Potential Relation between Plasma BDNF Levels and Human Coronary Plaque Morphology
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Collection, Plasma Preparation and BDNF Analyses
2.3. OCT Image Analysis
2.4. OCT Macrophage Analysis
2.5. Plaque Vulnerability Assessment
2.6. Statistical Analysis
3. Results
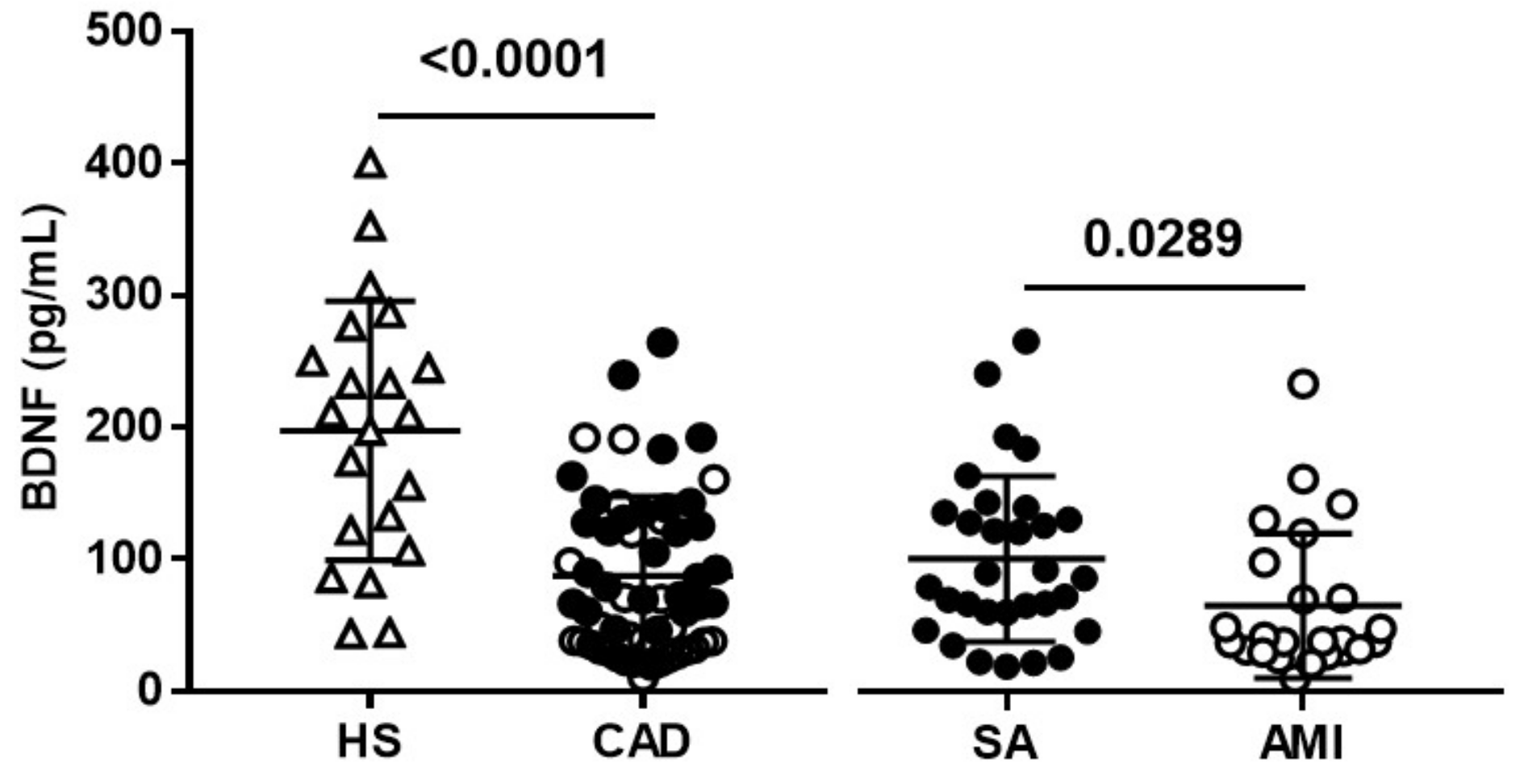
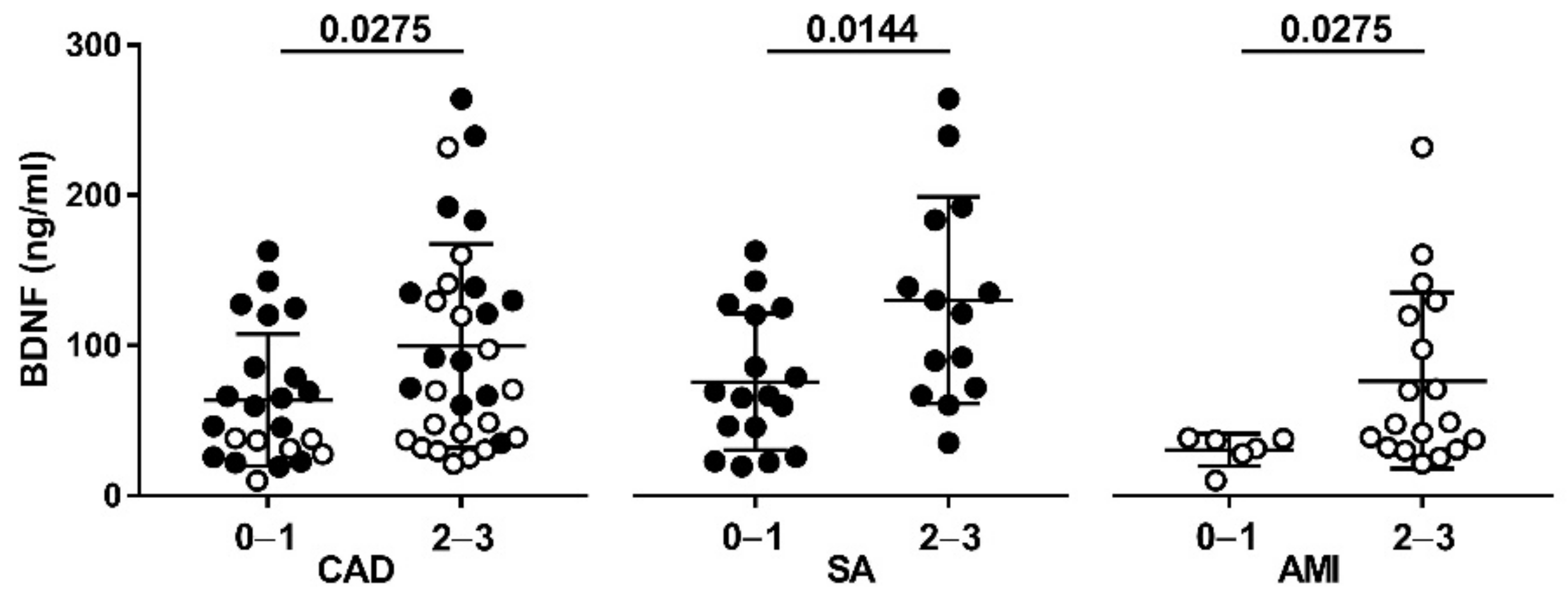
3.1. Clinical Characteristics and Plasma BDNF Levels in the Study Population
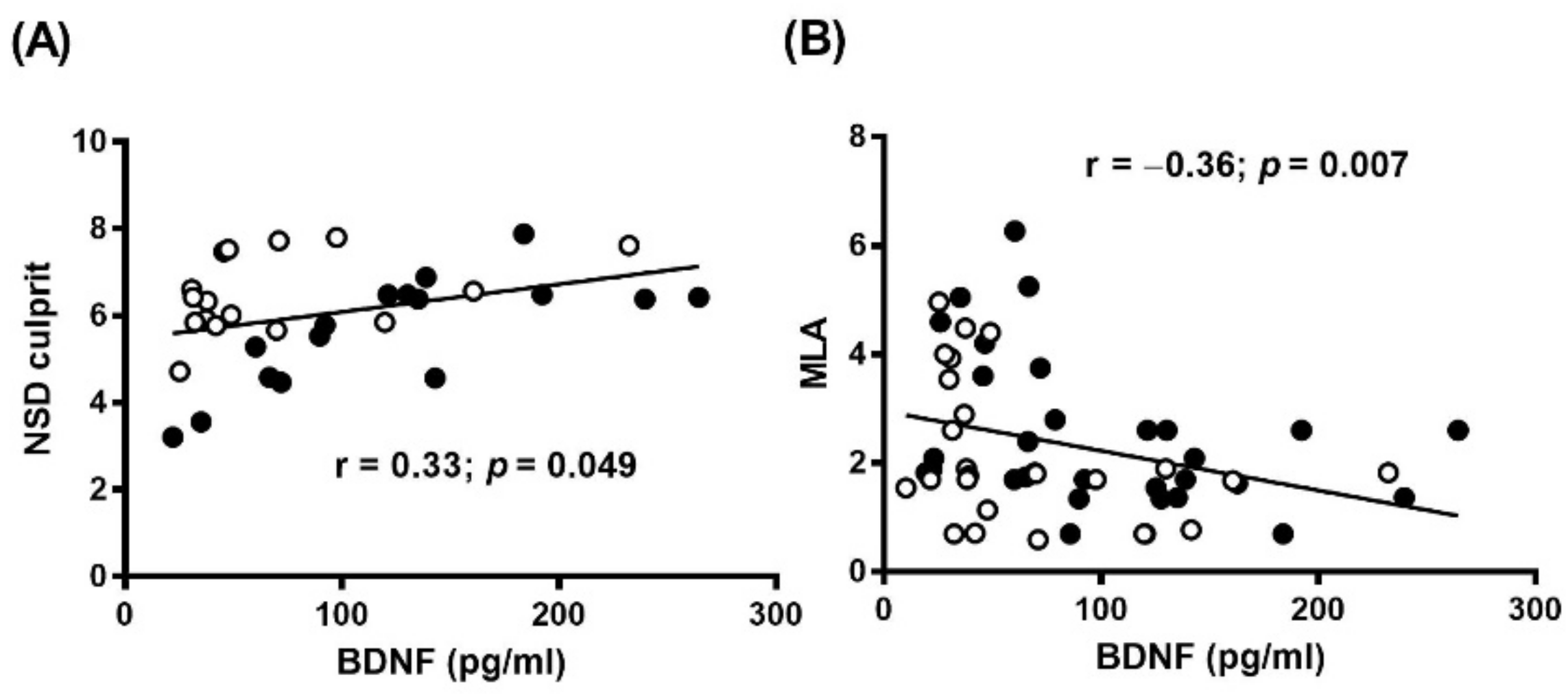
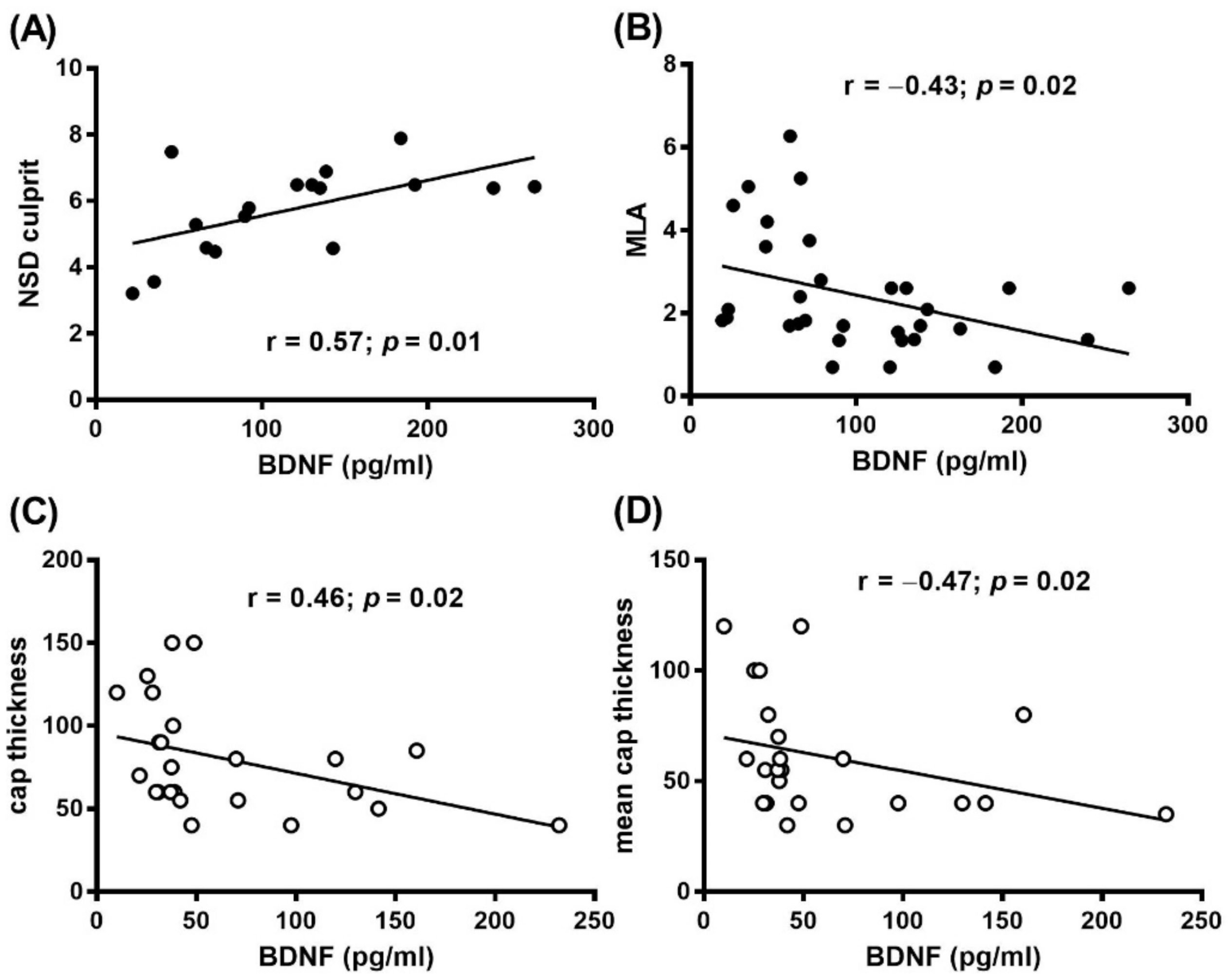
3.2. Plasma BDNF and OCT Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Anand, S.S.; Bosch, J.; Eikelboom, J.W.; Connolly, S.J.; Diaz, R.; Widimsky, P.; Aboyans, V.; Alings, M.; Kakkar, A.K.; Keltai, K.; et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 219–229. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Braunwald, E.; Sabatine, M.S. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1274–1275. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Morrow, D.A.; Braunwald, E.; Bonaca, M.P.; Ameriso, S.F.; Dalby, A.J.; Fish, M.P.; Fox, K.A.; Lipka, L.J.; Liu, X.; Nicolau, J.C.; et al. Vorapaxar in the secondary prevention of atherothrombotic events. N. Engl. J. Med. 2012, 366, 1404–1413. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Coppola, V.; Barrick, C.A.; Southon, E.A.; Celeste, A.; Wang, K.; Chen, B.; Haddad, B.; Yin, J.; Nussenzweig, A.; Subramaniam, A.; et al. Ablation of TrkA function in the immune system causes B cell abnormalities. Development 2004, 131, 5185–5195. [Google Scholar] [CrossRef]
- Kermani, P.; Hempstead, B. Brain-derived neurotrophic factor: A newly described mediator of angiogenesis. Trends Cardiovasc. Med. 2007, 17, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Anastasia, A.; Deinhardt, K.; Wang, S.; Martin, L.; Nichol, D.; Irmady, K.; Trinh, J.; Parada, L.; Rafii, S.; Hempstead, B.L.; et al. Trkb signaling in pericytes is required for cardiac microvessel stabilization. PLoS ONE 2014, 9, e87406. [Google Scholar] [CrossRef] [PubMed]
