Comparison of MRI Visualization Following Minimally Invasive and Open TLIF: A Retrospective Single-Center Study
Abstract
1. Introduction
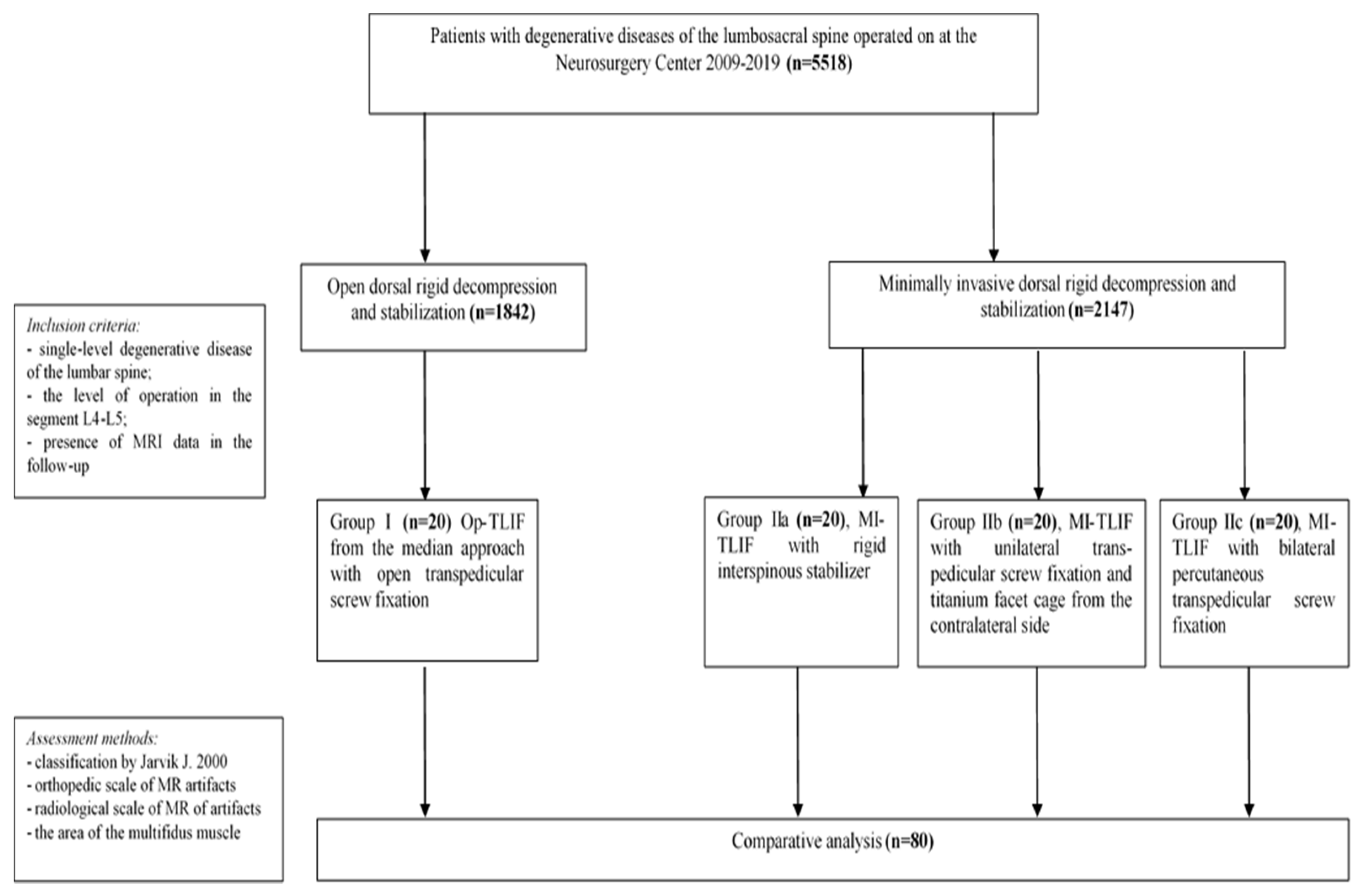
2. Materials and Methods
2.1. Patient Inclusion Criteria
- single-level degenerative disc disease of L4–L5;
- received TLIF and dorsal rigid stabilization performed for degenerative disc disease with foraminal stenosis or segmental instability;
- patient information (MRI data) was available in the follow-up.
