Potential Role and Impact of Peripheral Blood Mononuclear Cells in Radiographic Axial Spondyloarthritis-Associated Endothelial Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Sample Collection and Assessment of Biological Parameters
2.3. Endothelial Function
2.4. Markers Related to Inflammation and Cellular Adhesion
2.5. White-Blood-Cell Isolation
2.6. Total RNA Isolation
2.7. RT2 Profiler Atherosclerosis PCR Array
2.8. RT-qPCR Analysis
2.9. Determination of Plasma Oxidative Stress Biomarkers
2.10. Determination of Oxidative Stress Biomarkers in White Blood Cells
2.11. Protein Extraction and Western Blotting
2.12. In Vitro Studies
2.13. Statistical Analysis
3. Results
3.1. Study Population
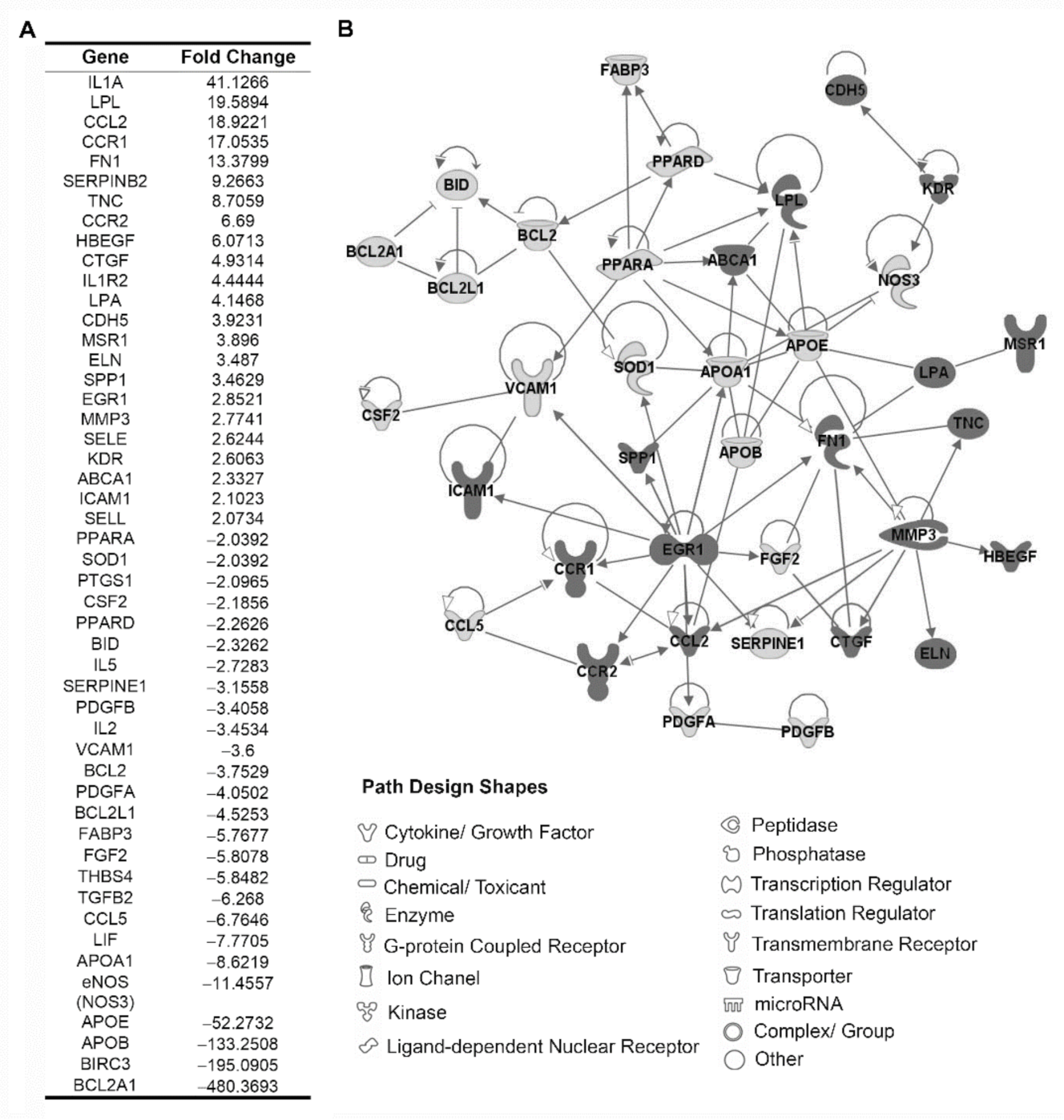
3.2. Identification of Proatherogenic Expression Profile in Circulating Leukocytes from r-axSpA Patients
3.3. Plasma Atherogenic Markers in r-axSpA Patients
3.4. Analysis of Inflammatory Expression Profile in Circulating Leukocytes from r-axSpA Patients
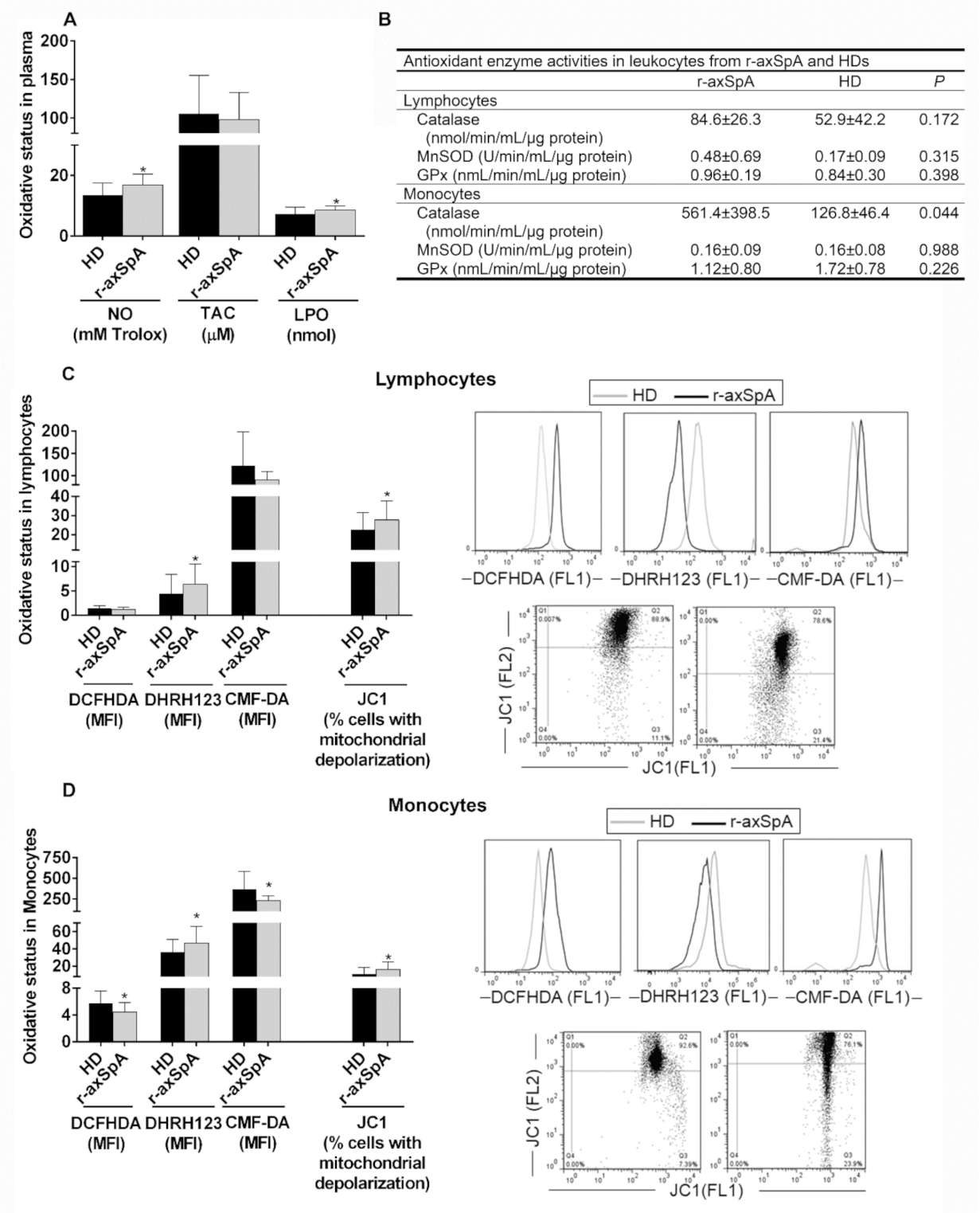
3.5. Oxidative Status in Plasma and Circulating Leukocyte Subsets from r-axSpA Patients
3.6. Correlation Studies between Atherogenic Status and Endothelial Function
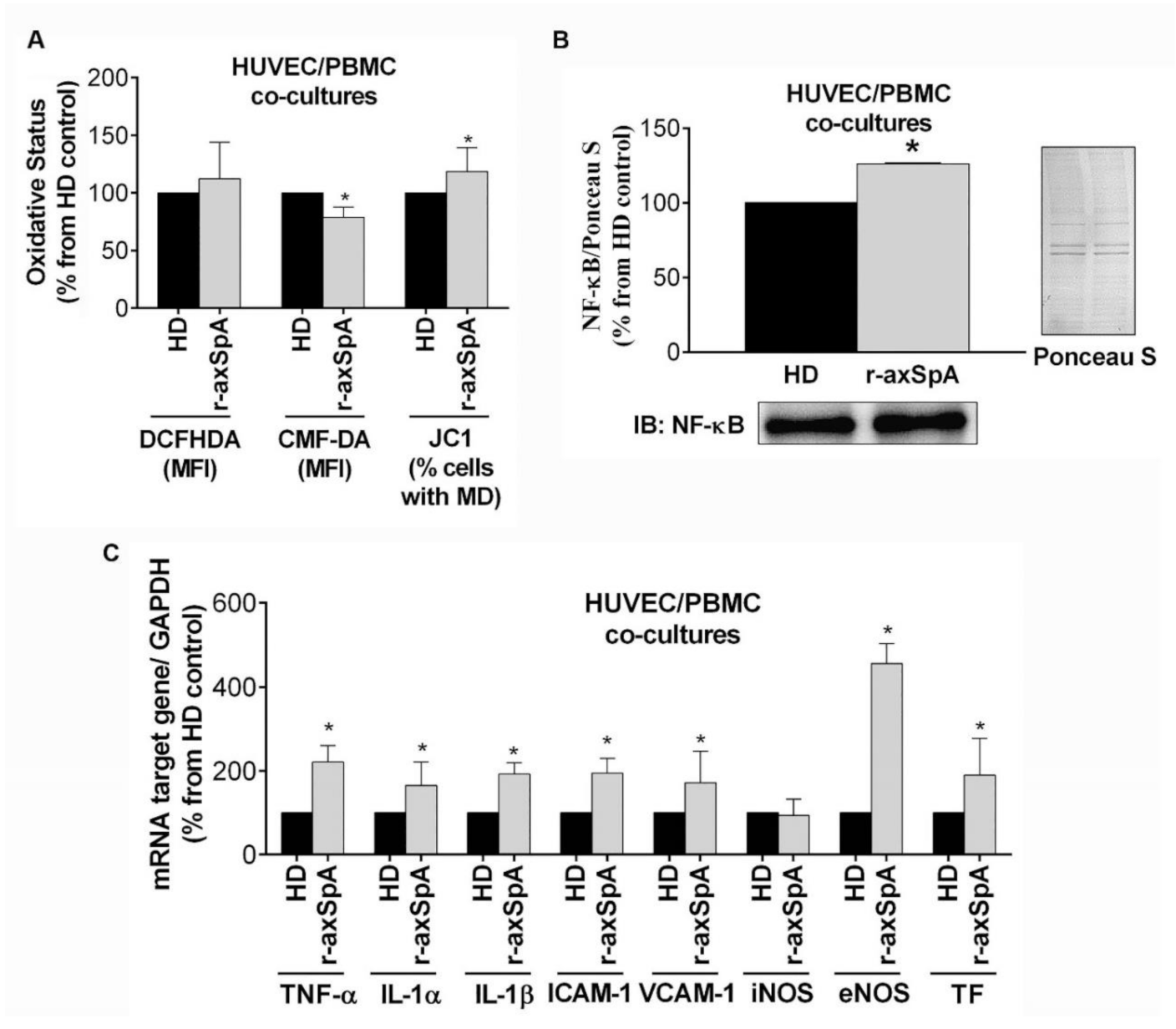
3.7. In Vitro Study of the Effect of PBMCs from r-axSpA Patients on Endothelium Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Lautermann, D.; Braun, J. Ankylosing spondylitis-cardiac manifestations. Clin. Exp. Rheumatol. 2002, 20, S11–S15. [Google Scholar]
- Peters, M.J.; van der Horst-Bruinsma, I.E.; Dijkmans, B.A.; Nurmohamed, M.T. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin. Arthritis Rheum. 2004, 34, 585–592. [Google Scholar] [CrossRef]
- Eriksson, J.K.; Jacobsson, L.; Bengtsson, K.; Askling, J. Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Ann. Rheum. Dis. 2017, 76, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Divecha, H.; Sattar, N.; Rumley, A.; Cherry, L.; Lowe, G.D.; Sturrock, R. Cardiovascular risk parameters in men with ankylosing spondylitis in comparison with non-inflammatory control subjects: Relevance of systemic inflammation. Clin. Sci. 2005, 109, 171–176. [Google Scholar] [CrossRef]