- Amadio, P.; Porro, B.; Sandrini, L.; Fiorelli, S.; Bonomi, A.; Cavalca, V.; Brambilla, M.; Camera, M.; Veglia, F.; Tremoli, E.; et al. Patho-physiological role of BDNF in fibrin clotting. Sci. Rep. 2019, 9, 389. [Google Scholar] [CrossRef]
- Amoureux, S.; Lorgis, L.; Sicard, P.; Girard, C.; Rochette, L.; Vergely, C. Vascular BDNF expression and oxidative stress during aging and the development of chronic hypertension. Fundam. Clin. Pharmacol. 2012, 26, 227–234. [Google Scholar] [CrossRef]
- Sharma, E.; Behl, T.; Mehta, V.; Kumar, A.; Setia, D.; Uddin, M.S.; Zengin, G.; Arora, S. Exploring the various aspects of Brain Derived Neurotropic Factor (BDNF) in Diabetes Mellitus. CNS Neurol. Disord. Drug Targets 2020. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.S.; Lima, M.M.O.; Figueiredo, P.H.S.; Martinelli, P.M.; Camargos, E.R.; Chaves, A.T.; Nunes, M.C.P.; Rocha, M.O.C. Prognostic value of serum brain-derived neurotrophic factor levels in patients with Chagas cardiomyopathy. Mem. Inst. Oswaldo Cruz 2018, 113, e180224. [Google Scholar] [CrossRef]
- Shibata, A.; Hanatani, A.; Izumi, Y.; Kitada, R.; Iwata, S.; Yoshiyama, M. Serum brain-derived neurotrophic factor level and exercise tolerance complement each other in predicting the prognosis of patients with heart failure. Heart Vessel. 2018, 33, 1325–1333. [Google Scholar] [CrossRef]
- Kaess, B.M.; Preis, S.R.; Lieb, W.; Beiser, A.S.; Yang, Q.; Chen, T.C.; Hengstenberg, C.; Erdmann, J.; Schunkert, H.; Seshadri, S.; et al. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J. Am. Heart Assoc. 2015, 4, e001544. [Google Scholar] [CrossRef]
- Lee, I.T.; Lee, W.J.; Tsai, I.C.; Liang, K.W.; Lin, S.Y.; Wan, C.J.; Fu, C.P.; Sheu, W.H. Brain-derived neurotrophic factor not associated with metabolic syndrome but inversely correlated with vascular cell adhesion molecule-1 in men without diabetes. Clin. Chim. Acta 2012, 413, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Malan, L.; Uys, A.S.; Malan, N.T.; Harvey, B.H.; Ziemssen, T. Attenuated brain-derived neurotrophic factor and hypertrophic remodelling: The SABPA study. J. Hum. Hypertens. 2015, 29, 33–39. [Google Scholar] [CrossRef]