2.2. Patient Exclusion Criteria
- two-level degenerative disc disease of the lumbar spine;
- operative level was L1–L2/L2–L3/L3–L4/L5–S1;
- anterior or lateral lumbar interbody fusion with dorsal rigid stabilization;
- previously performed operations at the lumbar level;
- inability to conduct MRI in patients in the postoperative period (fear of confined spaces, the presence of foreign metal objects, etc.);
- competing pathological process in the lumbar spine (traumatic injuries, systemic diseases of connective tissue, infectious and inflammatory diseases, tumor lesions, etc.);
- lack of patient consent to participate in the study;
- absence of the patient′s neuroimaging archive in the follow-up.
2.3. Surgical Technique
2.4. MR Imaging and Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
4.2. Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kim, Y.H.; Ha, K.Y.; Rhyu, K.W.; Park, H.Y.; Cho, C.H.; Kim, H.C.; Lee, H.J.; Kim, S.I. Lumbar Interbody Fusion: Techniques, Pearls and Pitfalls. Asian Spine J. 2020, 14, 730–741. [Google Scholar] [CrossRef]
- Li, A.; Li, X.; Zhong, Y. Is minimally invasive superior than open transforaminal lumbar interbody fusion for single-level de-generative lumbar diseases: A meta-analysis. J. Orthop. Surg. Res. 2018, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; Yoo, J.S.; Karmarkar, S.S.; Lamoutte, E.H.; Singh, K. Interbody options in lumbar fusion. J. Spine Surg. 2019, 5 (Suppl. 1), S19–S24. [Google Scholar] [CrossRef]
- Mao, J.Z.; Fritz, A.G.; Lucas, J.-M.P.; Khan, A.; Popoola, D.O.; Becker, A.B.; Adetunji, A.; Levy, B.R.; Agyei, J.O.; O’Connor, T.E.; et al. Assessment of Rod Material Types in Spine Surgery Outcomes: A Systematic Review. World Neurosurg. 2021, 146, e6–e13. [Google Scholar] [CrossRef] [PubMed]
- Byvaltsev, V.A.; Kalinin, A.A.; Aliyev, M.A.; Riew, K.D. Postoperative MRI Visualization of the Cervical Spine Following Cervical Disc Arthroplasty: A Prospective Single-Center Comparison of a Titanium and Cobalt-Chromium Prosthesis. Glob. Spine J. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Irmola, T.M.; Häkkinen, A.; Järvenpää, S.; Marttinen, I.; Vihtonen, K.; Neva, M. Reoperation Rates Following Instrumented Lumbar Spine Fusion. Spine 2018, 43, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Kim, H.J. Comparative Study Between Cobalt Chrome and Titanium Alloy Rods for Multilevel Spinal Fusion: Proximal Junctional Kyphosis More Frequently Occurred in Patients Having Cobalt Chrome Rods. World Neurosurg. 2017, 103, 404–409. [Google Scholar] [CrossRef]
