Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes
Abstract
:1. Introduction
2. Materials and Methods
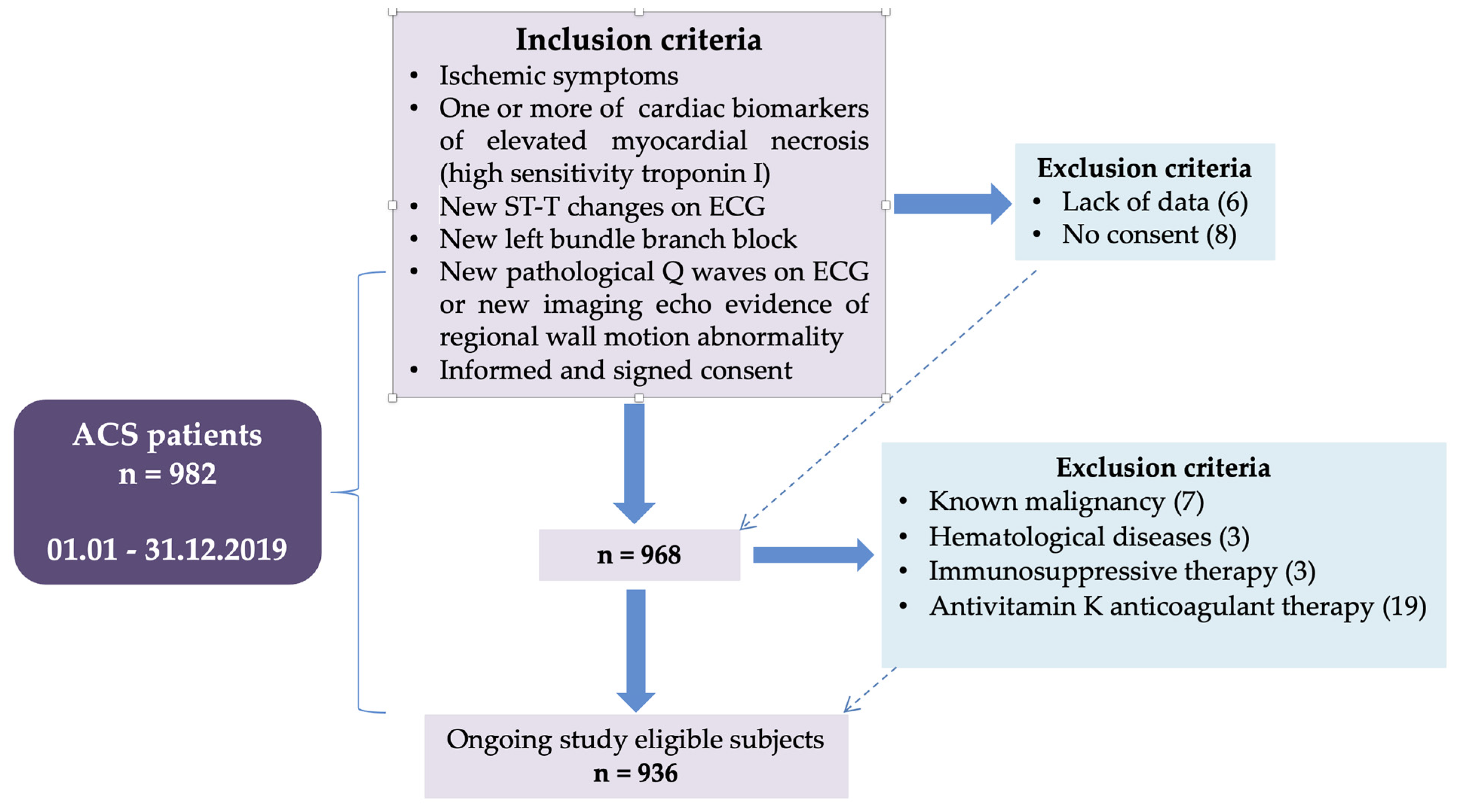
2.1. Study Design
2.2. Methodology
2.3. Statistical Analysis
3. Results
3.1. Hematological and Coagulation Parameters as Prognostic Factors for Mortality
3.2. Hematological and Coagulation Parameters as Prognostic Factors for Complications
3.3. Hematological and Coagulation Parameters as Prognostic Factors for Ventricular Rhythm Disturbances
3.4. Hematological and Coagulation Parameters as Prognostic Factors for Atrial Fibrillation
3.5. Hematological and Coagulation Parameters as Prognostic Factors for Heart Failure
3.6. Hematological and Coagulation Parameters as Prognostic Factors for New Ischemic Event
3.7. Hematological and Coagulation Parameters as Prognostic Factors for Stroke
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, R.W.; Sidney, S.; Chandra, M.; Sorel, M.; Selby, J.V.; Go, A.S. Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med. 2010, 362, 2155–2165. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, J.; Pieszko, K.; Burchardt, P.; Rzeźniczak, J.; Hiczkiewicz, J. The Role of Hematological Indices in Patients with Acute Coronary Syndrome. Dis. Markers 2017, 2017, 3041565. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Morrow, D.A.; Cannon, C.P.; Murphy, S.A.; Demopoulos, L.A.; DiBattiste, P.M.; McCabe, C.H.; Braunwald, E.; Gibson, C.M. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 2002, 40, 1761–1768. [Google Scholar] [CrossRef] [Green Version]
- Monteiro Júnior, J.G.D.M.; Torres, D.D.O.C.; da Silva, M.C.F.C.; Martins, C.M.D.H.; da Silva, I.K.; do Nascimento, M.E.M.; Dos Santos, A.C.O.; Montarroyos, U.R.; Filho, D.C.S. Prognostic value of hematological parameters in patients with acute myocardial infarction: Intrahospital outcomes. PLoS ONE 2018, 13, e0194897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez, J.; Núñez, E.; Bodí, V.; Sanchis, J.; Mainar, L.; Miñana, G.; Fácila, L.; Bertomeu, V.; Merlos, P.; Darmofal, H.; et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myocardial infarction. Coron. Artery Dis. 2010, 21, 1–7. [Google Scholar] [CrossRef]
- Dabbah, S.; Hammerman, H.; Markiewicz, W.; Aronson, D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am. J. Cardiol. 2010, 105, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Akin, F.; Köse, N.; Ayça, B.; Katkat, F.; Duran, M.; Uysal, O.K.; Arinc, H. Relation between red cell distribution width and severity of coronary artery disease in patients with acute myocardial infarction. Angiology 2013, 64, 592–596. [Google Scholar] [CrossRef]
- Kırış, T.; Vatansever, S.; Yazıcı, S.; Çelik, A.; Karaca, E.V.M.; Bayata, M.F.; Nazlı, C. Prognostic Value of Prothrombin Time in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Koşuyolu Heart J. 2018, 21, 98–107. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. ESC Scientific Document Group 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. ESC Scientific Document Group 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Lancellotti, P.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. ESC Scientific Document Group. Fourth universal definition of myocardial infarction. Eur. Heart J. 2018, 40, 237–269. [Google Scholar] [CrossRef] [Green Version]
- Uyarel, H.; Ergelen, M.; Cicek, G.; Kaya, M.G.; Ayhan, E.; Turkkan, C.; Yıldırım, E.; Kırbas, V.; Onturk, E.T.; Erer, H.B.; et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron. Artery Dis. 2011, 22, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.; Ahmad, A.; Malik, Q.Z.; Shaukat, H.; Jafarizade, M.; Kahe, F.; Kalayci, A. Prognostic Value of Red Cell Distribution Width in Acute Coronary Syndrome. Open Access Blood Res. Transfus. J. 2018, 1, 86–92. [Google Scholar] [CrossRef]
