Survival Rates of Patients with Non-Small Cell Lung Cancer Depending on Lymph Node Metastasis: A Focus on Saliva
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Saliva Analysis
2.3. Statistical Analysis
3. Results
3.1. Overall Survival Rates Depending on the Stage of the Disease, Histological Type, and Morphological Growth Forms of NSCLC
3.2. The Predictive Value of the Type of Treatment
3.3. Predictive Value of Saliva Biochemical Indicators
3.4. Multivariate Survival Analysis Using the Cox Regression Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Akcam, T.I.; Kaya, S.O.; Akcay, O.; Samancılar, O.; Ceylan, K.C.; Sevınc, S.; Unsal, S. Is there a survival difference between single station and multi-station N2 disease in operated non-small cell lung cancer patients? Cancer Treat. Commun. 2015, 4, 165–168. [Google Scholar] [CrossRef]
- Ferguson, M.K. Optimal management when unsuspected N2 nodal disease is identifi ed during thoracotomy for lung cancer: Cost-effectiveness analysis. J. Thorac. Cardiovasc. Surg. 2003, 126, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Yokomise, H.; Gotoh, M.; Okamoto, T.; Yamamoto, Y.; Ishikawa, S.; Nakashima, T.; Masuya, D.; Liu, D.; Huang, C.L. Induction chemoradiotherapy (carboplatin-taxane and concurrent 50 Gy radiation) for bulky cN2, N3 non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2007, 133, 1179–1185. [Google Scholar] [CrossRef][Green Version]
- Robinson, L.; Ruckdeschel, J.; Wagner, H., Jr.; Stevens, C.W. Treatment of Non-small Cell Lung Cancer-Stage III A (ACCP evidence-based clinical practice guidelines). Chest 2007, 132, 243S–265S. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, F.; Matsuo, K.; Fukui, T.; Mori, S.; Hatooka, S.; Mitsudomi, T. Effect of selective lymph node dissection based on patterns of lobespecific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: A large-scale retrospective cohort study applying a propensity score. J. Thorac. Cardiovasc. Surg. 2010, 139, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, T.; Okumura, T.; Isaka, M.; Nakagawa, K.; Ohde, Y.; Kondo, H. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2013, 44, 59–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsutani, Y.; Miyata, Y.; Nakayama, H.; Okumura, S.; Adachi, S.; Yoshimura, M.; Okada, M. Prediction of pathological node-negative clinical stage IA lung adenocarcinoma for optimal candidates undergoing sublobar resection. J. Thorac. Cardiovasc. Surg. 2012, 14, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-X.; Lu, T.-Y.; Lin, Y.-S.; Fang, H.-Y.; Shih, P.