Updated Incidence of Thyroid Cancer in the North East Region of Romania after 35 Years of Chernobyl Fallout. Is There a Link between?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Li, H.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L.; et al. Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw. Open 2020, 3, e208759. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, E.; Moslem, A.; Feizhadad, H.; Jarrahi, A.M.; Adineh, H.A.; Sohrabivafa, M.; Khazaei, Z. Epidemiology, incidence and mortality of thyroid cancer and their relationship with the human development index in the world: An ecology study in 2018. Adv. Hum. Biol. 2019, 9, 162–167. [Google Scholar]
- Dal Maso, L.; Tavilla, A.; Pacini, F.; Serraino, D.; van Dijk, B.A.C.; Chirlaque, M.D.; Capocaccia, R.; Larrañaga, N.; Colonna, M.; Agius, D.; et al. EUROCARE-5 Working Group. Survival of 86,690 patients with thyroid cancer: A population-based study in 29 European countries from EUROCARE-5. Eur. J. Cancer 2017, 77, 140–152. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef]
- Bazyka, D.; Prysyazhnyuk, A.; Gudzenko, N.; Dyagil, I.; Belyi, D.; Chumak, V.; Buzunov, V. Epidemiology of Late Health Effects in Ukrainian Chornobyl Cleanup Workers. Health Phys. 2018, 115, 161–169. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Baldari, S.; Trimarchi, F.; Trovato, M. What is New on Thyroid Cancer Biomarkers. Biomark. Insights 2008, 3, 237–252. [Google Scholar] [CrossRef]
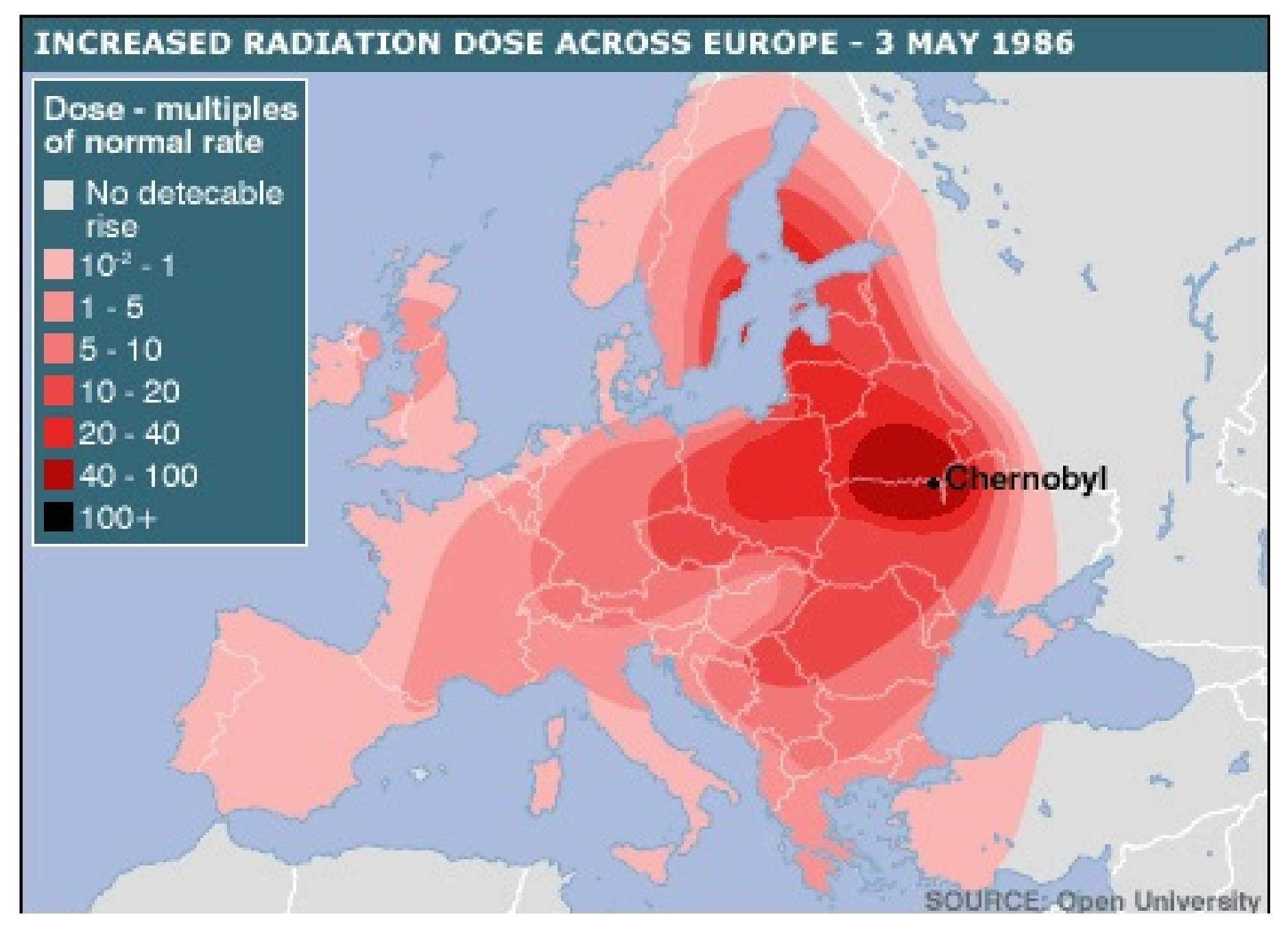
