadhanakul, B. The relationship between circulating estradiol and thyroid autoimmunity in males. Eur. J. Endocrinol. 2014, 170, 63–67. [Google Scholar] [CrossRef]
- Hazarika, N.; Archana, M. The Psychosocial Impact of Acne Vulgaris. Indian J. Dermatol. 2016, 61, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A review of diagnosis and treatment of acne in adult female patients. Int. J. Womens Dermatol. 2018, 4, 56–71. [Google Scholar] [CrossRef]
- Chelmea, L.; Di Modugno, F.; Samota, I.; Bobescu, E.; Floroian, L.; Restani, P.; Cioca, G.; Bungau, S.; Badea, M. New Electrochemical Detection Strategies for Iodinated Compounds. Rev. Chim. 2019, 70, 919–924. [Google Scholar] [CrossRef]


| Characteristics | AIT + Acne (n = 170) | Acne (n = 66) | p Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Gender | |||||
| Women | 149 | 87.65 | 38 | 57.58 | 0.012 * |
| Men | 21 | 12.35 | 28 | 42.42 | <0.001 * |
| Age (years) | |||||
| 12–21 | 26 | 32.05 | 52 | 67.54 | 0.002 * |
| >21 | 144 | 87.34 | 14 | 9.22 | <0.001 * |
| Average | 29 ± 8 years | 18 ± 6 years | <0.001 ** | ||
| Diagnostic | |||||
| Mild acne | 2 | 11.11 | 16 | 88.89 | <0.001 * |
| Moderate acne | 60 | 63.16 | 35 | 36.84 | 0.010 * |
| Severe acne | 108 | 87.80 | 15 | 12.20 | <0.001 * |
| Thyroid function | |||||
| Hyperfunction | 20 | 11.76 | 0 | 0 | <0.001 * |
| Normal function | 103 | 60.59 | 66 | 100 | |
| Hypofunction | 47 | 27.65 | 0 | 0 | |
| Antibodies | |||||
| Anti-TG | <0.001 * | ||||
| <115 UI/mL | 30 | 17.65 | 66 | 100 | |
| ≥115 UI/mL | 140 | 82.35 | 0 | 0 | |
| Anti-TPO | <0.001 * | ||||
| <35 UI/mL | 23 | 13.53 | 66 | 100 | |
| ≥35 UI/mL | 147 | 86.47 | 0 | 0 | |
| TSH | <0.001 * | ||||
| <0.39 (0.4) UI/mL | 20 | 11.76 | 0 | 0 | |
| 0.39 (0.4)–4 UI/mL | 103 | 60.59 | 66 | 100 | |
| ≥4 UI/mL | 47 | 27.65 | 0 | 0 | |
| Characteristics | AIT + Acne (n = 170) | Acne (n = 66) | p Value | ||
|---|---|---|---|---|---|
| M (UI/mL) | SD (UI/mL) | M (UI/mL) | SD (UI/mL) | ||
| Anti-TG | |||||
| Total | 439.4 | 264.9 | 19.29 | 16 | <0.001 * |
| Mild acne | 58.50 | 6.36 | 12.62 | 11.92 | <0.001 * |
| Moderate acne | 456.57 | 259.20 | 20 | 14.82 | <0.001 * |
| Severe acne | 436.85 | 266.22 | 23.8 | 20.67 | <0.001 * |
| Anti-TPO | |||||
| Total | 289.8 | 224.6 | 8 | 4.59 | <0.001 * |
| Mild acne | 55.30 | 47.65 | 5 | 3.99 | <0.001 * |
| Moderate acne | 228.63 | 210.70 | 8 | 4.46 | <0.001 * |
| Severe acne | 328.12 | 224.60 | 10 | 4.15 | <0.001 * |
| TSH | |||||
| Total | 2.79 | 1.97 | 2.11 | 0.79 | <0.001 * |
| Mild acne | 2.77 | 1.72 | 1.81 | 0.84 | 0.183 * |
| Moderate acne | 3.03 | 2.29 | 2.36 | 0.67 | 0.095 * |
| Severe acne | 2.79 | 1.97 | 1.85 | 0.85 | 0.071 * |
| Type of Acne | Degree of Acne | Average Difference (I–J) | Average Standard Error | p | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Mild | Moderate | −173.327 | 157.645 | 0.614 | −553.42 | 206.77 |
| Severe | −272.820 | 156.511 | 0.228 | −650.18 | 104.54 | |
| Moderate | Mild | 173.327 | 157.645 | 0.614 | −206.77 | 553.42 |
| Severe | −99.494 * | 35.314 | 0.016 | −184.64 | −14.35 | |
| Severe | Mild | 272.820 | 156.511 | 0.228 | −104.54 | 650.18 |
| moderate | 99.494 * | 35.314 | 0.016 | 14.35 | 184.64 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endres, L.; Tit, D.M.; Bungau, S.; Pascalau, N.A.; Maghiar Țodan, L.; Bimbo-Szuhai, E.; Iancu, G.M.; Negrut, N. Incidence and Clinical Implications of Autoimmune Thyroiditis in the Development of Acne in Young Patients. Diagnostics 2021, 11, 794. https://doi.org/10.3390/diagnostics11050794
Endres L, Tit DM, Bungau S, Pascalau NA, Maghiar Țodan L, Bimbo-Szuhai E, Iancu GM, Negrut N. Incidence and Clinical Implications of Autoimmune Thyroiditis in the Development of Acne in Young Patients. Diagnostics. 2021; 11(5):794. https://doi.org/10.3390/diagnostics11050794
Chicago/Turabian StyleEndres, Laura, Delia Mirela Tit, Simona Bungau, Nicoleta Anamaria Pascalau, Laura Maghiar Țodan, Erika Bimbo-Szuhai, Gabriela Mariana Iancu, and Nicoleta Negrut. 2021. "Incidence and Clinical Implications of Autoimmune Thyroiditis in the Development of Acne in Young Patients" Diagnostics 11, no. 5: 794. https://doi.org/10.3390/diagnostics11050794
APA StyleEndres, L., Tit, D. M., Bungau, S., Pascalau, N. A., Maghiar Țodan, L., Bimbo-Szuhai, E., Iancu, G. M., & Negrut, N. (2021). Incidence and Clinical Implications of Autoimmune Thyroiditis in the Development of Acne in Young Patients. Diagnostics, 11(5), 794. https://doi.org/10.3390/diagnostics11050794