-K. Prognostic effect of incongruous lymph node status in early-stage non-small cell lung cancer. Eur. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K.; Gundyrev, I.A. Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives. Diagnostics 2020, 10, 186. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K.; Sarf, E.A. Chronophysiological features of the normal mineral composition of human saliva. Arch. Oral Biol. 2017, 82, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Okiror, L.; Harling, L.; Toufektzian, L.; King, J.; Routledge, T.; Harrison-Phipps, K.; Pilling, J.; Veres, L.; Lal, R.; Bille, A. Prognostic factors including lymphovascular invasion on survival for resected non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2018, 156, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Molinier, O.; Goupil, F.; Debieuvre, D.; Auliac, J.-B.; Jeandeau, S.; Lacroix, S.; Martin, F.; Grivaux, M. Five-year survival and prognostic factors according to histology in 6,101 Non-Small-Cell Lung Cancer patients. Respir. Med. Res. 2020, 77, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Grosu, H.B.; Manzanera, A.; Shivakumar, S.; Sun, S.; Gonzalez, G.N.; Ost, D.E. Survival disparities following surgery among patients with different histological types of non-small cell lung cancer. Lung Cancer 2020, 140, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.E.; Alhanafy, A.M.; Habib, M.S.E.; Hagag, M.; Ibrahem, R.A.L. Serum circulating cell free DNA as potential diagnostic and prognostic biomarker in non-small cell lung cancer. Biochem. Biophys. Rep. 2018, 15, 45–51. [Google Scholar] [CrossRef]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Bæk, R.; Jakobsen, K.R.; Meldgaard, P.; Folkersen, B.H.; Rasmussen, T.R.; Varming, K.; Jørgensen, M.M.; Sorensen, B.S. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 2016, 10, 1595–1602. [Google Scholar] [CrossRef]
- Pérez-Ramírez, C.; Cańadas-Garre, M.; Alnatsha, A.; Molina, M.Á.; Robles, A.I.; Villar, E.; Delgado, J.R.; Faus-Dáder, M.J.; Calleja-Hernández, M.Á. Interleukins as new prognostic genetic biomarkers in non-small cell lung cancer. Surg. Oncol. 2017, 26, 278–285. [Google Scholar] [CrossRef]
- Targowski, T.; Jahnz-Rozyk, K.; Owczarek, W.; Raczka, A.; Janda, P.; Szkoda, T.; Płusa, T. Telomerase activity and serum levels of p53 protein as prognostic factors of survival in patients with advanced non-small cell lung cancer. Respir. Med. 2010, 104, 1356–1361. [Google Scholar] [CrossRef]
- Cedrés, S.; Nuñez, I.; Longo, M.; Martinez, P.; Checa, E.; Torrejón, D.; Felip, E. Serum Tumor Markers CEA, CYFRA21-1, and CA-125 Are Associated with Worse Prognosis in Advanced Non–Small-Cell Lung Cancer (NSCLC). Clin. Lung Cancer 2011, 12, 172–179. [Google Scholar] [CrossRef]