- van Halm, V.P.; van Denderen, J.C.; Peters, M.J.; Twisk, J.W.; van der Paardt, M.; van der Horst-Bruinsma, I.E.; van de Stadt, R.J.; de Koning, M.H.; Dijkmans, B.A.; Nurmohamed, M.T. Increased disease activity is associated with a deteriorated lipid profile in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2006, 65, 1473–1477. [Google Scholar] [CrossRef]
- Han, C.; Robinson, D.W., Jr.; Hackett, M.V.; Paramore, L.C.; Fraeman, K.H.; Bala, M.V. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J. Rheumatol. 2006, 33, 2167–2172. [Google Scholar] [CrossRef]
- Libby, P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am. J. Med. 2008, 121, S21–S31. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Gonzalez-Juanatey, C.; Martin, J. Rheumatoid arthritis: A disease associated with accelerated atherogenesis. Semin. Arthritis Rheum. 2005, 35, 8–17. [Google Scholar] [CrossRef]
- van Leuven, S.I.; Franssen, R.; Kastelein, J.J.; Levi, M.; Stroes, E.S.; Tak, P.P. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology 2008, 47, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Crouse, J.R.; Hsu, F.C.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef]
- Steyers, C.M., 3rd; Miller, F.J., Jr. Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 2014, 15, 11324–11349. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Okan, T.; Akar, S.; Cece, H.; Altay, C.; Secil, M.; Birlik, M.; Onen, F.; Akkoc, N. Impaired endothelial function in patients with ankylosing spondylitis. Rheumatology 2006, 45, 283–286. [Google Scholar] [CrossRef]
- van Eijk, I.C.; Peters, M.J.; Serne, E.H.; van der Horst-Bruinsma, I.E.; Dijkmans, B.A.; Smulders, Y.M.; Nurmohamed, M.T. Microvascular function is impaired in ankylosing spondylitis and improves after tumour necrosis factor alpha blockade. Ann. Rheum. Dis. 2009, 68, 362–366. [Google Scholar] [CrossRef]
- Batko, B.; Maga, P.; Urbanski, K.; Ryszawa-Mrozek, N.; Schramm-Luc, A.; Koziej, M.; Mikolajczyk, T.; McGinnigle, E.; Czesnikiewicz-Guzik, M.; Ceranowicz, P.; et al. Microvascular dysfunction in ankylosing spondylitis is associated with disease activity and is improved by anti-TNF treatment. Sci. Rep. 2018, 8, 13205. [Google Scholar] [CrossRef]
- Reinherz, E.L.; Schlossman, S.F. The differentiation and function of human T lymphocytes. Cell 1980, 19, 821–827. [Google Scholar] [CrossRef]