- Sun, X.P.; Chen, W.M.; Sun, Z.J.; Ding, X.S.; Gao, X.Y.; Liang, S.W.; Zhao, H.Q.; Yao, D.K.; Chen, H.; Li, H.W.; et al. Impact of red blood cell distribution width on long-term mortality in patients with ST-elevation myocardial infarction. Cardiology 2014, 128, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Uyarel, H.; Ergelen, M.; Karacimen, D.; Ugur, M.; Turer, A.; Bozbay, M.; Ayhan, E.; Akgul, O.; Uslu, N. Relationship between red Blood Cell Distribution Width and the Clinical Outcomes in non-ST Elevation Myocardial Infarction and Unstable Angina Pectoris: A 3-Year Follow. Coron. Artery Dis. 2012, 23, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Abrahan, L.L., 4th; Ramos, J.D.A.; Cunanan, E.L.; Tiongson, M.D.A.; Punzalan, F.E.R. Red Cell Distribution Width and Mortality in Patients With Acute Coronary Syndrome: A Meta-Analysis on Prognosis. Cardiol Res. 2018, 9, 144–152. [Google Scholar] [CrossRef]
- Tunçez, A.; Çetin, M.S.; Çetin, E.H.; Ylmaz, S.; Korkmaz, A.; Uçar, F.M. Association between RDW and stent thrombosis in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Medicine 2017, 96, e5986. [Google Scholar] [CrossRef]
- Chang, X.W.; Zhang, S.Y.; Wang, H.; Zhang, M.M.; Zheng, W.F.; Ma, H.F.; Gu, Y.F.; Wei, J.H.; Qiu, C.G. Combined value of red blood cell distribution width and global registry of acute coronary events risk score on predicting long-term major adverse cardiac events in STEMI patients undergoing primary PCI. Oncotarget 2018, 9, 13971–13980. [Google Scholar] [CrossRef]
- Acet, H.; Ertaş, F.; Akıl, M.A.; Özyurtlu, F.; Polat, N.; Bilik, M.Z.; Aydın, M.; Oylumlu, M.; Yüksel, M.; Yıldız, A.; et al. Relationship Between Hematologic Indices and Global Registry of Acute Coronary Events Risk Score in Patients With ST-Segment Elevation Myocardial Infarction. Clin. Appl. Thromb. Hemost. 2016, 22, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Timóteo, A.T.; Papoila, A.L.; Lousinha, A.; Alves, M.; Miranda, F.; Ferreira, M.L.; Ferreira, R.C. Predictive impact on medium-term mortality of hematological parameters in Acute Coronary Syndromes: Added value on top of GRACE risk score. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Filippozzi, L.; Montagnana, M.; Salvagno, G.L.; Franchini, M.; Guidi, G.C.; Targher, G. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin. Chem. Lab. Med. 2009, 47, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Barron, H.V.; Cannon, C.P.; Murphy, S.A.; Braunwald, E.; Gibson, C.M. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: A thrombolysis in myocardial infarction 10 sub study. Circulation 2000, 102, 2329–2334. [Google Scholar] [CrossRef]
- Grau, A.J.; Boddy, A.W.; Dukovic, D.A.; Buggle, F.; Lichy, C.; Brandt, T.; Hacke, W. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke 2004, 35, 1147–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, C.P.; McCabe, C.H.; Wilcox, R.G.; Bentley, J.H.; Braunwald, E. Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. Am. J. Cardiol. 2001, 87, 636–639. [Google Scholar] [CrossRef]
- Chia, S.; Nagurney, J.T.; Brown, D.F.; Raffel, O.C.; Bamberg, F.; Senatore, F.; Wackers, F.J.; Jang, I.K. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am. J. Cardiol. 2009, 103, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Anjarwani, S.; Nugraha, K.A.; FadLan, M.R. Leucocytosis as the Short-Term Predictor for Mortality in Acute Coronary Syndrome Patients Undergoing Percutaneous Coronary Intervention. Heart Sci. J. 2020, 1, 14–18. [Google Scholar] [CrossRef]