- Usui, T.; Naruo, A.; Okada, M.; Hayabe, Y.; Yamawaki, H. Brain-derived neurotrophic factor promotes angiogenic tube formation through generation of oxidative stress in human vascular endothelial cells. Acta Physiol. 2014, 211, 385–394. [Google Scholar] [CrossRef]
- Ejiri, J.; Inoue, N.; Kobayashi, S.; Shiraki, R.; Otsui, K.; Honjo, T.; Takahashi, M.; Ohashi, Y.; Ichikawa, S.; Terashima, M.; et al. Possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation 2005, 112, 2114–2120. [Google Scholar] [CrossRef]
- Amadio, P.; Baldassarre, D.; Sandrini, L.; Weksler, B.B.; Tremoli, E.; Barbieri, S.S. Effect of cigarette smoke on monocyte procoagulant activity: Focus on platelet-derived brain-derived neurotrophic factor (BDNF). Platelets 2017, 28, 60–65. [Google Scholar] [CrossRef]
- Amadio, P.; Sandrini, L.; Ieraci, A.; Tremoli, E.; Barbieri, S.S. Effect of Clotting Duration and Temperature on BDNF Measurement in Human Serum. Int. J. Mol. Sci. 2017, 18, 1987. [Google Scholar] [CrossRef]
- Serra-Millas, M.; Lopez-Vilchez, I.; Navarro, V.; Galan, A.M.; Escolar, G.; Penades, R.; Catalan, R.; Fananas, L.; Arias, B.; Gasto, C. Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacology 2011, 216, 1–8. [Google Scholar] [CrossRef]
- Tamura, S.; Suzuki, H.; Hirowatari, Y.; Hatase, M.; Nagasawa, A.; Matsuno, K.; Kobayashi, S.; Moriyama, T. Release reaction of brain-derived neurotrophic factor (BDNF) through PAR1 activation and its two distinct pools in human platelets. Thromb. Res. 2011, 128, e55–e61. [Google Scholar] [CrossRef]
- Niccoli, G.; Giubilato, S.; Di Vito, L.; Leo, A.; Cosentino, N.; Pitocco, D.; Marco, V.; Ghirlanda, G.; Prati, F.; Crea, F. Severity of coronary atherosclerosis in patients with a first acute coronary event: A diabetes paradox. Eur. Heart J. 2012, 34, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Camilli, M.; Del Buono, M.G.; Russo, M.; Rinaldi, R.; Canonico, F.; Pedicino, D.; Severino, A.; D’Amario, D.; Trani, C.; et al. Brain-derived neurotrophic factor in patients with acute coronary syndrome. Transl. Res. 2020. [Google Scholar]