- Open University. Available online: https://www.farlabs.edu.au/wp-content/uploads/2013/09/Chernobyl_Disaster_Zones.png (accessed on 8 May 2021).
- Jegerlehner, S.; Bulliard, J.L.; Aujesky, D.; Rodondi, N.; Germann, S.; Konzelmann, I.; Chiolero, A.; NICER Working Group. Overdiagnosis and overtreatment of thyroid cancer: A population-based temporal trend study. PLoS ONE 2017, 12, e0179387. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Statistics. Available online: http://statistici.insse.ro:8077/tempo-online (accessed on 8 March 2021).
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Piciu, D.; Irimie, A.; Piciu, A. Investigation of thyroid carcinoma over 40 years, using the database of the Ion Chiricuta Institute of Oncology Cluj-Napoca. J. BUON 2014, 19, 524–529. [Google Scholar]
- Mogoş, V.; Zbranca, E.; Strat, V.; Diaconescu, M.R.; Chifan, M.; Florea, N.; Costăchescu, G.; Găleşanu, M.R.; Rusu, V.; Georgescu, G. Les cancers thyroidiens [Thyroid cancers]. Rev. Med. Chir. Soc. Med. Nat. Iasi 1995, 99, 72–81. [Google Scholar]
- Buzduga, C.; Mogoş, V.; Găleşanu, C.; Vulpoi, C.; Ungureanu, M.; Cristea, C.; Preda, C.; Ciobanu, D.; Ferariu, D.; Florea, N.; et al. Epidemiology and histology of malignant thyroid nodules in North East Region of Romania (Moldavia) before and after alimentary salt universal iodination. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 45–48. [Google Scholar]
- WHO. 1986–2016: Chernobyl at 30. An Update. 2016. Available online: https://www.who.int/publications/m/item/1986-2016-chernobyl-at-30 (accessed on 8 March 2021).
