Bacterial Autofluorescence Digital Imaging Guides Treatment in Stage 4 Pelvic Pressure Injuries: A Preliminary Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Bacterial Autofluorescence Digital Imaging Procedure
2.3. Study Group Demographics
3. Results
3.1. Summary of Results
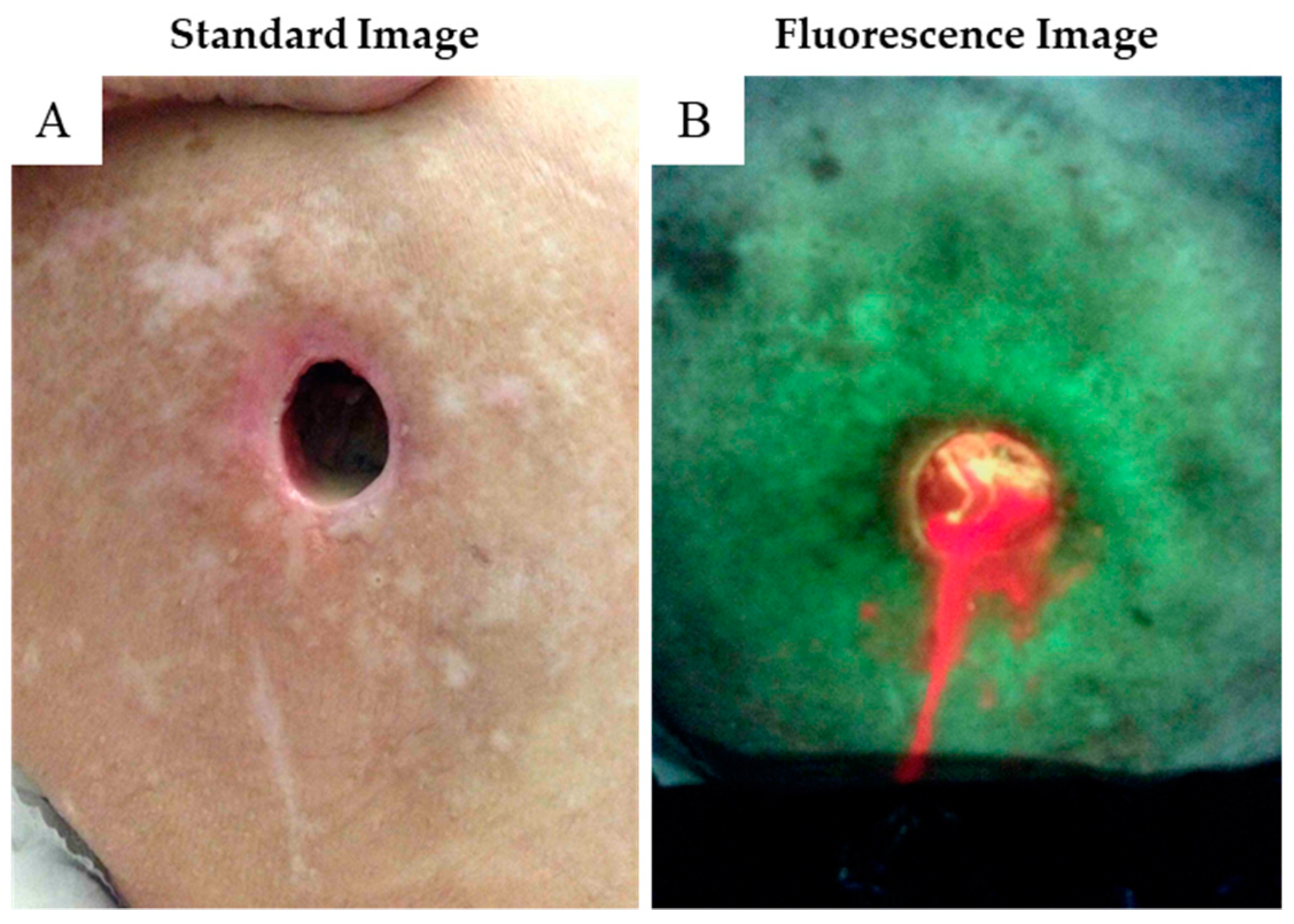
3.2. Case Reports
3.2.1. Case 1
3.2.2. Case 2
3.2.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White-Chu, E.F.; Flock, P.; Struck, B.; Aronson, L. Pressure ulcers in long-term care. Clin. Geriatr. Med. 2011, 27, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Winterberg, H.; Moffatt, C.J. Health-related quality of life and pressure ulceration assessment in patients treated in the community. Wound Repair Regen. 2002, 10, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Langemo, D.K.; Melland, H.; Hanson, D.; Olson, B.; Hunter, S. The lived experience of having a pressure ulcer: A qualitative analysis. Adv. Skin Wound Care 2000, 13, 225–235. [Google Scholar] [PubMed]
- Jaul, E.; Barron, J.; Rosenzweig, J.P.; Menczel, J. An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr. 2018, 18, 305. [Google Scholar] [CrossRef]
- Jump, R.L.P.; Levy, S.M.; Saltsman, W.S. Post-acute and Long-term Care Settings as First Responders for the Surviving Sepsis Campaign. J. Am. Med. Dir. Assoc. 2019, 20, 275–278. [Google Scholar] [CrossRef]
- Girard, T.D.; Opal, S.M.; Ely, E.W. Insights into severe sepsis in older patients: From epidemiology to evidence-based management. Clin. Infect. Dis. 2005, 40, 719–727. [Google Scholar] [CrossRef]
- Esper, A.M.; Moss, M.; Lewis, C.A.; Nisbet, R.; Mannino, D.M.; Martin, G.S. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit. Care Med. 2006, 34, 2576–2582. [Google Scholar] [CrossRef]
- Girard, T.D.; Ely, E.W. Bacteremia and sepsis in older adults. Clin. Geriatr. Med. 2007, 23, 633–647. [Google Scholar] [CrossRef]
- Hill, R.; Woo, K. A Prospective Multisite Observational Study Incorporating Bacterial Fluorescence Information Into the UPPER/LOWER Wound Infection Checklists. Wounds 2020, 32, 299–308. [Google Scholar]
- Hurley, C.M.; McClusky, P.; Sugrue, R.M.; Clover, J.A.; Kelly, J.E. Efficacy of a bacterial fluorescence imaging device in an outpatient wound care clinic: A pilot study. J. Wound Care 2019, 28, 438–443. [Google Scholar] [CrossRef]
- Raizman, R.; Little, W.; Smith, A.C. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing. Diagnostics 2021, 11, 280. [Google Scholar] [CrossRef]
- Rennie, M.Y.; Lindvere-Teene, L.; Tapang, K.; Linden, R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: A clinical study. J. Wound Care 2017, 26, 452–460. [Google Scholar] [CrossRef]
- Serena, T.E.; Harrell, K.; Serena, L.; Yaakov, R.A. Real-time bacterial fluorescence imaging accurately identifies wounds with moderate-to-heavy bacterial burden. J. Wound Care 2019, 28, 346–357. [Google Scholar] [CrossRef]
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv. Wound Care 2021, 10, 123–136. [Google Scholar] [CrossRef]
- Price, N. Routine fluorescence imaging to detect wound bacteria reduces antibiotic use and antimicrobial dressing expenditure while improving healing rates: Retrospective analysis of 229 foot ulcers. Diagnostics 2020, 10, 927. [Google Scholar] [CrossRef]
- Raizman, R. Fluorescence imaging guided dressing change frequency during negative pressure wound therapy: A case series. J. Wound Care 2019, 28 (Suppl. 9), S28–S37. [Google Scholar] [CrossRef]