- Brinjikji, W.; Diehn, F.; Jarvik, J.; Carr, C.; Kallmes, D.; Murad, M.; Luetmer, P. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2015, 36, 2394–2399. [Google Scholar] [CrossRef]
- Zou, Y.-F.; Chu, B.; Wang, C.-B.; Hu, Z.-Y. Evaluation of MR issues for the latest standard brands of orthopedic metal implants: Plates and screws. Eur. J. Radiol. 2015, 84, 450–457. [Google Scholar] [CrossRef]
- Rudisch, A.; Kremser, C.; Peer, S.; Kathrein, A.; Judmaier, W.; Daniaux, H. Metallic Artifacts in Magnetic Resonance Imaging of Patients wWith Spinal Fusion. Spine 1998, 23, 692–699. [Google Scholar] [CrossRef]
- Suzuki, A.K.; Campo, K.N.; Fonseca, E.B.; Araújo, L.C.; Gandra, F.C.G.; Lopes, É.S.N. Appraising the potential of Zr-based biomedical alloys to reduce magnetic resonance imaging artifacts. Sci. Rep. 2020, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Jarvik, J.G.; Robertson, W.D.; Wessbecher, F.; Reger, K.; Solomon, C.; Whitten, R.; Lumley, T.; Deyo, R.A. Variation in the Quality of Lumbar Spine MR Images in Washington State. Radiology 2000, 215, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Knott, P.T.; Mardjetko, S.M.; Kim, R.H.; Cotter, T.M.; Dunn, M.M.; Patel, S.T.; Spencer, M.J.; Wilson, A.S.; Tager, D.S. A comparison of magnetic and radiographic imaging artifact after using three types of metal rods: Stainless steel, titanium, and vitallium. Spine J. 2010, 10, 789–794. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.-H. Does lumbar paraspinal muscles improve after corrective fusion surgery in degenerative flat black? Indian J. Orthop. 2017, 51, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhang, J.; Lu, F.; Wu, H.; Chen, Z.; Jian, F. Minimally invasive versus open Transforaminal lumbar Interbody fusion in obese patients: A meta-analysis. BMC Musculoskelet. Disord. 2018, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Putzier, M.; Hartwig, T.; Hoff, E.K.; Streitparth, F.; Strube, P. Minimally invasive TLIF leads to increased muscle sparing of the multifidus muscle but not the longissimus muscle compared with conventional PLIF—A prospective randomized clinical trial. Spine J. 2016, 16, 811–819. [Google Scholar] [CrossRef]
- Sutherland-Smith, J.; Tilley, B. Magnetic resonance imaging metallic artifact of commonly encountered surgical implants and foreign material. Vet. Radiol. Ultrasound 2012, 53, 312–317. [Google Scholar] [CrossRef]
- Kajima, Y.; Takaichi, A.; Tsutsumi, Y.; Hanawa, T.; Wakabayashi, N.; Kawasaki, A. Influence of magnetic susceptibility and volume on MRI artifacts produced by low magnetic susceptibility Zr-14Nb alloy and dental alloys. Dent. Mater. J. 2020, 39, 256–261. [Google Scholar] [CrossRef]