- Prelaj, A.; Rebuzzi, S.E.; Pizzutilo, P.; Bilancia, M.; Montrone, M.; Pesola, F.; Longo, V.; Del Bene, G.; Lapadula, V.; Cassano, F.; et al. EPSILoN: A Prognostic Score Using Clinical and Blood Biomarkers in Advanced Non–Small-cell Lung Cancer Treated with Immunotherapy. Clin. Lung Cancer 2020, 21, 365–377. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, H.; Zhou, H.; Santiago, S.; Lee, J.M.; Garon, E.B.; Yang, J.; Brinkmann, O.; Yan, X.; Akin, D.; et al. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell. Mol. Life Sci. 2012, 69, 3341–3350. [Google Scholar] [CrossRef]
- Kisluk, J.; Ciborowski, M.; Niemira, M.; Kretowski, A.; Niklinski, J. Proteomics biomarkers for non-small cell lung cancer. J. Pharm. Biomed. Anal. 2014, 101, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, S.; Qiao, Z.; Shang, Z.; Xia, Z.; Niu, X.; Qian, L.; Zhang, Y.; Fan, L.; Cao, C.X.; et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal. Chim. Acta 2017, 982, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G. Endogenous Intoxication and Saliva Lipid Peroxidation in Patients with Lung Cancer. Diagnostics 2016, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K. A new field of application of saliva tests for prognostic purpose: Focus on lung cancer. Biomed. Chem. Res. Methods 2020, 3, e00133. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Yang, L.-P.; Qiu, M.-Z.; Wang, Z.-Q.; Zhou, Y.-X.; Wang, F.; Zhang, D.-S.; Wang, F.-H.; Li, Y.-H.; Xu, R.-H. Prognostic value of preoperative serum lactate dehydrogenase levels for resectable gastric cancer and prognostic nomograms. Oncotarget 2016, 26, 945–956. [Google Scholar] [CrossRef]
- Yao, F.; Zhao, T.; Zhong, C.; Zhu, J.; Zhao, H. LDHA is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumor Biol. 2013, 34, 25–31. [Google Scholar] [CrossRef]
- Koh, Y.W.; Lee, S.J.; Park, S.Y. Prognostic significance of lactate dehydrogenase B according to histologic type of non-small-cell lung cancer and its association with serum lactate dehydrogenase. Pathol. Res. Pract. 2017, 213, 1134–1138. [Google Scholar] [CrossRef]
- Huijgen, H.J.; Sanders, G.; Koster, R.W.; Vreeken, J.; Bossuyt, P. The clinical value of lactate dehydrogenase in serum: A quantitative review. Eur. Clin. Chem. Clin. Biochem. 1997, 35, 569–579. [Google Scholar]