- Atagunduz, P.; Appel, H.; Kuon, W.; Wu, P.; Thiel, A.; Kloetzel, P.M.; Sieper, J. HLA-B27-restricted CD8+ T cell response to cartilage-derived self peptides in ankylosing spondylitis. Arthritis Rheum. 2005, 52, 892–901. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.G.; Li, Y.H.; Qi, L.; Liu, X.G.; Yuan, C.Z.; Hu, N.W.; Ma, D.X.; Li, Z.F.; Yang, Q.; et al. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS ONE 2012, 7, e31000. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liao, Q.; Hu, Y.; Zhong, D. T lymphocyte subset imbalances in patients contribute to ankylosing spondylitis. Exp. Ther. Med. 2015, 9, 250–256. [Google Scholar] [CrossRef]
- Rezaiemanesh, A.; Abdolmaleki, M.; Abdolmohammadi, K.; Aghaei, H.; Pakdel, F.D.; Fatahi, Y.; Soleimanifar, N.; Zavvar, M.; Nicknam, M.H. Immune cells involved in the pathogenesis of ankylosing spondylitis. Biomed. Pharmacother. 2018, 100, 198–204. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, M.; Yang, R.; Liang, X.; Ma, Y.; Tang, Y.; Huang, L.; Ye, J.; Chen, K.; Wang, P.; et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+ Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res. Ther. 2011, 13, R29. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Ann. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Maggi, E.; Giudizi, M.G.; Biagiotti, R.; Annunziato, F.; Manetti, R.; Piccinni, M.P.; Parronchi, P.; Sampognaro, S.; Giannarini, L.; Zuccati, G.; et al. Th2-like CD8+ T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J. Exp. Med. 1994, 180, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 968549. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Gu, J.R.; Li, T.W.; Zhang, F.C.; Lin, Z.M.; Liao, Z.T.; Wei, Q.J.; Cao, S.Y.; Li, L. Value of the peripheral blood B-cells subsets in patients with ankylosing spondylitis. Chin. Med. J. 2009, 122, 1784–1789. [Google Scholar] [PubMed]
- Wright, C.; Edelmann, M.; diGleria, K.; Kollnberger, S.; Kramer, H.; McGowan, S.; McHugh, K.; Taylor, S.; Kessler, B.; Bowness, P. Ankylosing spondylitis monocytes show upregulation of proteins involved in inflammation and the ubiquitin proteasome pathway. Ann. Rheum. Dis. 2009, 68, 1626–1632. [Google Scholar] [CrossRef]