- Rahimi, K.; Watzlawek, S.; Thiele, H.; Secknus, M.A.; Hayerizadeh, B.F.; Niebauer, J.; Schuler, G. Incidence, time course, and predictors of early malignant ventricular arrhythmias after non-ST-segment elevation myocardial infarction in patients with early invasive treatment. Eur. Heart J. 2006, 27, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Adam, A.M.; Ali, M.A.; Shah, A.A.; Rizvi, A.H.; Rehan, A.; Godil, A.; Abbas, A.H.; Durrani, N.; Shaikh, A.T.; Mallick, M.; et al. Efficacy of Hematological and Coagulation Parameters in the Diagnosis and Prognosis of Patients with Acute Coronary Syndrome. J. Tehran Heart Cent. 2018, 13, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, D.; Curwin, J.; Gomes, J.A.; Fuster, V. Sudden death in coronary artery disease: Acute ischemia vs myocardial substrate. Circulation 1997, 96, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Núñez, E.; Bodí, V.; Sanchis, J.; Miñana, G.; Mainar, L.; Santas, E.; Merlos, P.; Rumiz, E.; Darmofal, H.; et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am. J. Cardiol. 2008, 101, 747–752. [Google Scholar] [CrossRef]
- Azab, B.; Zaher, M.; Weiserbs, K.F.; Torbey, E.; Lacossiere, K.; Gaddam, S.; Gobunsuy, R.; Jadonath, S.; Baldari, D.; McCord, D.; et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am. J. Cardiol. 2010, 106, 470–476. [Google Scholar] [CrossRef]
- Duffy, B.K.; Gurm, H.S.; Rajagopal, V.; Gupta, R.; Ellis, S.G.; Bhatt, D.L. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am. J. Cardiol. 2006, 97, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Tamhane, U.U.; Aneja, S.; Montgomery, D.; Rogers, E.K.; Eagle, K.A.; Gurm, H.S. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am. J. Cardiol. 2008, 102, 653–657. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chandra, P.; Guha, G.; Kalra, V.; Chakraborty, A.; Frankel, R.; Shani, J. Pre-procedural Elevated White Blood Cell Count and Neutrophil-Lymphocyte (N/L) Ratio are Predictors of Ventricular Arrhythmias During Percutaneous Coronary Intervention. Cardiovasc. Hematol. Disord. Drug Targets 2011, 11, 58–60. [Google Scholar] [CrossRef]
- Akpek, M.; Kaya, M.G.; Lam, Y.Y.; Sahin, O.; Elcik, D.; Celik, T.; Ergin, A.; Gibson, C.M. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am. J. Cardiol. 2010, 110, 621–627. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Jiang, X.; Zhu, H.; Lu, Z.; Xu, L. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: A meta-analysis of observational studies. Atherosclerosis 2014, 234, 206–213. [Google Scholar] [CrossRef]
- Ayça, B.; Akın, F.; Celik, O.; Sahin, I.; Yildiz, S.S.; Avci, I.I.; Gulsen, K.; Okuyan, E.; Dinckal, M.H. Neutrophil to lymphocyte ratio is related to stent thrombosis and high mortality in patients with acute myocardial infarction. Angiology 2015, 66, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Zouridakis, E.G.; Garcia-Moll, X.; Kaski, J.C. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am. J. Cardiol. 2000, 86, 449–451. [Google Scholar] [CrossRef]
- Rousseau, M.; Yan, R.T.; Tan, M.; Lefkowitz, C.J.; Casanova, A.; Fitchett, D.; Jolly, S.S.; Langer, A.; Goodman, S.G.; Yan, A.T. Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial Investigators Relation Between Haemoglobin Level and Recurrent Myocardial Ischemia in Acute Coronary Syndromes Detected by Continuous Electrocardiographic Monitoring. Am. J. Cardiol 2010, 106, 1417–1422. [Google Scholar] [CrossRef]
- Babes, E.E.; Babes, V.V. Detection of Silent Ischemia in Patients with Type 2 Diabetes. In Recent Advances in the Pathogenesis, Prevention and Management of Type 2 Diabetes and Its Complications; Zimering, M., Ed.; IntechOpen: London, UK, 2011; pp. 129–154. [Google Scholar] [CrossRef] [Green Version]
- Wester, A.; Attar, R.; Mohammad, A.; Andell, P.; Hofmann, R.; Jensen, J.; Szummer, K.; Erlinge, D.; Koul, S. Impact of Baseline Anaemia in Patients With Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: A Prespecified Analysis From the VALIDATE-SWEDEHEART Trial. J. Am. Heart Assoc. 2019, 8, e012741. [Google Scholar] [CrossRef]
- Azab, B.; Shah, N.; Akerman, M.; McGinn, J.T. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J. Thromb. Thrombolysis 2012, 34, 326–334. [Google Scholar] [CrossRef]