- Mazzaschi, G.; Minari, R.; Zecca, A.; Cavazzonib, A.; Ferria, V.; Mori, C.; Squadrilli, A.; Bordia, P.; Buti, S.; Bersanelli, M.; et al. Soluble PD-L1 and Circulating CD8+PD-1+ and NK Cells Enclose a Prognostic and Predictive Immune Effector Score in Immunotherapy Treated NSCLC patients. Lung Cancer 2020, 148, 1–11. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K. Correlation interrelations between the composition of saliva and blood plasma in norm. Klin. Lab. Diagn. Russ. Clin. Lab. Diagn. 2018, 63, 477–482. [Google Scholar]
- Xue, X.; Zang, X.; Liu, Y.; Lin, D.; Jiang, T.; Gao, J.; Wu, C.; Ma, X.; Deng, H.; Yu, Z.; et al. Independent risk factors for lymph node metastasis in 2623 patients with Non–Small cell lung cancer. Surg. Oncol. 2020, 34, 256–260. [Google Scholar] [CrossRef]
- Friedel, G.; Steger, V.; Kyriss, T.; Zoller, J.; Toomes, H. Prognosis in N2 NSCLC. Lung Cancer 2004, 45 (Suppl. 2), S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Aokage, K.; Ishii, G.; Nakasone, S.; Sakai, T.; Okada, S.; Miyoshi, T.; Tane, K.; Tsuboi, M. Prognostic Impact of the Number of Metastatic Lymph Nodes on the Eighth Edition of the TNM Classification of NSCLC. J. Thorac. Oncol. 2019, 14, 1408–1418. [Google Scholar] [CrossRef] [PubMed]

| LNS | Variables | HR (95% CI) | p-Value | OS, Months |
|---|---|---|---|---|
| pT | ||||
| pN0 | pT1, n = 18 | 1 | 0.03363 | 40.4 |
| pT2, n = 80 | 1.31 (0.50–3.43) | 36.8 | ||
| pT3, n = 22 | 2.18 (0.67–7.03) | 28.9 | ||
| pN1 | pT2, n = 20 | 1 | 0.00223 | 29.6 |
| pT3, n = 23 | 1.10 (0.31–3.93) | 19.7 | ||
| pT4, n = 8 | 2.40 (0.25–22.75) | 5.9 | ||
| pN2 | pT2, n = 25 | 1 | 0.18914 | 16.8 |
| pT3, n = 18 | 2.96 (0.57–15.20) | 10.2 | ||
| pT4, n = 25 | 3.85 (0.94–15.56) | 11.0 | ||
| Histological Subtype | ||||
| pN0 | ADC, n = 75 | 1 | 0.03366 | 36.5 |
| SCC, n = 45 | 2.75 (1.28–5.86) * | 32.7 | ||
| pN1 | ADC, n = 22 | 1 | 0.03069 | 26.3 |
| SCC, n = 29 | 2.13 (0.30–4.27) | 9.6 | ||
| pN2 | ADC, n = 34 | 1 | 0.07122 | 16.6 |
| SCC, n = 34 | 2.21 (0.51–9.55) | 9.7 | ||
| Morphological Growth Forms | ||||
| pN0 | Peripheral, n = 106 ADC-71, SCC-35 | 1 | 0.80773 | 36.7 |
| Central, n = 14 ADC-3, SCC-11 | 1.29 (0.46–3.59) | 31.2 | ||
| pN1 | Peripheral, n = 28 ADC-19, SCC-9 | 1 | 0.08248 | 25.3 |
| Central, n = 23 ADC-3, SCC-20 | 1.26 (0.36–4.34) | 13.5 | ||
| pN2 | Peripheral, n = 30 ADC-22, SCC-8 | 1 | 0.20789 | 16.0 |
| Central, n = 31 ADC-10, SCC-21 | 2.22 (0.62–7.90) | 10.8 | ||
| Mediastinal, n = 7 ADC-0, SCC-7 | 2.89 (0.33–24.84) | 9.8 | ||
| Degree of Differentiation | ||||
| pN0 | G1, n = 30 | 1 | 0.06772 | 38.7 |
| G2, n = 38 | 1.61 (0.59–4.37) | 36.7 | ||
| G3, n = 27 | 1.22 (0.41–3.62) | 32.3 | ||
| G4, n = 25 | 1.42 (0.45–4.43) | 22.5 | ||
| pN1 | G1, n = 10 | 1 | 0.06314 | 37.9 |
| G2, n = 13 | 0.80 (0.10–6.27) | 16.0 | ||