- Raizman, R.; Dunham, D.; Lindvere-Teene, L.; Jones, L.M.; Tapang, K.; Linden, R.; Rennie, M.Y. Use of a bacterial fluorescence imaging device: Wound measurement, bacterial detection and targeted debridement. J. Wound Care 2019, 28, 824–834. [Google Scholar] [CrossRef]
- Rennie, M.Y.; Dunham, D.; Lindvere-Teene, L.; Raizman, R.; Hill, R.; Linden, R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X(TM). Diagnostics 2019, 9, 22. [Google Scholar] [CrossRef]
- Meyer, J.M.; Neely, A.; Stintzi, A.; Georges, C.; Holder, I.A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 1996, 64, 518–523. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Bay, L.; Kragh, K.N.; Eickhardt, S.R.; Poulsen, S.S.; Gjerdrum, L.M.R.; Ghathian, K.; Calum, H.; Ågren, M.S.; Bjarnsholt, T. Bacterial Aggregates Establish at the Edges of Acute Epidermal Wounds. Adv. Wound Care 2018, 7, 105–113. [Google Scholar] [CrossRef]
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. N. Am. 2020, 100, 757–776. [Google Scholar] [CrossRef]



| Case | Age | Charlson Index 1 | Sepsis Diagnosis | Autofluorescence Imaging Findings | Impact of Imaging |
|---|---|---|---|---|---|
| 1 | 71 | 7 | Yes, informed by images | Gross wound contamination (red fluorescence) was found suggesting low-grade wound sepsis, despite the wound physically looking benign | (1) Demonstrated objectively the insufficiency of the prescribed cleansing regime, (2) led to a switch to cleanser with irrigation and enabled immediate evaluation of its efficacy, (3) prompted collection of a wound culture |
| 2 | 95 | 6 | Yes, informed by images | Widespread red fluorescence indicative of heavy bacterial contamination of the wound bed | Severity of imaging findings prompted immediate wound debridement, which uncovered a large, deep abscess and led to antibiotic prescription |
| 3 | 71 | 8 | Yes, informed by images | Red fluorescent pus readily observed on images of sero-sanguinous wound fluid | Large wound fluid amount prompted further clinical investigation revealing probable periprosthetic total join infection |
| 4 | 76 | 9 | no | Initial images and follow-up scans were negative for bacterial loads of concern. | Indicated that advanced treatments were unnecessary; wound healed without incident |
| 5 | 88 | 4 | no | Across multiple wounds, initial images and follow-up scans were negative for bacterial loads of concern. | Indicated that advanced treatments were unnecessary; wound continues to progress well |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiehl, J.B. Bacterial Autofluorescence Digital Imaging Guides Treatment in Stage 4 Pelvic Pressure Injuries: A Preliminary Case Series. Diagnostics 2021, 11, 839. https://doi.org/10.3390/diagnostics11050839
Stiehl JB. Bacterial Autofluorescence Digital Imaging Guides Treatment in Stage 4 Pelvic Pressure Injuries: A Preliminary Case Series. Diagnostics. 2021; 11(5):839. https://doi.org/10.3390/diagnostics11050839
Chicago/Turabian StyleStiehl, James B. 2021. "Bacterial Autofluorescence Digital Imaging Guides Treatment in Stage 4 Pelvic Pressure Injuries: A Preliminary Case Series" Diagnostics 11, no. 5: 839. https://doi.org/10.3390/diagnostics11050839
APA StyleStiehl, J. B. (2021). Bacterial Autofluorescence Digital Imaging Guides Treatment in Stage 4 Pelvic Pressure Injuries: A Preliminary Case Series. Diagnostics, 11(5), 839. https://doi.org/10.3390/diagnostics11050839