- Sun, X.P.; Li, J.; Zhu, W.W.; Li, D.B.; Chen, H.; Li, H.W.; Chen, W.M.; Hua, Q. Impact of platelet-to-lymphocyte ratio on clinical outcomes in patients with ST-segment elevation myocardial infarction. Angiology 2017, 68, 346–353. [Google Scholar] [CrossRef]
- Engelmann, M.D.M.; Svendsen, J.H. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur. Heart J. 2005, 26, 2083–2092. [Google Scholar] [CrossRef]
- Dong, G.; Huang, A.; Liu, L. Platelet-to-lymphocyte ratio and prognosis in STEMI: A meta-analysis Review. Eur. J. Clin. Investig. 2021, 51, e13386. [Google Scholar] [CrossRef]
- Yildiz, A.; Yuksel, M.; Oylumlu, M.; Polat, N.; Akyuz, A.; Acet, H.; Aydin, M.; Ülgen, M.S. The utility of the platelet–lymphocyte ratio for predicting no reflow in patients with ST-segment elevation myocardial infarction. Clin. Appl. Thromb. Hemost. 2015, 21, 223–228. [Google Scholar] [CrossRef]
- Pantea-Roșan, L.R.; Pantea, V.A.; Bungau, S.; Tit, D.M.; Behl, T.; Vesa, C.M.; Bustea, C.; Moleriu, R.D.; Rus, M.; Popescu, M.I.; et al. No-Reflow after PPCI—A Predictor of Short-Term Outcomes in STEMI Patients. J. Clin. Med. 2020, 9, 2956. [Google Scholar] [CrossRef]
- Celik, T.; Kaya, M.G.; Akpek, M.; Gunebakmaz, O.; Balta, S.; Sarli, B.; Duran, M.; Demirkol, S.; Uysal, O.K.; Oguzhan, A.; et al. Predictive value of admission platelet volume indices for in-hospital major adverse cardiovascular events in acute ST-segment elevation myocardial infarction. Angiology 2015, 66, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bekler, A.; Ozkan, M.T.; Tenekecioglu, E.; Gazi, E.; Yener, A.U.; Temiz, A.; Altun, B.; Barutcu, A.; Erbag, G.; Binnetoglu, E. Increased platelet distribution width is associated with severity of coronary artery disease in patients with acute coronary syndrome. Angiology 2015, 66, 638–643. [Google Scholar] [CrossRef]
- De Luca, G.; Venegoni, L.; Iorio, S.; Secco, G.G.; Cassetti, E.; Verdoia, M.; Schaffer, A.; Coppo, L.; Bellomo, G.; Marino, P.; et al. Platelet distribution width and the extent of coronary artery disease: Results from a large prospective study. Platelets 2010, 21, 508–514. [Google Scholar] [CrossRef]
- Kowara, M.; Grodecki, K.; Huczek, Z.; Puchta, D.; Paczwa, K.; Rymuza, B.; Zbroński, K.; Filipiak, K.J.; Opolski, G. Platelet distribution width predicts left ventricular dysfunction in patients with acute coronary syndromes treated with percutaneous coronary intervention. Kardiol. Pol. 2017, 75, 42–47. [Google Scholar] [CrossRef]
- Huczek, Z.; Kochman, J.; Filipiak, K.J.; Horszczaruk, G.J.; Grabowski, M.; Piatkowski, R.; Wilczynska, J.; Zielinski, A.; Meier, B.; Opolski, G. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Coll. Cardiol. 2005, 46, 284–290. [Google Scholar] [CrossRef] [Green Version]
- López-Cuenca, A.A.; Tello-Montoliu, A.; Roldán, V.; Pérez-Berbel, P.; Valdés, M.; Marín, F. Prognostic value of mean platelet volume in patients with non-ST-elevation acute coronary syndrome. Angiology 2012, 63, 241–244. [Google Scholar] [CrossRef]
- Klovaite, J.; Benn, M.; Yazdanyar, S.; Nordestgaard, B.G. High platelet volume and increased risk of myocardial infarction: 39,531 participants from the general population. J. Thromb. Haemost. 2011, 9, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Butkiewicz, A.M.; Kemona, H.; Dymicka-Piekarska, V.; Bychowski, J. Beta-thromboglobulin and platelets in unstable angina. Kardiol. Pol. 2003, 58, 449–455. [Google Scholar]
- Stoicescu, M.; Csepento, C.; Mutiu, G.; Bungau, S. The role of increased plasmatic renin level in the pathogenesis of arterial hypertension in young adults. Rom. J. Morphol. Embriol. 2011, 52 (Suppl. 1), 419–423. [Google Scholar]
- Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Pantea Stoian, A.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics 2020, 10, 483. [Google Scholar] [CrossRef]