- Gábora, K.; Bărbuş, E.; Peştean, C.; Larg, M.I.; Bonci, E.A.; Bădulescu, C.I.; Piciu, A. Radiation induced thyroid carcinoma in Romania—Effects of the Chernobyl fallout, a systematic review of observational studies. Clujul Med. 2018, 91, 372–375. [Google Scholar] [CrossRef]
- Bogdanova, T.I.; Saenko, V.A.; Hashimoto, Y.; Hirokawa, M.; Zurnadzhy, L.Y.; Hayashi, T.; Ito, M.; Iwadate, M.; Mitsutake, N.; Rogounovitch, T.I.; et al. Papillary Thyroid Carcinoma in Ukraine After Chernobyl and in Japan After Fukushima: Different Histopathological Scenarios. Thyroid 2020. [Google Scholar] [CrossRef]
- Hatch, M.; Brenner, A.V.; Cahoon, E.K.; Drozdovitch, V.; Little, M.P.; Bogdanova, T.; Shpak, V.; Bolshova, E.; Zamotayeva, G.; Terekhova, G.; et al. Thyroid Cancer and Benign Nodules After Exposure in Utero to Fallout from Chernobyl. J. Clin. Endocrinol. Metab. 2019, 104, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Paulson, V.A.; Rudzinski, E.R.; Hawkins, D.S. Thyroid Cancer in the Pediatric Population. Genes 2019, 10, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatch, M.; Cardis, E. Somatic health effects of Chernobyl: 30 years on. Eur. J. Epidemiol. 2017, 32, 1047–1054. [Google Scholar] [CrossRef]
- Ștefan, A.I.; Piciu, A.; Căinap, S.S.; Gabora, K.; Piciu, D. Differentiated Thyroid Cancer in Children in the Last 20 Years: A Regional Study in Romania. J. Clin. Med. 2020, 9, 3617. [Google Scholar] [CrossRef]
- Bazyka, D.A.; Prysyazhnyuk, A.Y.; Fuzik, M.M.; Fedorenko, Z.P. Thyroid Cancer and the Chornobyl Accident in Ukraine: Experience with the Implementation of a Follow-Up Programme. Radiat. Prot. Dosim. 2016, 171, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Szanto, Z.; Kun, I.Z.; Borda, A.; Jung, J. Thyroid cancer in two representative medical centers in Mures County between 1984–2007. Acta Endocrinol. 2009, 5, 199–211. [Google Scholar] [CrossRef]
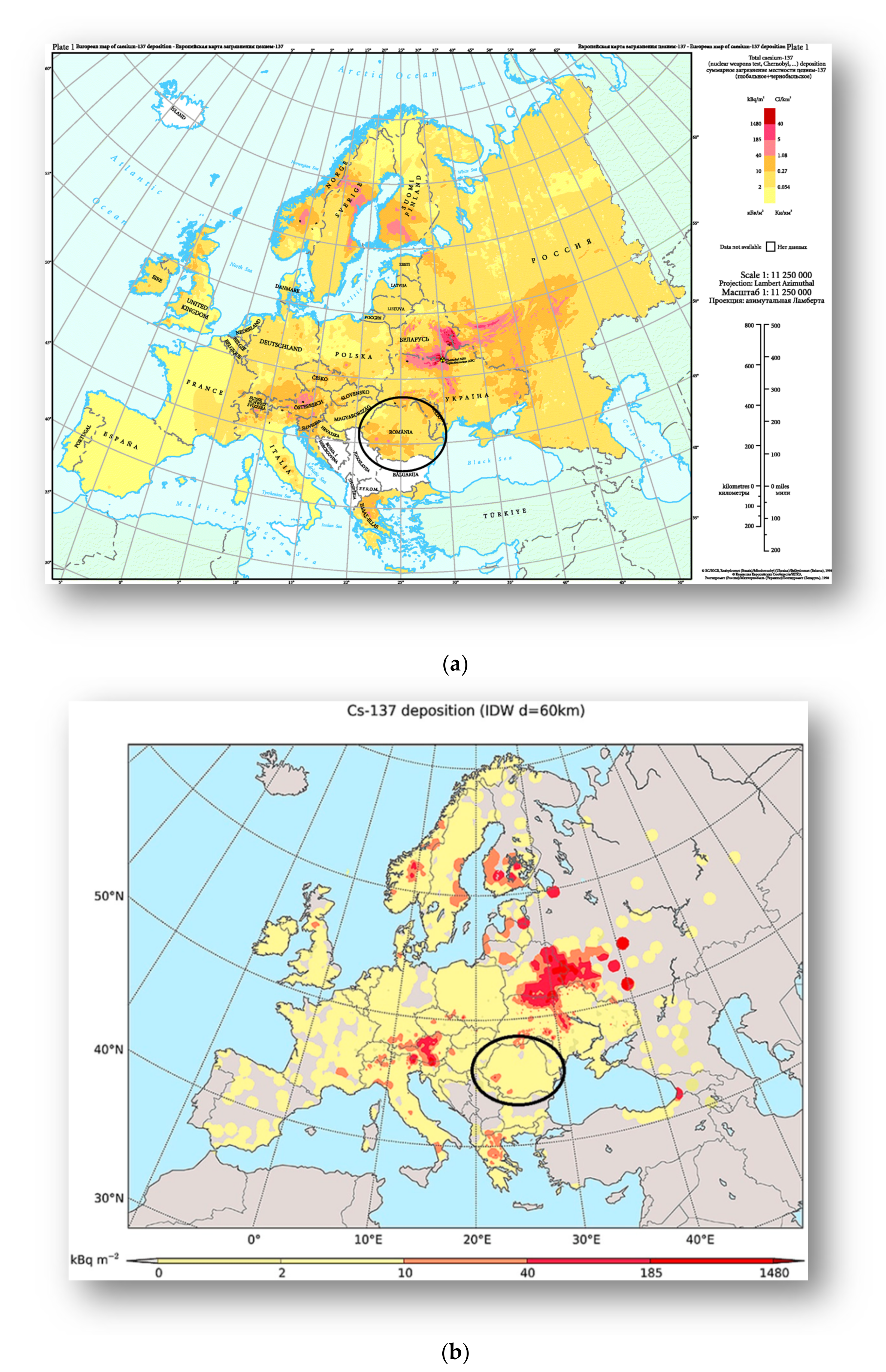
- Begy, R.C.; Simon, H.; Vasilache, D.; Kelemen, S.; Cosma, C. Cs contamination over Transylvania region (Romania) after Chernobyl Nuclear Power Plant Accident. Sci. Total Environ. 2017, 599–600, 627–636. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/figures/deposition-from-chernobyl-in-europe (accessed on 8 May 2021).