- Rupp, R.; Ebraheim, N.A.; Savolaine, E.R.; Jackson, W.T. Magnetic resonance imaging evaluation of the spine with metal implants. General safety and superior imaging with titanium. Spine 1993, 18, 379–385. [Google Scholar] [CrossRef]
- Massaad, E.; Fatima, N.; Kiapour, A.; Hadzipasic, M.; Shankar, G.M.; Shin, J.H. Polyetheretherketone Versus Titanium Cages for Posterior Lumbar Interbody Fusion: Meta-Analysis and Review of the Literature. Neurospine 2020, 17, 125–135. [Google Scholar] [CrossRef]
- Hak, D.J.; Mauffrey, C.; Seligson, D.; Lindeque, B. Use of Carbon-Fiber-Reinforced Composite Implants in Orthopedic Surgery. Orthopedics 2014, 37, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Seaman, S.; Kerezoudis, P.; Bydon, M.; Torner, J.C.; Hitchon, P.W. Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J. Clin. Neurosci. 2017, 44, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ernstberger, T.; Buchhorn, G.; Heidrich, G. Artifacts in spine magnetic resonance imaging due to different intervertebral test spacers: An in vitro evaluation of magnesium versus titanium and carbon-fiber-reinforced polymers as biomaterials. Neuroradiology 2009, 51, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Massaad, E.; Shankar, G.M.; Shin, J.H. Structural Allograft versus Polyetheretherketone Implants in Patients Undergoing Spinal Fusion Surgery: A Systematic Review and Meta-Analysis. World Neurosurg. 2020, 136, 101–109. [Google Scholar] [CrossRef]
- Tahal, D.; Madhavan, K.; Chieng, L.O.; Ghobrial, G.M.; Wang, M.Y. Metals in Spine. World Neurosurg. 2017, 100, 619–627. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Buckley, J.M.; Ames, C.; Deviren, V. The fatigue life of contoured cobalt chrome posterior spinal fusion rods. Proc. Inst. Mech. Eng. H 2011, 225, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Wayer, D.; Kim, N.; Otto, B.; Grayev, A.; Kuner, A. Unintended Consequences: Review of New Artifacts Introduced by Iterative Reconstruction CT Metal Artifact Reduction in Spine Imaging. AJNR Am. J. Neuroradiol. 2019, 40, 1973–1975. [Google Scholar] [CrossRef] [PubMed]
- Trammell, T.R.; Flint, K.; Ramsey, C.J. A Comparison of MRI and CT Imaging Clarity of Titanium Alloy and Titanium Alloy with Cobalt-Chromium-Alloy Pedicle Screw and Rod Implants in the Lumbar Spine. J. Bone Joint Surg. Am. 2012, 94, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-W.; Liu, L.; Wang, J.; Dong, A.-S.; Lu, J.-P.; He, S.-S.; Li, M. Magnetic resonance imaging of artificial lumbar disks: Safety and metal artifacts. Chin. Med. J. 2009, 122, 911–916. [Google Scholar] [PubMed]