- Eligini, S.; Cosentino, N.; Fiorelli, S.; Fabbiocchi, F.; Niccoli, G.; Refaat, H.; Camera, M.; Calligaris, G.; De Martini, S.; Bonomi, A.; et al. Biological profile of monocyte-derived macrophages in coronary heart disease patients: Implications for plaque morphology. Sci. Rep. 2019, 9, 8680. [Google Scholar] [CrossRef] [PubMed]
- Scalone, G.; Niccoli, G.; Refaat, H.; Vergallo, R.; Porto, I.; Leone, A.M.; Burzotta, F.; D’Amario, D.; Liuzzo, G.; Fracassi, F.; et al. Not all plaque ruptures are born equal: An optical coherence tomography study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1271–1277. [Google Scholar] [CrossRef]
- Prati, F.; Regar, E.; Mintz, G.S.; Arbustini, E.; Di Mario, C.; Jang, I.K.; Akasaka, T.; Costa, M.; Guagliumi, G.; Grube, E.; et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 2010, 31, 401–415. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef]
- Jang, I.K.; Tearney, G.J.; MacNeill, B.; Takano, M.; Moselewski, F.; Iftima, N.; Shishkov, M.; Houser, S.; Aretz, H.T.; Halpern, E.F.; et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 2005, 111, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Chen, Y.; Wang, B.; Zhu, Y.; Chen, L.; Han, X.; Ma, G.; Liu, N. Association between brain-derived neurotrophic factor and von Willebrand factor levels in patients with stable coronary artery disease. BMC Cardiovasc. Disord. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Lin, M.I.; Wiegn, P.; Ringstedt, T.; Kraemer, R.; Hahn, R.; Wang, S.; Ibanez, C.F.; Rafii, S.; Hempstead, B.L. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 2000, 127, 4531–4540. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Miranda, R.C.; Kraemer, R.; McCaffrey, T.A.; Tessarollo, L.; Mahadeo, D.; Sharif, S.; Kaplan, D.R.; Tsoulfas, P.; Parada, L.; et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am. J. Pathol. 1995, 147, 309–324. [Google Scholar]