- Hermida, N.; Balligand, J.L. Low-density lipoprotein-cholesterol-induced endothelial dysfunction and oxidative stress: The role of statins. Antioxid. Redox Signal. 2014, 20, 1216–1237. [Google Scholar] [CrossRef]
- Karakoc, M.; Altindag, O.; Keles, H.; Soran, N.; Selek, S. Serum oxidative-antioxidative status in patients with ankylosing spondilitis. Rheumatol. Int. 2007, 27, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Ozgocmen, S.; Sogut, S.; Ardicoglu, O.; Fadillioglu, E.; Pekkutucu, I.; Akyol, O. Serum nitric oxide, catalase, superoxide dismutase, and malondialdehyde status in patients with ankylosing spondylitis. Rheumatol. Int. 2004, 24, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Tunez, I.; Feijoo, M.; Huerta, G.; Montilla, P.; Munoz, E.; Ruiz, A.; Collantes, E. The effect of infliximab on oxidative stress in chronic inflammatory joint disease. Curr. Med. Res. Opin. 2007, 23, 1259–1267. [Google Scholar] [CrossRef]
- Feijoo, M.; Tunez, I.; Tasset, I.; Montilla, P.; Perez-Guijo, V.; Munoz-Gomariz, E.; Ruiz, A.; Schiotis, R.; Collantes, E. Infliximab reduces myeloperoxidase concentration in chronic inflammatory joint diseases. Pharmacology 2009, 83, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Jenkinson, T.R.; Mallorie, P.A.; Whitelock, H.C.; Kennedy, L.G.; Garrett, S.L.; Calin, A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J. Rheumatol. 1994, 21, 1694–1698. [Google Scholar]
- Calin, A.; Garrett, S.; Whitelock, H.; Kennedy, L.G.; O’Hea, J.; Mallorie, P.; Jenkinson, T. A new approach to defining functional ability in ankylosing spondylitis: The development of the Bath Ankylosing Spondylitis Functional Index. J. Rheumatol. 1994, 21, 2281–2285. [Google Scholar]
- Creemers, M.C.; Franssen, M.J.; van’t Hof, M.A.; Gribnau, F.W.; van de Putte, L.B.; van Riel, P.L. Assessment of outcome in ankylosing spondylitis: An extended radiographic scoring system. Ann. Rheum. Dis. 2005, 64, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Millan, J.; Pinto, X.; Munoz, A.; Zuniga, M.; Rubies-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernandez-Mijares, A.; Gonzalez-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- Perez-Sanchez, C.; Font-Ugalde, P.; Ruiz-Limon, P.; Lopez-Pedrera, C.; Castro-Villegas, M.C.; Abalos-Aguilera, M.C.; Barbarroja, N.; Arias-de la Rosa, I.; Lopez-Montilla, M.D.; Escudero-Contreras, A.; et al. Circulating microRNAs as potential biomarkers of disease activity and structural damage in ankylosing spondylitis patients. Hum. Mol. Genet. 2018, 27, 875–890. [Google Scholar] [CrossRef]