- Sivri, N.; Tekin, G.; Yalta, K.; Aksoy, Y.; Senen, K.; Yetkin, E. Statins decrease mean platelet volume irrespective of cholesterol lowering effect. Kardiol. Pol. 2013, 71, 1042–1047. [Google Scholar] [CrossRef]
- Ault, K.A.; Rinder, H.M.; Mitchell, J.; Carmody, M.B.; Vary, C.P.; Hillman, R.S. The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am. J. Clin. Pathol. 1992, 98, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Prokisch, H.; Gieger, C.; Soranzo, N.; Mehta, D.; Rosskopf, D.; Lichtner, P.; Klopp, N.; Stephens, J.; Watkins, N.A.; et al. A genome-wide association study identifies three loci associated with mean platelet volume. Am. J. Hum. Genet. 2009, 84, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrmann, V.; Kneidinger, N.; Herkner, H.; Heinz, G.; Nikfardjam, M.; Bojic, A.; Schellongowski, P.; Angermayr, B.; Kitzberger, R.; Warszawska, J.; et al. Hypoxic hepatitis: Underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009, 35, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Fei, A.; Lin, Q.; Liu, J.; Wang, F.; Wang, H.; Pan, S. The relationship between coagulation abnormality and mortality in ICU patients: A prospective, observational study. Sci. Rep. 2015, 5, 9391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, E.L.; Qian, F.; Pine, M.; Fry, D.E.; Whitman, K.; Dennison, B.A. The value of adding laboratory data to coronary artery bypass grafting registry data to improve models for risk-adjusting provider mortality rates. Ann. Thorac. Surg. 2015, 99, 495–501. [Google Scholar] [CrossRef] [PubMed]


| Data | Clinical/Paraclinical Characteristics | Discharged (n = 849) | Mortality (n = 87) | p |
|---|---|---|---|---|
| Demographics | Age (Years) | 64.90 ± 11.56 | 72.98 ± 11.60 | <0.001 |
| Male | 559/849 (65.84%) | 42/87 (48.27%) | <0.001 | |
| BMI (kg/m²) | 30.07 ± 5.33 | 29.12 ± 4.59 | 0.06 | |
| Previous History | Smoking | 201/849 (23.67%) | 23/87 (26.44%) | 0.232 |
| Diabetes Mellitus | 261/849 (30.74%) | 33/87 (37.93%) | 0.172 | |
| Hypertension | 595/849 (70.08%) | 30/87 (34.48%) | <0.001 | |
| Dyslipidemia | 360/849 (42.40%) | 64/87 (73.56%) | <0.001 | |
| Diagnosis | UA | 438/849 (51.59%) | 3/87 (3.45%) | <0.001 |
| NSTEMI | 101/849 (11.89%) | 13/87 (14.94%) | 0.409 | |
| STEMI | 310/849 (36.51%) | 71/87 (81.61%) | <0.001 | |
| Number of Diseased Vessels | 1 | 236/692 (34.10%) | 5/28 (17.86%) | <0.001 |
| 2 | 177/692 (25.58%) | 1/28 (3.57%) | <0.001 | |
| 3 | 207/692 (29.91%) | 21/28 (75%) | <0.001 | |
| CBC | HGB (g/dL) | 13.78 ± 1.79 | 12.63 ± 2.19 | <0.001 |
| WBC (×10³/μL) | 10.45 ± 4.11 | 14.45 ± 6.90 | 0.006 | |
| NEU (×10³/μL) | 7.40 ± 3.84 | 11.19 ± 6.50 | 0.003 | |
| LYM (×10³/μL) | 2.16 ± 1.01 | 2.19 ± 1.59 | 0.391 | |
| PLT (×10³/μL) | 225.68 ± 70.91 | 237.84 ± 91.36 | 0.993 | |
| PDW (%) | 20.12 ± 1.24 | 19.95 ± 1.41 | 0.417 | |
| MPV (fl) | 8.39 ± 1.91 | 7.74 ± 1.52 | 0.124 | |
| NLR | 3.52 ± 4.08 | 6.45 ± 6.79 | 0.013 | |
| PLR | 128.17 ± 82.60 | 169.56 ± 117.23 | 0.010 | |
| RDW (%) | 12.39 ± 1.48 | 13.07 ± 1.26 | 0.001 | |
| Coagulation Tests | APTT(s) | 31.82 ± 17.00 | 36.49 ± 16.77 | 0.096 |
| PT(s) | 15.36 ± 6.69 | 17.85 ± 1.01 | <0.001 | |
| Creatinine (mg/dL) | - | 1.04 ± 0.56 | 1.72 ± 1.07 | <0.001 |
| LVEF (%) | 47.09 ± 8.63 | 34,4 ± 9.87 | <0.001 |
| NSTEMI CBC | Discharged (n = 101) | Mortality (n = 13) | p |
|---|---|---|---|
| HGB (g/dL) | 13.43 ± 1.87 | 12.36 ± 1.82 | 0.054 |
| WBC (×10³/μL) | 10.57 ± 4.196 | 17.00 ± 7.80 | <0.001 |
| NEU (×10³/μL) | 7.60 ± 3.94 | 12.72 ± 7.75 | 0.001 |
| LYM (×10³/μL) | 2.09 ± 0.95 | 3.10 ± 2.33 | 0.06 |
| PLT (×10³/μL) | 222.88 ± 63.35 | 235.90 ± 78.76 | 0.49 |
| PDW (%) | 20.08 ± 0.99 | 20.03 ± 1.30 | 0.71 |
| MPV (fl) | 8.11 ± 1.53 | 7.50 ± 1.54 | 0.26 |
| NLR | 3.66 ± 5.22 | 5.29 ± 6.32 | 0.23 |