- Evangeliou, N.; Hamburger, T.; Talerko, N.; Zibtsev, S.; Bondar, Y.; Stohl, A.; Balkanski, Y.; Mousseau, T.A.; Møller, A.P. Reconstructing the Chernobyl Nuclear Power Plant (CNPP) accident 30 years after. A unique database of air concentration and deposition measurements over Europe. Environ. Pollut. 2016, 216, 408–418. [Google Scholar] [CrossRef]



| Histological Characteristics | Number of Patients (%) |
|---|---|
| Papillary carcinoma: | |
| Classical variant | 672(62.92) |
| MEN 1 associated | * 2 (0.18) |
| Medullary carcinoma: | |
| Sporadic | 69 (6.46) |
| MEN 2 associated | * 3 (0.28) |
| Follicular carcinoma: | |
| Classical variant | 157 (14.7) |
| Follicular variant of papillary carcinoma | 114 (10.67) |
| Hürthle cell carcinoma | 26 (2.43) |
| Anaplastic carcinoma | 11(1.02) |
| Poor differentiated carcinoma | 3 (0.28) |
| Primary thyroid lymphoma | 6 (0.56) |
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aget at diagnosis | ||||||||||||
| Overall | 52.0 | 52.1 | 55.5 | 53.7 | 54.6 | 53.3 | 55.9 | 55.8 | 57.0 | 55.1 | 57.4 | 1.000 |
| Male | 53.9 | 57.6 | 58.8 | 57.5 | 54.6 | 53.7 | 57.3 | 55.5 | 51.3 | 55.3 | 56.2 | 1.000 |
| Female | 51.7 | 50.9 | 54.6 | 52.6 | 54.7 | 53.2 | 55.5 | 55.9 | 57.9 | 55.0 | 57.6 | 1.000 |
| p-value | 0.911 | 0.590 | 0.773 | 0.717 | 0.909 | 0.956 | 0.949 | 0.886 | 0.462 | 0.939 | 0.812 | |
| Sex | ||||||||||||
| Male | 27 | 14 | 17 | 18 | 19 | 17 | 22 | 23 | 14 | 11 | 9 | 0.528 |
| Female | 177 | 64 | 68 | 62 | 74 | 89 | 89 | 102 | 89 | 94 | 60 | 0.998 |
| Male to Female ratio | 0.15 | 0.22 | 0.25 | 0.29 | 0.26 | 0.19 | 0.25 | 0.23 | 0.16 | 0.12 | 0.15 | |
| Total | 204 | 78 | 85 | 80 | 93 | 106 | 111 | 125 | 103 | 105 | 69 | |
| 0–20 years old in 1986 (26.91%) | 48 | 16 | 23 | 18 | 16 | 33 | 29 | 39 | 27 | 39 | 24 | |
| Pathological classification | ||||||||||||
| Papillary | 120 | 35 | 37 | 26 | 38 | 70 | 83 | 76 | 62 | 75 | 52 | 0.259 |
| Follicular | 20 | 9 | 11 | 8 | 8 | 13 | 10 | 22 | 15 | 24 | 17 | |
| Medullary | 14 | 2 | 6 | 6 | 9 | 4 | 4 | 9 | 5 | 7 | 6 | |
| PDTC* | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| <10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–80 | ≥81 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Both sexes | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| Papillary | 0 | 0 | 6 | 85.7 | 16 | 72.7 | 71 | 75.5 | 135 | 75.4 | 222 | 75 | 162 | 71.4 | 51 | 67.1 | 11 | 84.6 |