- Jiang, H.; Huang, S.; Li, X.; Li, X.; Zhang, Y.; Chen, Z.Y. Tyrosine kinase receptor B protects against coronary artery disease and promotes adult vasculature integrity by regulating Ets1-mediated VE-cadherin expression. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 580–588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manni, L.; Nikolova, V.; Vyagova, D.; Chaldakov, G.N.; Aloe, L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int. J. Cardiol. 2005, 102, 169–171. [Google Scholar] [CrossRef]
- Lorgis, L.; Amoureux, S.; de Maistre, E.; Sicard, P.; Bejot, Y.; Zeller, M.; Vergely, C.; Sequeira-Le Grand, A.; Lagrost, A.C.; Berchoud, J.; et al. Serum brain-derived neurotrophic factor and platelet activation evaluated by soluble P-selectin and soluble CD-40-ligand in patients with acute myocardial infarction. Fundam. Clin. Pharmacol. 2010, 24, 525–530. [Google Scholar] [CrossRef]
- Wu, H.; Cao, G.; Wang, Y.; Tian, H.; Du, R. Increased Serum CA125 and Brain-Derived Neurotrophic Factor (BDNF) Levels on Acute Myocardial Infarction: A Predictor for Acute Heart Failure. Med. Sci. Monit. 2019, 25, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Bahls, M.; Konemann, S.; Markus, M.R.P.; Wenzel, K.; Friedrich, N.; Nauck, M.; Volzke, H.; Steveling, A.; Janowitz, D.; Grabe, H.J.; et al. Brain-derived neurotrophic factor is related with adverse cardiac remodeling and high NTproBNP. Sci. Rep. 2019, 9, 15421. [Google Scholar] [CrossRef]
- Sandrini, L.; Castiglioni, L.; Amadio, P.; Werba, J.P.; Eligini, S.; Fiorelli, S.; Zara, M.; Castiglioni, S.; Bellosta, S.; Lee, F.S.; et al. Impact of BDNF Val66Met Polymorphism on Myocardial Infarction: Exploring the Macrophage Phenotype. Cells 2020, 9, 1084. [Google Scholar] [CrossRef]
- Hang, P.; Zhao, J.; Cai, B.; Tian, S.; Huang, W.; Guo, J.; Sun, C.; Li, Y.; Du, Z. Brain-derived neurotrophic factor regulates TRPC3/6 channels and protects against myocardial infarction in rodents. Int. J. Biol. Sci. 2015, 11, 536–545. [Google Scholar] [CrossRef]