- Farrelly, C.; Davarpanah, A.; Brennan, S.A.; Sampson, M.; Eustace, S.J. Imaging of soft tissues adjacent to orthopedic hard-ware: Comparison of 3-T and 1.5-T MRI. AJR Am. J. Roentgenol. 2010, 194, W60–W64. [Google Scholar] [CrossRef]
- Tohtz, S.W.; Rogalla, P.; Taupitz, M.; Perka, C.; Winkler, T.; Putzier, M. Inter- and intraobserver variability in the postoperative evaluation of transpedicular stabilization: Computed tomography versus magnetic resonance imaging. Spine J. 2010, 10, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lim, D.; Kim, E.; Kim, S.; Song, H.-T.; Suh, J.-S. Usefulness of slice encoding for metal artifact correction (SEMAC) for reducing metallic artifacts in 3-T MRI. Magn. Reson. Imaging 2013, 31, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Cha, J.G.; Lee, M.H.; Lee, Y.K.; Lee, E.H.; Jeon, C.H. Usefulness of IDEAL T2-weighted FSE and SPGR imaging in reducing metallic artifacts in the postoperative ankles with metallic hardware. Skelet. Radiol. 2013, 42, 239–247. [Google Scholar] [CrossRef] [PubMed]




| Criteria | Group I (n = 20) | Group II (n = 60) | F | p | ||
|---|---|---|---|---|---|---|
| Subgroup IIa (n = 20) | Subgroup IIb (n = 20) | Subgroup IIc (n = 20) | ||||
| Age, years | 46.4 ± 5.7 | 44.5 ± 3.9 | 43.4 ± 6.2 | 47.4 ± 7.3 | 0.31 | 0.63 |
| Male/female ratio, n (%) | 11 (55)/9 (45) | 12 (60)/8 (40) | 13 (65)/7 (35) | 10 (50)/10 (50) | 0.25 | 0.85 |
| Observation period, months | 79.5 ± 2.54 | 78.1 ± 1.85 | 77.0 ± 0.81 | 78.3 ± 3.23 | 0.88 | 0.45 |
| Criteria | Group I (n = 20) | Group II (n = 60) | ||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup IIa, n = 20 | Subgroup IIb, n = 20 | Subgroup IIc, n = 20 | ||||||
| Kappa ± SE | 95% Confidence Interval | Kappa ± SE | 95% Confidence Interval | Kappa ± SE | 95% Confidence Interval | Kappa ± SE | 95% Confidence Interval | |
| Dural sac at operation level | 0.86 ± 0.15 | 0.74–0.93 | 0.95 ± 0.04 | 0.85–1.00 | 0.75 ± 0.13 | 0.69–0.90 | 0.85 ± 0.14 | 0.76–0.94 |
| Interbody space at operation level | 0.79 ± 0.12 | 0.66–0.91 | 0.81 ± 0.11 | 0.68–0.92 | 0.80 ± 0.11 | 0.77–0.92 | 0.95 ± 0.14 | 0.87–1.00 |
| Central canal at operation level | 0.80 ± 0.12 | 0.77–0.93 | 0.85 ± 0.10 | 0.64–0.95 | 0.74 ± 0.14 | 0.66–0.84 | 0.95 ± 0.04 | 0.85–1.00 |
| Right foramen | 0.95 ± 0.04 | 0.85–1.00 | 0.80 ± 0.09 | 0.63–0.92 | 0.85 ± 0.14 | 0.76–0.94 | 0.75 ± 0.10 | 0.64–0.90 |