| PLR | 139.24 ± 110.94 | 134.19 ± 99.78 | 0.78 |
| RDW (%) | 12.47 ± 1.43 | 13.50 ± 1.11 | 0.01 |
| APTT (s) | 34.44 ± 14.48 | 36.56 ± 15.38 | 0.36 |
| PT (s) | 16.13 ± 6.39 | 17.76 ± 3.24 | 0.42 |
| STEMI-CBC | Discharged (n = 310) | Mortality (n = 71) | p |
|---|---|---|---|
| HGB | 14.10 ± 1.852 | 12.66 ± 2.29 | 0.017 |
| WBC | 12.36 ± 4.47 | 14.26 ± 6.72 | 0.006 |
| NEU | 9.19 ± 4.21 | 11.15 ± 6.32 | 0.003 |
| LYM | 2.21 ± 1.18 | 2.06 ± 1.43 | 0.130 |
| PLT | 241.94 ± 74.47 | 242.03 ± 92.33 | 0.099 |
| PDW | 20.12 ± 1.19 | 19.97 ± 1.44 | 0.655 |
| MPV | 8.18 ± 1.86 | 7.84 ± 1.52 | 0.133 |
| NLR | 5.16 ± 4.52 | 6.84 ± 6.97 | 0.013 |
| PLR | 138.68 ± 95.04 | 175.48 ± 121.61 | 0.01 |
| RDW | 12.29 ± 1.53 | 13.01 ± 1.29 | 0.001 |
| APTT | 31.54 ± 19.73 | 36.56 ± 17.35 | 0.218 |
| PT | 14.16 ± 5.29 | 17.92 ± 6.26 | 0.001 |
| Data | Clinical/Paraclinical Parameters | No Complications (n = 743) | Complications (n = 193) | p |
|---|---|---|---|---|
| Demographics | Age (Years) | 64.51 ± 11.66 | 70.03 ± 11.28 | <0.001 |
| Male | 488/743 (65.68%) | 113/193 (58.55%) | 0.085 | |
| BMI (kg/m²) | 31.15 ± 5.38 | 29.90 ± 4.82 | 0.002 | |
| Previous history | Smoking | 190/743 (25.57%) | 34/193 (17.61%) | 0.021 |
| Diabetes Mellitus | 225/743 (30.28%) | 69/193 (35.75%) | 0.148 | |
| Hypertension | 524/743 (70.52%) | 101/193 (52.33%) | <0.001 | |
| Dyslipidemia | 360/743 (48.45%) | 64/193 (33.16%) | <0.001 | |
| Diagnosis | UA | 39/743 (53.56%) | 43/193 (22.28%) | <0.001 |
| NSTEMI | 83/743 (11.17%) | 30/193 (15.54%) | 0.109 | |
| STEMI | 261/743 (35.13%) | 120/193 (62.17%) | <0.001 | |
| Number of Diseased Vessels | 1 | 198/605 (32.73%) | 43/115 (37.39%) | 0.146 |
| 2 | 154/605 (25.45%) | 24/115 (20.87%) | 0.06 | |
| 3 | 184/605 (30.41%) | 44/115 (38.26%) | 0.003 | |
| CBC | HGB (g/dL) | 13.84 ± 1.76 | 12.04 ± 2.09 | <0.001 |
| WBC(×10³/μL) | 10.18 ± 3.75 | 13.38 ± 6.38 | <0.001 | |
| NEU(×10³/μL) | 7.16 ± 3.52 | 10.13 ± 5.98 | <0.001 | |
| LYM(×10³/μL) | 2.15 ± 0.94 | 2.23 ± 1.49 | 0.37 | |
| PLT (×10³/μL) | 225.83 ± 71.45 | 230.56 ± 79.12 | 0.42 | |
| PDW (%) | 20.08 ± 1.21 | 20.15 ± 1.42 | 0.53 | |
| MPV(fl) | 8.37 ± 1.88 | 8.16 ± 1.88 | 0.21 | |
| NLR | 3.41 ± 3.94 | 5.30 ± 5.90 | <0.001 | |
| PLR | 1267.21 ± 80.39 | 150.59 ± 107.62 | 0.013 | |
| RDW (%) | 12.31 ± 1.41 | 13.02 ± 1.56 | <0.001 | |
| Coagulation Tests | APTT(s) | 31.80 ± 17.49 | 34.20 ± 15.38 | 0.18 |
| PT(s) | 15.20 ± 6,69 | 16.86 ± 6.27 | 0.018 | |
| Cholesterol (mg/dL) | - | 177.34 ± 47.98 | 173.81 ± 45.72 | 0.34 |
| Creatinine (mg/dL) | - | 1.01 ± 0.51 | 1.45 ± 0.96 | <0.001 |
| LVEF% | - | 47.74 ± 8.16 | 38.89 ± 10.90 | <0.001 |
| NSTEMI CBC | No Complications (n = 84) | Complications (n = 30) | p |
|---|---|---|---|
| HGB (g/dL) | 13.46 ± 1.82 | 12.88 ± 2.06 | 0.15 |
| WBC (×10³/μL) | 10.29 ± 3.77 | 14.93 ± 7.07 | <0.001 |
| NEU (×10³/μL) | 7.39 ± 3.70 | 11.18 ± 6.53 | 0.002 |
| LYM (×10³/μL) | 2.09 ± 0.96 | 2.69 ± 1.82 | 0.059 |
| PLT (×10³/μL) | 223.44 ± 65.45 | 226.95 ± 64.83 | 0.80 |
| PDW (%) | 20.09 ± 0.99 | 19.87 ± 1.20 | 0.43 |
| MPV (fl) | 8.23 ± 1.51 | 7.44 ± 1.41 | 0.58 |
| NLR | 3.81 ± 5.45 | 4.26 ± 5.03 | 0.70 |
| PLR | 133.86 ± 104.45 | 149.69 ± 119.92 | 0.57 |
| RDW (%) | 12.24 ± 0.99 | 13.77 ± 1.88 | <0.001 |
| APTT (s) | 31.95 ± 6.90 | 40.83 ± 21.24 | 0.03 |
| PT (s) | 15.50 ± 4.60 | 17.70 ± 7.46 | 0.21 |
| STEMI-CBC | No Complications (n = 261) | Complications (n = 120) | p |
|---|---|---|---|
| HGB (g/dL) | 14.22 ± 1.78 | 12.98 ± 2.24 | <0.001 |
| WBC (×10 ³/μL) | 12.00 ± 3.96 | 14.25 ± 6.49 | <0.001 |
| NEU (×10³/μL) | 8.90 ± 3.72 | 10.97 ± 6.16 | <0.001 |
| LYM (×10³/μL) | 2.17 ± 1.05 | 2.23 ± 1.57 | 0.67 |
| PLT (×10³/μL) | 243.29 ± 75.55 | 239.05 ± 83.24 | 0.62 |
| PDW (%) | 20.09 ± 1.14 | 20.10 ± 1.56 | 0.95 |
| MPV (fl) | 8.16 ± 1.83 | 8.05 ± 1.76 | 0.62 |