- Cai, D.; Holm, J.M.; Duignan, I.J.; Zheng, J.; Xaymardan, M.; Chin, A.; Ballard, V.L.; Bella, J.N.; Edelberg, J.M. BDNF-mediated enhancement of inflammation and injury in the aging heart. Physiol. Genom. 2006, 24, 191–197. [Google Scholar] [CrossRef]
- Fiorelli, S.; Porro, B.; Cosentino, N.; Di Minno, A.; Manega, C.M.; Fabbiocchi, F.; Niccoli, G.; Fracassi, F.; Barbieri, S.; Marenzi, G.; et al. Activation of Nrf2/HO-1 Pathway and Human Atherosclerotic Plaque Vulnerability: An In Vitro and In Vivo Study. Cells 2019, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, J.; Ma, L.; Liu, F.; Zhang, C.; Zhang, Y.; Ni, M. Overexpression of tissue factor induced atherothrombosis in apolipoprotein E−/− mice via both enhanced plaque thrombogenicity and plaque instability. J. Mol. Cell. Cardiol. 2019, 127, 1–10. [Google Scholar] [CrossRef]
- Camare, C.; Pucelle, M.; Negre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hu, Y.; Chu, Z.; Huang, J.; Zhang, L. The effect of brain-derived neurotrophic factor on angiogenesis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 139–143. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Martinet, W.; Schrijvers, D.M.; De Meyer, G.R. Necrotic cell death in atherosclerosis. Basic Res. Cardiol. 2011, 106, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Antoniou, C.K.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Pro-inflammatory atherogenic role of platelets. Nat. Rev. Cardiol. 2020, 17, 6–7. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.A.; Chatterjee, M.; Rath, D.; Geisler, T. Platelets, inflammation and anti-inflammatory effects of antiplatelet drugs in ACS and CAD. Thromb. Haemost. 2015, 114, 498–518. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | HS (n = 21) | CAD (n = 55) | p-Value |
|---|---|---|---|
| Age, years | 58.86 ± 12.84 | 63.05 ± 11.03 | 0.25 |
| Male sex, n (%) | 19 (90%) | 47 (85.5%) | 0.91 |
| BMI, kg/m2 | 28.23 ± 4.01 | 30.31 ± 4.86 | 0.19 |
| WBC, 103/μL | 5.97 ± 1.26 | 8.94 ± 3.22 | <0.0001 |
| PLT, 103/μL | 216.8 ± 49.06 | 226.1 ± 77.25 | 0.94 |