| G3, n = 8 | 2.70 (0.33–21.52) | 18.2 | ||
| G4, n = 20 | 3.30 (0.42–25.79) | 11.8 | ||
| pN2 | G1, n = 12 | 1 | 0.47036 | 25.2 |
| G2, n = 18 | 1.25 (0.09–17.63) | 9.7 | ||
| G3, n = 9 | 1.63 (0.14–18.12) | 11.8 | ||
| G4, n = 29 | 1.58 (0.13–19.04) | 10.1 | ||
| LNS | Variables | HR (95% CI) | p-Value | OS, Months |
|---|---|---|---|---|
| Treatment Type | ||||
| pN0 | Radical, n = 79 | 1 | 0.00196 | 37.7 |
| Combined, n = 36 | 2.66 (1.22–5.71) * | 30.9 | ||
| pN1 | Combined, n = 27 | 1 | 0.00738 | 26.3 |
| Palliative, n = 19 | 10.91 (1.29–89.53) * | 11.2 | ||
| pN2 | Combined, n = 19 | 1 | 0.08023 | 23.9 |
| Palliative, n = 36 | 2.63 (0.76–9.04) | 12.7 | ||
| The Extent of Surgical Treatment | ||||
| pN0 | Lobectomy, n = 75 | 1 | 0.00021 | 37.6 |
| Bilobectomy, n = 33 | 1.11 (0.49–2.55) | 31.2 | ||
| Pneumonectomy, n = 7 | 3.87 (0.86–17.10) | 18.5 | ||
| No surgery, n = 5 | 6.96 (1.31–36.00) * | 13.4 | ||
| pN1 | Lobectomy, n = 14 | 1 | 0.05689 | 32.8 |
| Bilobectomy, n = 7 | 0.62 (0.11–3.63) | 21.8 | ||
| Pneumonectomy, n = 9 | 0.25 (0.04–1.38) | 17.8 | ||
| No surgery, n = 21 | 8.31 (0.84–79.56) | 11.2 | ||
| pN2 | Lobectomy, n = 4 | 1 | 0.56381 | 22.6 |
| Bilobectomy, n = 8 | 2.00 (0.14–27.78) | 20.5 | ||
| Pneumonectomy, n = 12 | 4.33 (0.33–56.10) | 17.1 | ||
| No surgery, n = 44 | 2.63 (0.45–15.00) | 11.0 | ||
| Indicators | Variables | HR (95% CI) | p-Value | OS, Months |
|---|---|---|---|---|
| pN0 | ||||
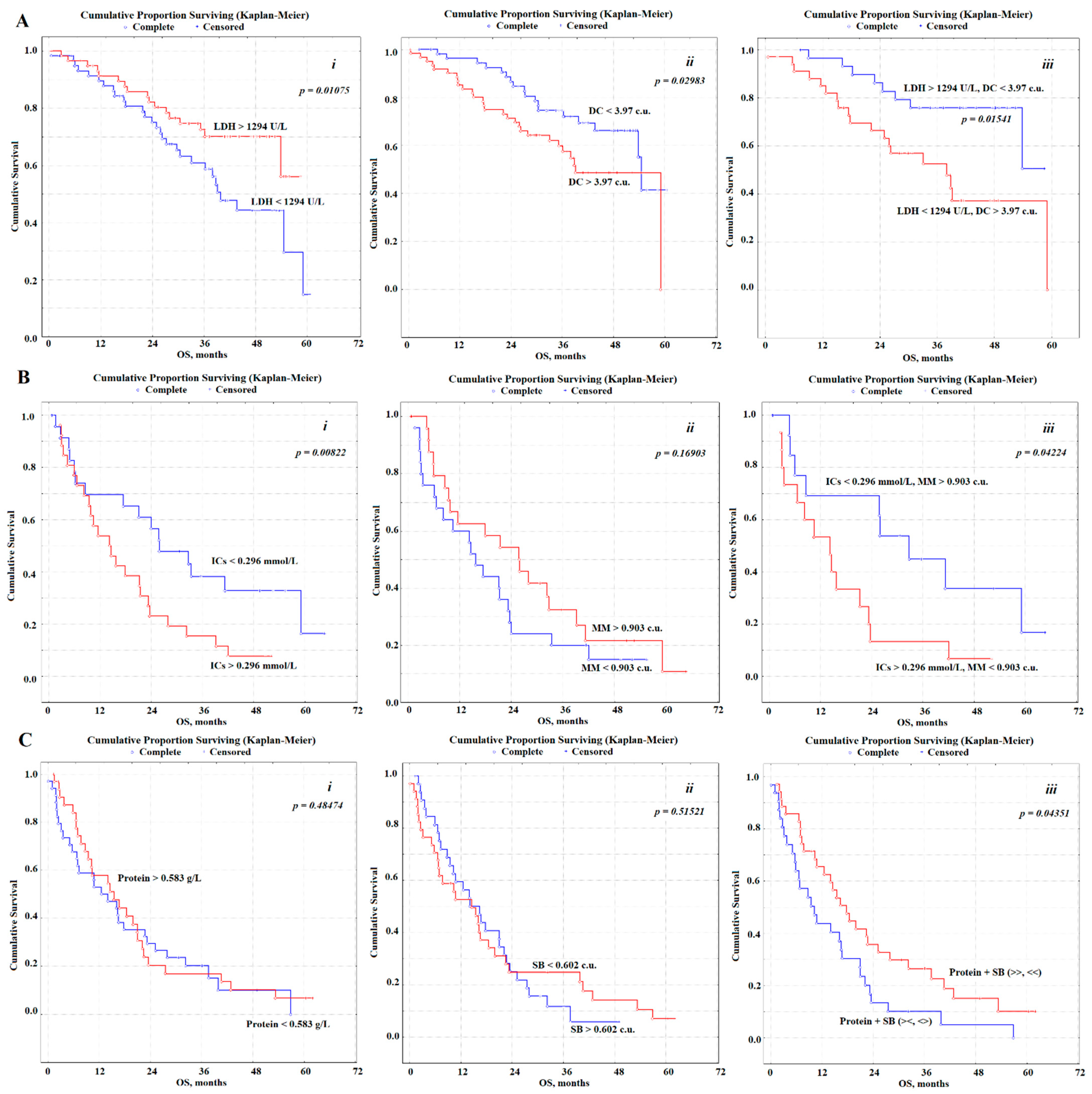
| LDH, U/L | <1294, n = 60 | 1 | 0.01075 | 30.6 |
| >1294, n = 60 | 0.42 (0.20–0.90) * | 36.6 | ||
| LDH, U/L | <636, n = 46 | 1 | 0.16263 | 30.1 |
| 636–1900, n = 47 | 0.47 (0.21–1.09) | 37.2 | ||
| >1900, n = 27 | 0.26 (0.09–0.77) * | 36.4 | ||
| DC, c.u. | <3.97, n = 56 | 1 | 0.02983 | 38.2 |
| >3.97, n = 64 | 1.64 (0.78–3.44) | 30.3 | ||
| DC, c.u. | <3.76, n = 29 | 1 | 0.14553 | 41.3 |
| 3.76–4.19, n = 70 | 1.48 (0.59–3.69) | 36.5 | ||
| >4.16, n = 21 | 1.67 (0.52–5.30) | 23.6 | ||
| LDH + DC | >1294, <3.97, n = 30 | 1 | 0.01541 | 39.6 |
| <1294, >3.97, n = 34 | 3.48 (1.21–9.85) * | 28.7 | ||
| pN1 | ||||
| ICs, mmol/L | <0.296, n = 25 | 1 | 0.00822 | 26.2 |
| >0.296, n = 26 | 6.75 (1.28–34.57) * | 14.7 | ||
| ICs, mmol/L | <0.182, n = 13 | 1 | 0.00639 | 26.2 |
| 0.182–0.455, n = 25 | 0.64 (0.14–2.95) | 23.7 | ||
| >0.455, n = 13 | 4.20 (1.38–45.31) * | 6.8 | ||
| MM, c.u. | <0.903, n = 25 | 1 | 0.16903 | 16.0 |
| <0.903, n = 26 | 0.52 (0.13–2.04) | 24.0 | ||
| MM, c.u. | <0.832, n = 13 | 1 | 0.10240 | 14.9 |
| 0.832–0.989, n = 26 | 0.28 (0.03–2.58) | 18.8 | ||
| >0.989, n = 12 | 0.17 (0.02–0.97) * | 28.7 | ||