| NLR | 5.01 ± 4.36 | 6.47 ± 6.31 | 0.01 |
| PLR | 138.59 ± 93.81 | 159.37 ± 114.39 | 0.07 |
| RDW (%) | 12.21 ± 1.45 | 12.89 ± 1.56 | <0.001 |
| APTT (s) | 32.00 ± 21.42 | 33.14 ± 14.07 | 0.61 |
| PT (s) | 14.23 ± 5.80 | 16.07 ± 5.09 | 0.01 |
| PCI-CBC | No Complications (n = 404) | Complications (n = 86) | p |
|---|---|---|---|
| HGB (g/dL) | 14.05 ± 1.68 | 13.59 ± 1.74 | 0.02 |
| WBC (×10³/μL) | 10.79 ± 3.82 | 12.60 ± 5.79 | 0.001 |
| NEU (×10³/μL) | 7.64 ± 3.58 | 9.22 ± 5.20 | 0.002 |
| LYM (×10³/μL) | 2.25 ± 0.97 | 2.49 ± 1.73 | 0.105 |
| PLT (×10³/μL) | 231.274 ± 73.43 | 231.44 ± 64.13 | 0.96 |
| PDW (%) | 20.12 ± 1.18 | 20.22 ± 1.33 | 0.55 |
| NLR | 3.55 ± 3.41 | 4.77 ± 5.62 | 0.01 |
| PLR | 120.39 ± 64.44 | 142.06 ± 118.14 | 0.03 |
| MPV (fl) | 8.38 ± 1.94 | 8.19 ± 1.81 | 0.42 |
| RDW (%) | 12.13 ± 1.10 | 12.65 ± 1.14 | <0.001 |
| APTT (s) | 32.09 ± 21.76 | 34.02 ± 18.15 | 0.56 |
| PT (s) | 14.09 ± 4.80 | 16.36 ± 7.15 | 0.007 |
| ACS-CBC | No Ventricular Arrhythmias (n = 880) | Ventricular Arrhythmias (n = 56) | p |
|---|---|---|---|
| HGB (g/dL) | 13.73 ± 1.820 | 12.79 ± 2.23 | <0.001 |
| WBC (×10³/μL) | 10.49 ± 4.18 | 15.89 ± 6.95 | <0.001 |
| NEU (×10³/μL) | 7.45 ± 3.92 | 12.57 ± 6.53 | <0.001 |
| LYM (×10³/μL) | 2.15 ± 1.01 | 2.40 ± 1.75 | 0.11 |
| PLT (×10³/μL) | 226.09 ± 72.63 | 238.09 ± 79.72 | 0.23 |
| PDW (%) | 20.11 ± 1.26 | 19.99 ± 1.24 | 0.55 |
| MPV (fl) | 8.37 ± 1.88 | 7.60 ± 1.72 | 0.664 |
| NLR | 3.60 ± 4.18 | 7.01 ± 7.11 | <0.001 |
| PLR | 130.04 ± 85.14 | 160.63 ± 109.29 | 0.01 |
| RDW (%) | 12.40 ± 1.42 | 13.27 ± 1.88 | <0.001 |
| APTT(s) | 32.19 ± 16.78 | 35.15 ± 18.09 | 0.29 |
| PT (s) | 15.57 ± 6.72 | 16.63 ± 5.52 | 0.26 |
| STEMI-CBC | No Ventricular Arrhythmias (n = 332) | Ventricular Arrhythmias (n = 49) | p |
|---|---|---|---|
| HGB (g/dL) | 13.99 ± 1.92 | 12.76 ± 2.35 | 0.074 |
| WBC (×10³/μL) | 12.27 ± 4.47 | 15.64 ± 6.97 | <0.001 |
| NEU (×10³/μL) | 9.12 ± 4.22 | 12.43 ± 6.57 | <0.001 |
| LYM (×10³/μL) | 2.17 ± 1.14 | 2.35 ± 1.72 | 0.364 |
| PLT (×10³/μL) | 242.99 ± 77.51 | 235.00 ± 81.54 | 0.862 |
| PDW (%) | 20.10 ± 1.23 | 20.06 ± 1.25 | 0.798 |
| MPV (fl) | 8.19 ± 1.81 | 7.67 ± 1.74 | 0.992 |
| NLR | 5.20 ± 4.66 | 7.30 ± 7.25 | 0.001 |
| PLR | 142.27 ± 98.94 | 162.79 ± 112.02 | 0.066 |
| RDW (%) | 12.29 ± 1.40 | 13.33 ± 1.94 | 0.458 |
| APTT (s) | 32.16 ± 20.07 | 33.68 ± 15.02 | 0.077 |
| PT (s) | 14.49 ± 5.58 | 16.78 ± 5.67 | 0.055 |
| PCI-CBC | No Ventricular Arrhythmias (n = 466) | Ventricular Arrhythmias (n = 24) | p |
|---|---|---|---|
| HGB (g/dL) | 13.99 ± 1.71 | 13.58 ± 1.64 | 0.23 |
| WBC (×10³/μL) | 10.79 ± 3.81 | 16.54 ± 7.40 | <0.001 |
| NEU (×10³/μL) | 7.64 ± 3.54 | 12.73 ± 6.78 | <0.001 |
| LYM (×10³/μL) | 2.26 ± 1.08 | 2.88 ± 2.02 | 0.11 |
| PLT(×10³/μL) | 230.77 ± 72.60 | 241.64 ± 54.62 | 0.35 |
| PDW (%) | 20.14 ± 1.20 | 20.09 ± 1.37 | 0.83 |
| MPV (fl) | 8.38 ± 1.92 | 7.80 ± 1.79 | 0.15 |
| NLR | 3.63 ± 3.74 | 6.29 ± 5.91 | 0.001 |
| PLR | 123.22 ± 73.66 | 139.70 ± 118.53 | 0.32 |
| RDW (%) | 12.20 ± 1.13 | 12.62 ± 1.00 | 0.07 |
| APTT (s) | 32.12 ± 20.45 | 37.02 ± 25.77 | 0.33 |
| PT (s) | 14.55 ± 5.54 | 15.62 ± 5.09 | 0.41 |
| ACS-CBC | No Heart Failure (n = 820) | Heart Failure (n = 116) | p |
|---|---|---|---|
| HGB (g/dL) | 13.77 ± 1.80 | 12.98 ± 2.11 | 0.006 |
| WBC (×10³/μL) | 10.48 ± 4.18 | 13.28 ± 6.35 | <0.001 |
| NEU (×10³/μL) | 7.44 ± 3.91 | 9.99 ± 6.01 | <0.001 |
| LYM (×10³/μL) | 2.16 ± 1.00 | 2.17 ± 1.46 | 0.981 |
| PLT (×10³/μL) | 225.49 ± 71.97 | 236.11 ± 80.22 | 0.207 |
| PDW (%) | 20.08 ± 1.22 | 20.21 ± 1.53 | 0.160 |
| MPV (fl) | 8.34 ± 1.87 | 8.21 ± 1.96 | 0.847 |
| NLR | 3.57 ± 4.32 | 5.39 ± 5.25 | <0.001 |
| PLR | 128.39 ± 82.54 | 157.35 ± 111.39 | <0.001 |
| RDW (%) | 12.36 ± 1.44 | 13.12 ± 1.49 | <0.001 |
| PT (s) | 15.38 ± 6.67 | 17.08 ± 6.35 | 0.302 |
| APTT (s) | 32.10 ± 17.26 | 34.21 ± 15.38 | 0.33 |