| Total cholesterol, mg/dL | 223.1 ± 41.03 | 193.4 ± 43.02 | 0.01 |
| HDL cholesterol, mg/dL | 52.19 ± 17.19 | 50.6 ± 13.86 | 0.69 |
| LDL cholesterol, mg/dL | 147.5 ± 36.26 | 113.7 ± 38.27 | 0.001 |
| Triglycerides, mmol/L | 125.9 ± 48.59 | 146.8 ± 66.11 | 0.26 |
| Glycaemia, mg/dL | 108.3 ± 24.5 | 137.1 ± 37.58 | 0.0003 |
| Hb, g/dL | 15.43 ± 0.90 | 13.41 ± 2.52 | <0.0001 |
| Smokers, n (%) | 4 (19.0%) | 31 (56.4%) | 0.008 |
| Diabetes, n (%) | 3 (14.3%) | 21 (38.2%) | 0.08 |
| Hypertension, n (%) | 8 (38.1%) | 31 (56.4%) | 0.24 |
| Drug treatment | |||
| ACE-inhibitors, n (%) | 4 (19%) | 17 (30.9%) | 0.45 |
| Statin, n (%) | 0 (0%) | 19 (34.5%) | <0.0001 |
| β-blockers, n (%) | 2 (9.52%) | 18 (32.7%) | 0.08 |
| Aspirin, n (%) | 0 (0%) | 17 (38.9%) | <0.0001 |
| OCT Parameters | BDNF (pg/mL) | p-Value | |
|---|---|---|---|
| No | Yes | ||
| Thrombus | 75.38 (38.43–130.1) (n = 40) | 41.94 (30.72–70.85) (n = 15) | 0.05 |
| Plaque rupture | 78.83 (42.91–140.1) (n = 29) | 46.04 (32.02–103.2) (n = 26) | 0.15 |
| Lipid plaque | 37.41 (22.74–66.27) (n = 14) | 78.83 (40.43–130) (n = 41) | 0.004 |
| TCFA | 65.78 (32.02–125.8) (n = 38) | 69.98 (40.43–135.6) (n = 17) | 0.32 |
| Micro-vessel | 66.43 (31.21–131.4) (n = 34) | 69.98 (37.84–120.1) (n = 21) | 0.64 |
| Multivessel | 46.47 (33.63–123.3) (n = 21) | 69.66 (40.92–131.4) (n = 34) | 0.39 |
| NSD culprit | 53.16 (27.43–94.41) (n = 22) | 71.92 (39.89–140.9) (n = 33) | 0.03 |
| OCT Parameters | BDNF (pg/mL) | p-Value | |
|---|---|---|---|
| No | Yes | ||
| SA patients | |||
| Thrombus | 85.73 (59.84–135.1) (n = 31) | (n = 0) | . |
| Plaque rupture | 78.83 (59.84–138.8) (n = 23) | 106.8 (51.49–129.5) (n = 8) | 0.58 |
| Lipid plaque | 46.47 (22.52–106.1) (n = 9) | 106.3 (66.48–136) (n = 22) | 0.01 |
| TCFA | 82.28 (49.92–137.9) (n = 26) | 85.73 (59.84–120.5) (n = 5) | 0.27 |
| Micro-vessel | 84.33 (31.21–131.4) (n = 24) | 69.98 (37.84–120.1) (n = 7) | 0.82 |
| Multivessel | 106.8 (43.61–139.8) (n = 6) | 78.83 (60.06–137) (n = 25) | 0.81 |