| ICs + MM | <0.296, >0.903, n = 15 | 1 | 0.04224 | 26.3 |
| >0.296, <0.903, n = 15 | 9.80 (1.04–89.91) * | 14.5 | ||
| pN2 | ||||
| Protein, g/L | <0.583, n = 34 | 1 | 0.48474 | 13.5 |
| >0.583, n = 33 | 0.75 (0.18–3.04) | 14.3 | ||
| SB, c.u. | <0.602, n = 34 | 1 | 0.51521 | 14.3 |
| >0.602, n = 34 | 1.29 (0.32–5.24) | 12.9 | ||
| Protein + SB | ><, <>, n = 33 | 1 | 0.04351 | 9.3 |
| >>, <<, n = 35 | 0.50 (0.12–0.98) * | 16.6 | ||
| Prognostic Factors | β | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| N0 (χ2 = 34.55, p < 0.00001) | ||||
| pT | 0.2020 | 0.2938 | 0.6875 | 0.4918 |
| Histological Subtype | 0.8011 | 0.3154 | 2.540 | 0.0111 |
| Morphological Growth Forms | −1.031 | 0.5382 | −1.915 | 0.0555 |
| Treatment Types | 1.220 | 0.2509 | 4.862 | 0.0000 |
| LDH | −1.094 | 0.3440 | −3.179 | 0.0015 |
| DC | 0.6192 | 0.3083 | 2.008 | 0.0447 |
| N1 (χ2 = 20.57, p = 0.00446) | ||||
| pT | 0.6598 | 0.3417 | 1.931 | 0.0535 |
| Histological Subtype | 0.6680 | 0.4306 | 1.551 | 0.1208 |
| Morphological Growth Forms | −0.8718 | 0.4470 | −1.951 | 0.0511 |
| Treatment Types | 0.8776 | 0.4488 | 1.956 | 0.0505 |
| ICs | 0.6709 | 0.3342 | 2.007 | 0.0448 |
| MM | −0.2486 | 0.3269 | −0.7606 | 0.4469 |
| N2 (χ2 = 18.80, p = 0.00884) | ||||
| pT | −0.1463 | 0.2107 | −0.6943 | 0.4875 |
| Histological Subtype | 0.4886 | 0.3275 | 1.492 | 0.1357 |
| Morphological Growth Forms | 0.2740 | 0.2860 | 0.9582 | 0.3380 |
| Treatment Types | 0.6314 | 0.2707 | 2.333 | 0.0197 |
| Protein | −0.0891 | 0.2878 | −0.3095 | 0.7569 |
| SB | 0.0806 | 0.2869 | 0.2808 | 0.7789 |
| Protein + SB | −0.6886 | 0.3007 | −2.290 | 0.0220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K. Survival Rates of Patients with Non-Small Cell Lung Cancer Depending on Lymph Node Metastasis: A Focus on Saliva. Diagnostics 2021, 11, 912. https://doi.org/10.3390/diagnostics11050912
Bel’skaya LV, Sarf EA, Kosenok VK. Survival Rates of Patients with Non-Small Cell Lung Cancer Depending on Lymph Node Metastasis: A Focus on Saliva. Diagnostics. 2021; 11(5):912. https://doi.org/10.3390/diagnostics11050912
Chicago/Turabian StyleBel’skaya, Lyudmila V., Elena A. Sarf, and Victor K. Kosenok. 2021. "Survival Rates of Patients with Non-Small Cell Lung Cancer Depending on Lymph Node Metastasis: A Focus on Saliva" Diagnostics 11, no. 5: 912. https://doi.org/10.3390/diagnostics11050912
APA StyleBel’skaya, L. V., Sarf, E. A., & Kosenok, V. K. (2021). Survival Rates of Patients with Non-Small Cell Lung Cancer Depending on Lymph Node Metastasis: A Focus on Saliva. Diagnostics, 11(5), 912. https://doi.org/10.3390/diagnostics11050912