| Follicular | 0 | 0 | 0 | 0 | 2 | 9.1 | 15 | 16 | 31 | 17.3 | 48 | 16.2 | 46 | 20.3 | 13 | 17.1 | 2 | 15.4 |
| Medullary | 0 | 0 | 1 | 14.3 | 4 | 18.2 | 8 | 8.5 | 12 | 6.7 | 22 | 7.4 | 16 | 7 | 9 | 11.8 | 0 | 0.0 |
| Anaplastic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.7 | 3 | 1.3 | 2 | 2.6 | 0 | 0.0 |
| PDTC* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 2 | 0.7 | 0 | 0 | 1 | 1.3 | 0 | 0.0 |
| Total | 0 | 0 | 7 | 100 | 22 | 100 | 94 | 100 | 179 | 100 | 296 | 100 | 227 | 100 | 76 | 100 | 13 | 100 |
| Males | ||||||||||||||||||
| Papillary | 0 | 0 | 0 | 0.0 | 3 | 60.0 | 6 | 85.7 | 18 | 78.3 | 27 | 67.5 | 25 | 67.6 | 11 | 50.0 | 0 | 0 |
| Follicular | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 13.0 | 8 | 20.0 | 10 | 27.0 | 5 | 22.7 | 0 | 0 |
| Medullary | 0 | 0 | 1 | 100.0 | 2 | 40.0 | 1 | 14.3 | 2 | 8.7 | 3 | 7.5 | 2 | 5.4 | 5 | 22.7 | 0 | 0 |
| Anaplastic | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 5.0 | 0 | 0.0 | 1 | 4.6 | 0 | 0 |
| PDTC* | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0 |
| Total | 0 | 0 | 1 | 100 | 5 | 100 | 7 | 100 | 23 | 100 | 40 | 100 | 37 | 100 | 22 | 100.0 | 0 | 0 |
| Females | ||||||||||||||||||
| Papillary | 0 | 0 | 6 | 100.0 | 13 | 76.5 | 65 | 74.7 | 117 | 75.0 | 195 | 76.2 | 137 | 72.1 | 40 | 74.1 | 11 | 84.6 |
| Follicular | 0 | 0 | 0 | 0.0 | 2 | 11.8 | 15 | 17.2 | 28 | 18.0 | 40 | 15.6 | 36 | 19.0 | 8 | 14.8 | 2 | 15.4 |
| Medullary | 0 | 0 | 0 | 0.0 | 2 | 11.8 | 7 | 8.1 | 10 | 6.4 | 19 | 7.4 | 14 | 7.4 | 4 | 7.4 | 0 | 0.0 |
| Anaplastic | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 1.6 | 1 | 1.9 | 0 | 0.0 |
| PDTC* | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 2 | 0.8 | 0 | 0.0 | 1 | 1.9 | 0 | 0.0 |
| Total | 0 | 0 | 6 | 100.0 | 17 | 100.0 | 87 | 100.0 | 156 | 100.0 | 256 | 100.0 | 190 | 100.0 | 54 | 100.0 | 13 | 100.0 |
| <45 | ≥45 | Total | χ2/F | p-Value | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 47 | 144 | 191 | 0.318 | 0.573 |
| Female | 220 | 748 | 968 | ||
| Histological Type | |||||
| Papillary | 153 | 521 | 674 | 104.903 | <0.001 |
| Follicular | 27 | 130 | 157 | ||
| Medullary | 20 | 52 | 72 | ||
| Anaplastic | 0 | 7 | 7 | ||
| PDTC | 0 | 4 | 4 | ||