- Andrews, N.C.; Faller, D.V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991, 19, 2499. [Google Scholar] [CrossRef]
- Romero-Calvo, I.; Ocon, B.; Martinez-Moya, P.; Suarez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef]
- Luczak, A.; Madej, M.; Kasprzyk, A.; Doroszko, A. Role of the eNOS Uncoupling and the Nitric Oxide Metabolic Pathway in the Pathogenesis of Autoimmune Rheumatic Diseases. Oxidative Med. Cell Longev. 2020, 2020, 1417981. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juanatey, C.; Vazquez-Rodriguez, T.R.; Miranda-Filloy, J.A.; Dierssen, T.; Vaqueiro, I.; Blanco, R.; Martin, J.; Llorca, J.; Gonzalez-Gay, M.A. The high prevalence of subclinical atherosclerosis in patients with ankylosing spondylitis without clinically evident cardiovascular disease. Medicine 2009, 88, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.; Krishan, P.; Syngle, A. Predictors of Atherosclerosis in Ankylosing Spondylitis. Rheumatol. Ther. 2015, 2, 173–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Autieri, M.V. Pro- and anti-inflammatory cytokine networks in atherosclerosis. IRSN Vasc. Med. 2012, 2012, 987629. [Google Scholar] [CrossRef]
- Tay, C.; Liu, Y.H.; Hosseini, H.; Kanellakis, P.; Cao, A.; Peter, K.; Tipping, P.; Bobik, A.; Toh, B.H.; Kyaw, T. B-cell-specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc. Res. 2016, 111, 385–397. [Google Scholar] [CrossRef]
- Bal, A.; Unlu, E.; Bahar, G.; Aydog, E.; Eksioglu, E.; Yorgancioglu, R. Comparison of serum IL-1 beta, sIL-2R, IL-6, and TNF-alpha levels with disease activity parameters in ankylosing spondylitis. Clin. Rheumatol. 2007, 26, 211–215. [Google Scholar] [CrossRef]
- Mei, Y.; Pan, F.; Gao, J.; Ge, R.; Duan, Z.; Zeng, Z.; Liao, F.; Xia, G.; Wang, S.; Xu, S.; et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin. Rheumatol. 2011, 30, 269–273. [Google Scholar] [CrossRef]