| Left foramen | 0.85 ± 0.10 | 0.65–0.92 | 0.80 ± 0.10 | 0.66–0,92 | 0.77 ± 0.15 | 0.65–0.85 | 0.80 ± 0.11 | 0.77–0.92 |
| Upper adjacent level | 0.72 ± 0.12 | 0.61–0.85 | 0.75 ± 0.10 | 0.64–0.90 | 0.85 ± 0.13 | 0.79–0.90 | 0.81 ± 0.11 | 0.68–0.92 |
| Lower adjacent level | 0.85 ± 0.14 | 0.76–0.94 | 0.85 ± 0.10 | 0.64–0.95 | 0.95 ± 0.14 | 0.87–.00 | 0.72 ± 0.12 | 0.61–0.85 |
| Criteria | Preoperative | Postoperative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I (n = 20) | Group IIa (n = 20) | Group IIb (n = 20) | Group IIc (n = 20) | F | p | Group I (n = 20) | Group IIa (n = 20) | Group IIb (n = 20) | Group IIc (n = 20) | F | p | |
| Operation level | 3.96 ± 0.12 | 3.96 ± 0.17 | 3.97 ± 0.17 | 3.98 ± 0.17 | 0.20 | 0.88 | 2.64 ± 0.29 | 3.94 ± 0.17 | 3.11 ± 0.12 | 2.96 ± 0.26 | 41.63 | <0.001 |
| Adjacent level | 3.98 ± 0.10 | 3.98 ± 0.15 | 3.98 ± 0.16 | 3.97 ± 0.16 | 0.53 | 0.66 | 3.62 ± 0.23 | 3.89 ± 0.12 | 3.87 ± 0.27 | 3.65 ± 0.46 | 0.34 | 0.61 |
| Overall score | 3.94 ± 0.36 | 3.97 ± 0.14 | 3.97 ± 0.14 | 3.97 ± 0.15 | 0.18 | 0.91 | 3.17 ± 0.26 | 3.91 ± 0.12 | 3.59 ± 0.16 | 3.34 ± 0.27 | 0.42 | 0.74 |
| Criteria | Group I (n = 20) | Group II (n = 60) | ||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup IIa, n = 20 | Subgroup IIb, n = 20 | Subgroup IIc, n = 20 | ||||||
| Kappa ± SE | 95% Confidence Interval | Kappa ± SE | 95% Confidence Interval | Kappa ± SE | 95% Confidence Interval | Kappa ± SE | 95% Confidence Interval | |
| Radiology scale: operation level | 0.86 ± 0.07 | 0.67–1.00 | 0.95 ± 0.10 | 0.78–1.00 | 0.85 ± 0.14 | 0.81–1.00 | 0.91 ± 0.12 | 0.87–1.00 |
| Radiology scale: upper adjacent level | 0.90 ± 0.16 | 0.82–1.00 | 0.85 ± 0.08 | 0.68–1.00 | 0.76 ± 0.10 | 0.68–0.95 | 0.84 ± 0.11 | 0.67–0.93 |
| Radiology scale: lower adjacent level | 0.78 ± 0.17 | 0.70–0.91 | 0.82 ± 0.10 | 0.66–0.95 | 0.73 ± 0.14 | 0.67–0.82 | 0.78 ± 0.15 | 0.69–0.83 |
| Orthopedic scale: operation level | 0.82 ± 0.10 | 0.66–0.92 | 0.80 ± 0.09 | 0.71–0.98 | 0.92 ± 0.15 | 0.85–1.00 | 0.90 ± 0.18 | 0.80–0.99 |
| Orthopedic scale: upper adjacent level | 0.81 ± 0.10 | 0.72–0.94 | 0.85 ± 0.08 | 0.68–1.00 | 0.75 ± 0.10 | 0.64–0.95 | 0.72 ± 0.16 | 0.61–0.92 |
| Orthopedic scale: lower adjacent level | 0.75 ± 0.20 | 0.67–0.92 | 0.75 ± 0.10 | 0.64–0.92 | 0.80 ± 0.14 | 0.71–0.91 | 0.79 ± 0.19 | 0.70–0.90 |
| Criteria | Group I (n = 20) | Group II (n = 60) | F | p | ||
|---|---|---|---|---|---|---|
| Subgroup IIa (n = 20) | Subgroup IIb (n = 20) | Subgroup IIc (n = 20) | ||||