| Others | 92 | 255 | 347 | ||
| LN metastasis | |||||
| Yes | 18 | 44 | 62 | 1.434 | 0.231 |
| No | 44 | 159 | 203 | ||
| Metastasis | |||||
| Yes | 0 | 2 | 2 | 0.600 | 0.439 |
| No | 1 | 3 | 4 |
| <21 | 21–45 | 46–59 | ≥60 | Total | χ2/F | p-Value | |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 3 | 46 | 56 | 86 | 191 | 8015 | 0.046 |
| Female | 9 | 227 | 380 | 352 | 968 | ||
| Histological Type | |||||||
| Papillary | 6 | 161 | 253 | 254 | 674 | 110,375 | <0.001 |
| Follicular | 0 | 30 | 58 | 69 | 157 | ||
| Medullary | 1 | 20 | 23 | 28 | 72 | ||
| Anaplastic | 0 | 0 | 1 | 6 | 7 | ||
| PDTC | 0 | 0 | 2 | 2 | 4 | ||
| Others | 5 | 89 | 133 | 119 | 346 | ||
| LN metastasis | |||||||
| Yes | 1 | 19 | 22 | 20 | 62 | 55.880 | <0.001 |
| No | 1 | 46 | 74 | 82 | 203 | ||
| Metastasis | |||||||
| Yes | 0 | 0 | 1 | 2 | 3 | 254.545 | <0.001 |
| No | 0 | 4 | 0 | 0 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teodoriu, L.; Ungureanu, M.C.; Leustean, L.; Preda, C.; Ciobanu, D.; Grierosu, I.; Matei, M.; Iacob, R.; Stefanescu, C. Updated Incidence of Thyroid Cancer in the North East Region of Romania after 35 Years of Chernobyl Fallout. Is There a Link between? Diagnostics 2021, 11, 907. https://doi.org/10.3390/diagnostics11050907
Teodoriu L, Ungureanu MC, Leustean L, Preda C, Ciobanu D, Grierosu I, Matei M, Iacob R, Stefanescu C. Updated Incidence of Thyroid Cancer in the North East Region of Romania after 35 Years of Chernobyl Fallout. Is There a Link between? Diagnostics. 2021; 11(5):907. https://doi.org/10.3390/diagnostics11050907
Chicago/Turabian StyleTeodoriu, Laura, Maria Christina Ungureanu, Letitia Leustean, Cristina Preda, Delia Ciobanu, Irena Grierosu, Mioara Matei, Roxana Iacob, and Cipriana Stefanescu. 2021. "Updated Incidence of Thyroid Cancer in the North East Region of Romania after 35 Years of Chernobyl Fallout. Is There a Link between?" Diagnostics 11, no. 5: 907. https://doi.org/10.3390/diagnostics11050907
APA StyleTeodoriu, L., Ungureanu, M. C., Leustean, L., Preda, C., Ciobanu, D., Grierosu, I., Matei, M., Iacob, R., & Stefanescu, C. (2021). Updated Incidence of Thyroid Cancer in the North East Region of Romania after 35 Years of Chernobyl Fallout. Is There a Link between? Diagnostics, 11(5), 907. https://doi.org/10.3390/diagnostics11050907