- Perpetuo, I.P.; Caetano-Lopes, J.; Vieira-Sousa, E.; Campanilho-Marques, R.; Ponte, C.; Canhao, H.; Ainola, M.; Fonseca, J.E. Ankylosing Spondylitis Patients Have Impaired Osteoclast Gene Expression in Circulating Osteoclast Precursors. Front. Med. 2017, 4, 5. [Google Scholar] [CrossRef]
- Kamari, Y.; Werman-Venkert, R.; Shaish, A.; Werman, A.; Harari, A.; Gonen, A.; Voronov, E.; Grosskopf, I.; Sharabi, Y.; Grossman, E.; et al. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis 2007, 195, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh-Fard, M.; Abolhalaj, M.; Amiri, P.; Zaki, M.; Taheri, Z.; Qorbani, M.; Bazzaz, J.T.; Amoli, M.M. IL-23 gene expression in PBMCs of patients with coronary artery disease. Dis. Markers 2012, 33, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, S.; Mooteri, S.; Peckham, N.; Pai, R.G. Atherogenic effect of interleukin-2 and antiatherogenic effect of interleukin-2 antibody in apo-E-deficient mice. Angiology 2004, 55, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef]
- Surdacki, A.; Sulicka, J.; Korkosz, M.; Mikolajczyk, T.; Telesinska-Jasiowka, D.; Klimek, E.; Kierzkowska, I.; Guzik, T.; Grodzicki, T.K. Blood monocyte heterogeneity and markers of endothelial activation in ankylosing spondylitis. J. Rheumatol. 2014, 41, 481–489. [Google Scholar] [CrossRef]
- Sari, I.; Alacacioglu, A.; Kebapcilar, L.; Taylan, A.; Bilgir, O.; Yildiz, Y.; Yuksel, A.; Kozaci, D.L. Assessment of soluble cell adhesion molecules and soluble CD40 ligand levels in ankylosing spondylitis. Jt. Bone Spine 2010, 77, 85–87. [Google Scholar] [CrossRef]
- Blann, A.D.; Sanders, P.A.; Herrick, A.; Jayson, M.I. Soluble L-selectin in the connective tissue diseases. Br. J. Haematol. 1996, 95, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Rittling, S.R.; Okamoto, H.; Inobe, M.; Jia, N.; Shimizu, T.; Akino, M.; Sugawara, T.; Morimoto, J.; Kimura, C.; et al. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1029–1034. [Google Scholar] [CrossRef]
- Choi, S.T.; Kim, J.H.; Kang, E.J.; Lee, S.W.; Park, M.C.; Park, Y.B.; Lee, S.K. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology 2008, 47, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Giachelli, C.M.; Rittling, S.R. Role of osteopontin in cellular signaling and toxicant injury. Ann. Rev. Pharmacol. Toxicol. 2001, 41, 723–749. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Corrales, J.J.; Almeida, M.; Burgo, R.M.; Hernandez, P.; Miralles, J.M.; Orfao, A. Decreased production of inflammatory cytokines by circulating monocytes and dendritic cells in type 2 diabetic men with atherosclerotic complications. J. Diabetes Complicat. 2007, 21, 41–49. [Google Scholar] [CrossRef]