| Radiology scale: operation level | 2.83 ± 0.37 | 1.11 ± 0.30 | 1.99 ± 0.40 | 2.95 ± 0.48 | 37.17 | <0.001 |
| Radiology scale: upper adjacent level | 1.21 ± 0.31 | 1.04 ± 0.22 | 1.08 ± 0.24 | 1.15 ± 0.36 | 0.76 | 0.23 |
| Radiology scale: lower adjacent level | 1.13 ± 0.34 | 1.15 ± 0.33 | 1.16 ± 0.43 | 1.17 ± 0.47 | 0.88 | 0.14 |
| Orthopedic scale: operation level | 2.39 ± 0.52 | 1.13 ± 0.30 | 2.04 ± 0.30 | 2.50 ± 0.50 | 14.49 | <0.001 |
| Orthopedic scale: upper adjacent level | 1.25 ± 0.36 | 1.05 ± 0.22 | 1.02 ± 0.24 | 1.23 ± 0.47 | 0.63 | 0.34 |
| Orthopedic scale: lower adjacent level | 1.21 ± 0.72 | 1.03 ± 0.33 | 1.05 ± 0.41 | 1.10 ± 0.77 | 0.51 | 0.63 |
| Criteria | Group I (n = 20) | Group II (n = 60) | F | p | F | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup IIa, n = 20 | Subgroup IIb, n = 20 | Subgroup IIc, n = 20 | ||||||||||||||
| Preoperative | Postoperative | Mean Changes, % | Preoperative | Postoperative | Mean Changes, % | Preoperative | Postoperative | Mean Changes, % | Preoperative | Postoperative | Mean Changes, % | Preoperative | Postoperative | |||
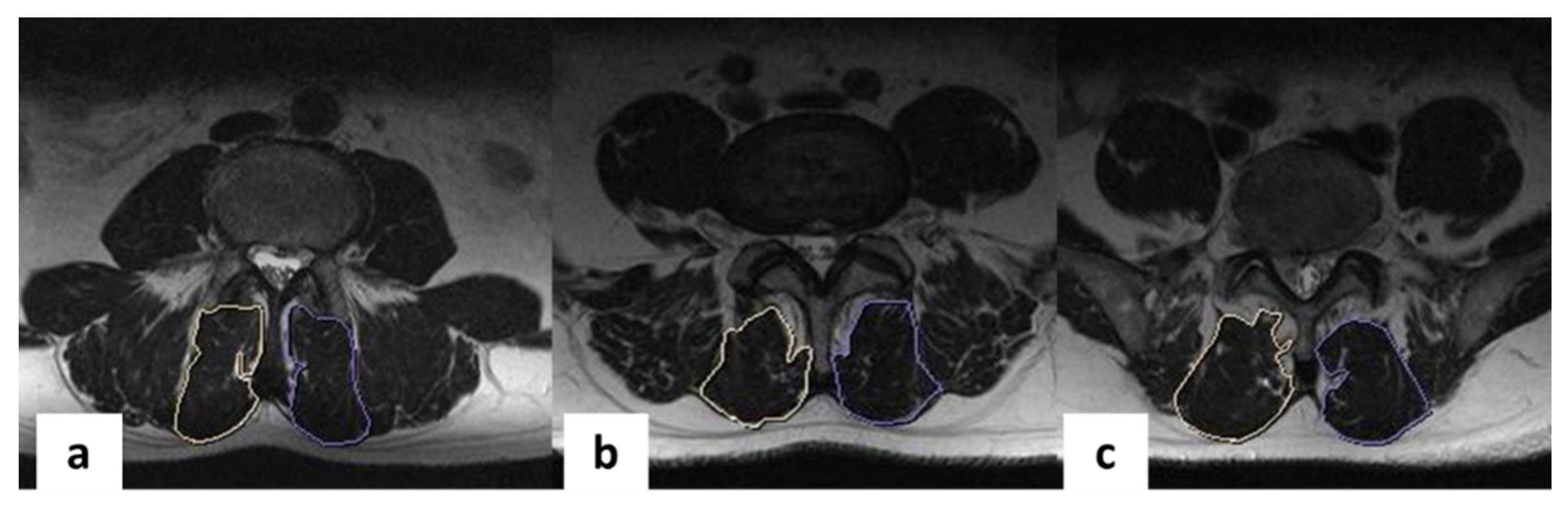
| Average multifidus muscle area, mm2 | 6.6 ± 1.2 | 3.0 ± 1.5 * | 54.5 | 6.3 ± 0.7 | 6.1 ± 1.6 | 3.2 | 6.4 ± 1.2 | 5.8 ± 1.1 | 9.4 | 6.4 ± 1.9 | 5.0 ± 1.4 * | 21.9 | 1.74 | 0.17 | 23.82 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byvaltsev, V.A.; Kalinin, A.A.; Giers, M.B.; Shepelev, V.V.; Pestryakov, Y.Y.; Biryuchkov, M.Y. Comparison of MRI Visualization Following Minimally Invasive and Open TLIF: A Retrospective Single-Center Study. Diagnostics 2021, 11, 906. https://doi.org/10.3390/diagnostics11050906
Byvaltsev VA, Kalinin AA, Giers MB, Shepelev VV, Pestryakov YY, Biryuchkov MY. Comparison of MRI Visualization Following Minimally Invasive and Open TLIF: A Retrospective Single-Center Study. Diagnostics. 2021; 11(5):906. https://doi.org/10.3390/diagnostics11050906
Chicago/Turabian StyleByvaltsev, Vadim A., Andrei A. Kalinin, Morgan B. Giers, Valerii V. Shepelev, Yurii Ya. Pestryakov, and Mikhail Yu. Biryuchkov. 2021. "Comparison of MRI Visualization Following Minimally Invasive and Open TLIF: A Retrospective Single-Center Study" Diagnostics 11, no. 5: 906. https://doi.org/10.3390/diagnostics11050906
APA StyleByvaltsev, V. A., Kalinin, A. A., Giers, M. B., Shepelev, V. V., Pestryakov, Y. Y., & Biryuchkov, M. Y. (2021). Comparison of MRI Visualization Following Minimally Invasive and Open TLIF: A Retrospective Single-Center Study. Diagnostics, 11(5), 906. https://doi.org/10.3390/diagnostics11050906