| NSTEMI CBC | No Heart Failure (n = 92) | Heart Failure (n = 22) | p |
|---|---|---|---|
| HGB (g/dL) | 13.491 ± 1.769 | 12.563 ± 2.219 | 0.338 |
| WBC (×10³/μL) | 10.432 ± 3.912 | 15.476 ± 7.459 | 0.005 |
| NEU (×10³/μL) | 7.453 ± 3.713 | 11.785 ± 7.039 | 0.001 |
| LYM (×10³/μL) | 2.164 ± 1.091 | 2.564 ± 1.746 | 0.027 |
| PLT (×10³/μL) | 224.495 ± 64.075 | 223.818 ± 70.423 | 0.885 |
| PDW (%) | 20.105 ± 1.033 | 19.878 ± 1.012 | 0.886 |
| MPV (fl) | 8.210 ± 1.536 | 7.336 ± 1.317 | 0.276 |
| NLR | 3.601 ± 5.297 | 5.076 ± 5.495 | 0.111 |
| PLR | 131.574 ± 102.688 | 163.572 ± 130.222 | 0.034 |
| RDW (%) | 12.236 ± 0.984 | 14.158 ± 1.883 | 0.026 |
| APTT (s) | 34.148 ± 14.356 | 36.184 ± 3.597 | 0.317 |
| PT (s) | 15.553 ± 6.669 | 18.021 ± 7.966 | 0.231 |
| STEMI-CBC | No Heart Failure (n = 303) | Heart Failure (n = 78) | p |
|---|---|---|---|
| HGB (g/dL) | 14.00 ± 1.96 | 13.16 ± 2.12 | 0.152 |
| WBC (×10³/μL) | 12.44 ± 4.62 | 13.69 ± 6.13 | 0.001 |
| NEU (×10³/μL) | 9.31 ± 4.33 | 10.45 ± 5.91 | 0.001 |
| LYM (×10³/μL) | 2.20 ± 1.16 | 2.13 ± 1.48 | 0.083 |
| PLT (×10³/μL) | 241.34 ± 78.08 | 244.36 ± 78.01 | 0.932 |
| PDW (%) | 20.09 ± 1.16 | 20.12 ± 1.52 | 0.297 |
| MPV (fl) | 8.12 ± 1.83 | 8.15 ± 1.71 | 0.564 |
| NLR | 5.29 ± 5.01 | 6.17 ± 5.42 | 0.027 |
| PLR | 140.22 ± 96.90 | 164.11 ± 114.12 | 0.024 |
| RDW (%) | 12.29 ± 1.40 | 12.84 ± 1.31 | 0.333 |
| APTT (s) | 31.90 ± 20.19 | 34.25 ± 15.98 | 0.539 |
| PT (s) | 14.51 ± 5.84 | 15.98 ± 4.61 | 0.305 |
| PCI-CBC | No Heart Failure (n = 448) | Heart Failure (n = 42) | p |
|---|---|---|---|
| HGB (g/dL) | 14.00 ± 1.70 | 13.64 ± 1.74 | 0.18 |
| WBC (×10³/μL) | 11.03 ± 4.22 | 11.83 ± 4.76 | 0.26 |
| NEU (×10³/μL) | 7.84 ± 3.91 | 8.59 ± 4.22 | 0.26 |
| LYM (×10³/μL) | 2.29 ± 1.06 | 2.33 ± 1.71 | 0.83 |
| PLT (×10³/μL) | 231.74 ± 72.36 | 226.62 ± 66.46 | 0.63 |
| PDW (%) | 20.10 ± 1.18 | 20.49 ± 1.37 | 0.09 |
| MPV (fl) | 8.34 ± 1.91 | 8.43 ± 2.02 | 0.79 |
| NLR | 3.64 ± 3.77 | 5.05 ± 4.99 | 0.02 |
| PLR | 121.54 ± 69.04 | 149.09 ± 127.84 | 0.03 |
| RDW (%) | 12.16 ± 1.09 | 12.86 ± 1.25 | <0.001 |
| APTT (s) | 32.47 ± 21.38 | 33.01 ± 17.77 | 0.88 |
| PT (s) | 14.36 ± 5.06 | 16.54 ± 7.90 | 0.04 |
| PCI-CBC | No Re Infarction (n = 486) | Re Infarction (n = 4) | p |
|---|---|---|---|
| HGB (g/dL) | 13.97 ± 1.70 | 13.74 ± 1.00 | 0.67 |
| WBC (×10³/μL) | 11.10 ± 4.28 | 10.66 ± 3.28 | 0.83 |
| NEU (×10³/μL) | 7.90 ± 3.94 | 9.09 ± 3.46 | 0.60 |
| LYM (×10³/μL) | 2.29 ± 1.13 | 0.94 ± 0.44 | 0.03 |
| PLT (×10³/μL) | 231.36 ± 71.96 | 224.00 ± 59.23 | 0.82 |
| PDW (%) | 20.142 ± 1.207 | 19.64 ± 0.327 | 0.10 |
| MPV (fl) | 8.35 ± 1.92 | 7.49 ± 0.33 | 0.12 |
| NLR | 3.71 ± 3.79 | 10.13 ± 10.76 | 0.001 |
| PLR | 123.41 ± 76.05 | 217.58 ± 115.22 | 0.03 |
| RDW (%) | 12.22 ± 1.13 | 12.77 ± 0.75 | 0.32 |
| APTT (s) | 32.56 ± 20.98 | 27.80 ± 15.02 | 0.82 |
| PT (s) | 14.52 ± 5.29 | 38.6 ± 6.55 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babes, E.E.; Zaha, D.C.; Tit, D.M.; Nechifor, A.C.; Bungau, S.; Andronie-Cioara, F.L.; Behl, T.; Stoicescu, M.; Munteanu, M.A.; Rus, M.; et al. Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes. Diagnostics 2021, 11, 850. https://doi.org/10.3390/diagnostics11050850
Babes EE, Zaha DC, Tit DM, Nechifor AC, Bungau S, Andronie-Cioara FL, Behl T, Stoicescu M, Munteanu MA, Rus M, et al. Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes. Diagnostics. 2021; 11(5):850. https://doi.org/10.3390/diagnostics11050850
Chicago/Turabian StyleBabes, Elena Emilia, Dana Carmen Zaha, Delia Mirela Tit, Aurelia Cristina Nechifor, Simona Bungau, Felicia Liana Andronie-Cioara, Tapan Behl, Manuela Stoicescu, Mihai Alexandru Munteanu, Marius Rus, and et al. 2021. "Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes" Diagnostics 11, no. 5: 850. https://doi.org/10.3390/diagnostics11050850