- Adhikari, N.; Charles, N.; Lehmann, U.; Hall, J.L. Transcription factor and kinase-mediated signaling in atherosclerosis and vascular injury. Curr. Atheroscler. Rep. 2006, 8, 252–260. [Google Scholar] [CrossRef]
- Yang, J.; Chatterjee-Kishore, M.; Staugaitis, S.M.; Nguyen, H.; Schlessinger, K.; Levy, D.E.; Stark, G.R. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005, 65, 939–947. [Google Scholar]
- Yang, J.; Liao, X.; Agarwal, M.K.; Barnes, L.; Auron, P.E.; Stark, G.R. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007, 21, 1396–1408. [Google Scholar] [CrossRef]

| r-axSpA Patients (n = 30) | Healthy Donors (n = 32) | p | |
|---|---|---|---|
| Clinical parameters | |||
| Women/men, n/n | 5/25 | 10/22 | 0.180 |
| Age, years | 46.40 ± 13.41 | 41.06 ± 10.04 | 0.080 |
| BASDAI | 4.42 ± 2.49 | … | |
| BASFI | 4.73 ± 3.32 | … | |
| BASMI | 3.22 ± 1.68 | … | |
| mSASSS | 19.16 ± 21.14 | … | |
| Disease duration, years | 13.23 ± 11.33 | … | |
| Obesity (%) | 6/30 (20.00%) | 1/30 (3.33%) | 0.103 |
| Type 2 diabetes (%) | 4/29 (13.79%) | 0 | 0.112 |
| Dyslipidemia | 8/30 (26.67%) | 3/31 (9.68%) | 0.084 |
| Hypertension (%) | 8/30 (26.67%) | 2/27 (7.41%) | 0.083 |
| Smoker (%) | 11/28 (39.28%) | 5/22 (22.73%) | 0.213 |
| Atherogenic risk (%) | 8/30 (26.67%) | 6/32 (18.75%) | 0.456 |
| Endothelial function | |||
| Rest flow (PU) | 11.28 ± 2.81 | 15.74 ± 5.88 | 0.017 |
| Peak flow (PU) | 51.36 ± 21.85 | 80.54 ± 29.11 | 0.006 |
| Area of hyperemia (PU/s) | 1730.93 ± 1398.64 | 2962.88 ± 1458.60 | 0.032 |
| Peak flow − rest flow (PU) | 39.27 ± 21.13 | 64.80 ± 25.25 | 0.008 |
| Biological zero-peak flow (PU) | 963.96 ± 584.07 | 1433.68 ± 565.25 | 0.040 |
| Laboratory parameters | |||
| HLA-B27 (%) | 26/30 (86.67%) | … | |
| CRP a, nmol/L | 122.57 ± 171.90 | 13.62 ± 18.38 | <0.001 |
| ESR a, mm/h | 19.84 ± 22.42 | 7.48 ± 5.66 | 0.006 |
| TNF-α, pg/mL | 10.89 ± 10.00 | 4.02 ± 6.78 | 0.021 |
| IL-1β, pg/mL | 2.46 ± 1.20 | 1.57 ± 1.55 | 0.047 |