| NSD culprit | 66.32 (36.22–103.1) (n = 13) | 125.8 (64.97–168) (n = 18) | 0.04 |
| AMI patients | |||
| Thrombus | 38.27 (30.62–124.8) (n = 9) | 41.94 (30.72–70.85) (n = 15) | 0.74 |
| Plaque rupture | 88.65 (31.21–164.1) (n = 6) | 37.63 (30.51–70.2) (n = 18) | 0.29 |
| Lipid plaque | 31.37 (18.97–37.41) (n = 5) | 47.55 (32.23–119.8) (n = 19) | 0.03 |
| TCFA | 34.82 (25.98–46.2) (n = 12) | 58.77 (37.47–121.7) (n = 12) | 0.07 |
| Micro-vessel | 34.61 (29.38–61.03) (n = 10) | 40.43 (35.79–122.3) (n = 14) | 0.69 |
| Multivessel | 38.27 (29.86–119.8) (n = 15) | 41.94 (31.05–70.42) (n = 9) | 0.92 |
| NSD culprit | 36.98 (24.68–84.33) (n = 9) | 47.55 (32.23–97.6) (n = 15) | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amadio, P.; Cosentino, N.; Eligini, S.; Barbieri, S.; Tedesco, C.C.; Sandrini, L.; Zarà, M.; Fabiocchi, F.; Niccoli, G.; Magnani, G.; et al. Potential Relation between Plasma BDNF Levels and Human Coronary Plaque Morphology. Diagnostics 2021, 11, 1010. https://doi.org/10.3390/diagnostics11061010
Amadio P, Cosentino N, Eligini S, Barbieri S, Tedesco CC, Sandrini L, Zarà M, Fabiocchi F, Niccoli G, Magnani G, et al. Potential Relation between Plasma BDNF Levels and Human Coronary Plaque Morphology. Diagnostics. 2021; 11(6):1010. https://doi.org/10.3390/diagnostics11061010
Chicago/Turabian StyleAmadio, Patrizia, Nicola Cosentino, Sonia Eligini, Simone Barbieri, Calogero Claudio Tedesco, Leonardo Sandrini, Marta Zarà, Franco Fabiocchi, Giampaolo Niccoli, Giulia Magnani, and et al. 2021. "Potential Relation between Plasma BDNF Levels and Human Coronary Plaque Morphology" Diagnostics 11, no. 6: 1010. https://doi.org/10.3390/diagnostics11061010
APA StyleAmadio, P., Cosentino, N., Eligini, S., Barbieri, S., Tedesco, C. C., Sandrini, L., Zarà, M., Fabiocchi, F., Niccoli, G., Magnani, G., Fracassi, F., Crea, F., Veglia, F., Marenzi, G., & Barbieri, S. S. (2021). Potential Relation between Plasma BDNF Levels and Human Coronary Plaque Morphology. Diagnostics, 11(6), 1010. https://doi.org/10.3390/diagnostics11061010