| Cholesterol, nmol/L | 4.90 ± 1.10 | 4.97 ± 0.71 | 0.800 |
| HDL, nmol/L | 1.09 ± 0.24 | 1.27 ± 0.30 | 0.014 |
| LDL, nmol/L | 3.26 ± 0.91 | 3.16 ± 0.66 | 0.619 |
| Triglycerides, nmol/L | 1.17 ± 0.60 | 1.14 ± 0.61 | 0.829 |
| Apolipoprotein A, g/L | 1.32 ± 0.25 | 1.42 ± 0.24 | 0.129 |
| Apolipoprotein B, g/L | 0.87 ± 0.21 | 0.85 ± 0.20 | 0.842 |
| Treatments | |||
| NSAIDs (%) | 28/30 (93.33%) | … | |
| Sulfasalazine (%) | 2/30 (6.67%) | … |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Limon, P.; Ladehesa-Pineda, M.L.; Lopez-Medina, C.; Lopez-Pedrera, C.; Abalos-Aguilera, M.C.; Barbarroja, N.; Arias-Quiros, I.; Perez-Sanchez, C.; Arias-de la Rosa, I.; Ortega-Castro, R.; et al. Potential Role and Impact of Peripheral Blood Mononuclear Cells in Radiographic Axial Spondyloarthritis-Associated Endothelial Dysfunction. Diagnostics 2021, 11, 1037. https://doi.org/10.3390/diagnostics11061037
Ruiz-Limon P, Ladehesa-Pineda ML, Lopez-Medina C, Lopez-Pedrera C, Abalos-Aguilera MC, Barbarroja N, Arias-Quiros I, Perez-Sanchez C, Arias-de la Rosa I, Ortega-Castro R, et al. Potential Role and Impact of Peripheral Blood Mononuclear Cells in Radiographic Axial Spondyloarthritis-Associated Endothelial Dysfunction. Diagnostics. 2021; 11(6):1037. https://doi.org/10.3390/diagnostics11061037
Chicago/Turabian StyleRuiz-Limon, Patricia, Maria L. Ladehesa-Pineda, Clementina Lopez-Medina, Chary Lopez-Pedrera, Maria C. Abalos-Aguilera, Nuria Barbarroja, Isabel Arias-Quiros, Carlos Perez-Sanchez, Ivan Arias-de la Rosa, Rafaela Ortega-Castro, and et al. 2021. "Potential Role and Impact of Peripheral Blood Mononuclear Cells in Radiographic Axial Spondyloarthritis-Associated Endothelial Dysfunction" Diagnostics 11, no. 6: 1037. https://doi.org/10.3390/diagnostics11061037
APA StyleRuiz-Limon, P., Ladehesa-Pineda, M. L., Lopez-Medina, C., Lopez-Pedrera, C., Abalos-Aguilera, M. C., Barbarroja, N., Arias-Quiros, I., Perez-Sanchez, C., Arias-de la Rosa, I., Ortega-Castro, R., Escudero-Contreras, A., Collantes-Estevez, E., & Jimenez-Gomez, Y. (2021). Potential Role and Impact of Peripheral Blood Mononuclear Cells in Radiographic Axial Spondyloarthritis-Associated Endothelial Dysfunction. Diagnostics, 11(6), 1037. https://doi.org/10.3390/diagnostics11061037







