Diagnostic Value of Whole-Body MRI Short Protocols in Bone Lesion Detection in Multiple Myeloma Patients
Abstract
:1. Introduction
2. Materials and Methods
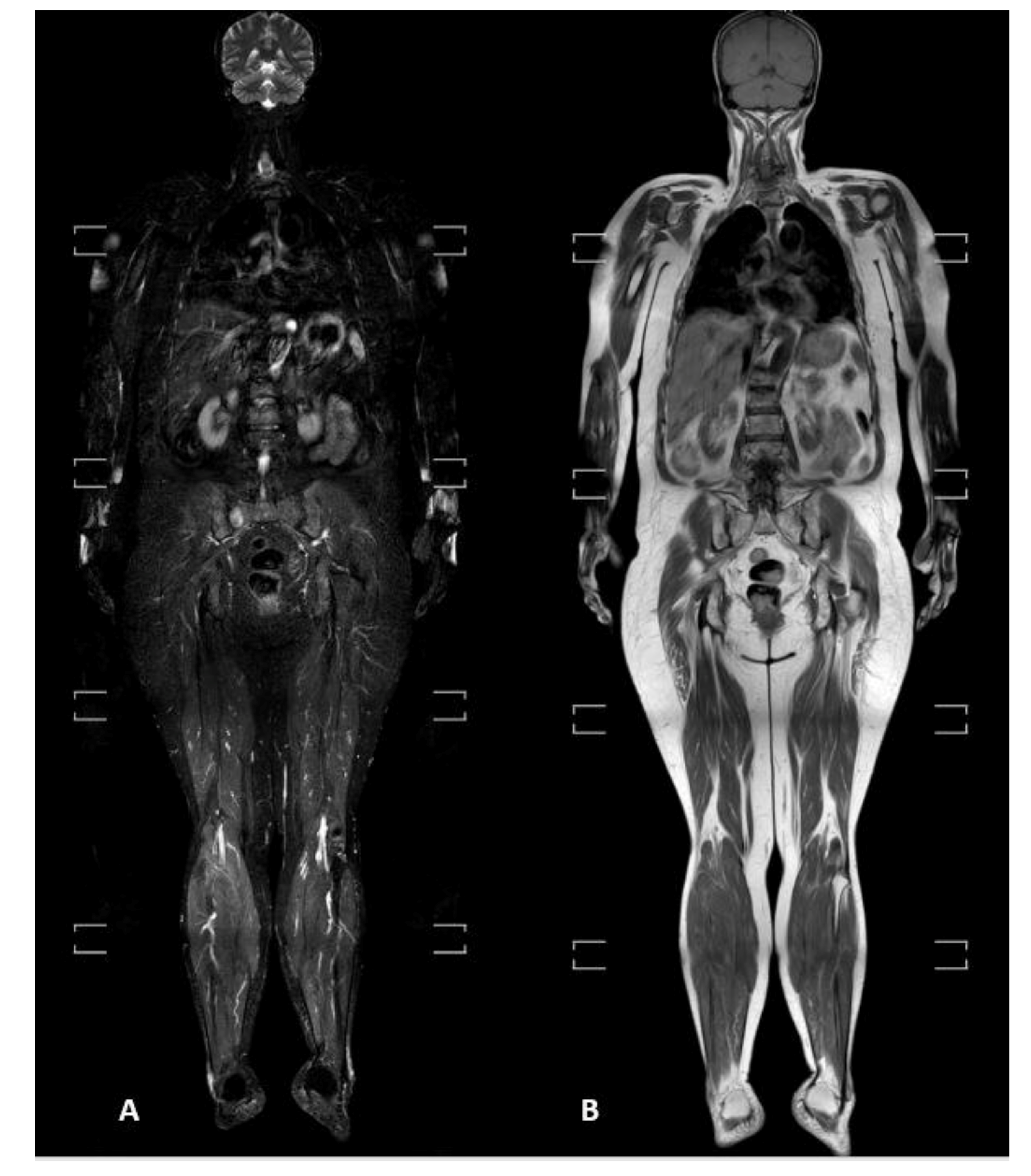
2.1. WBMRI Protocol
2.2. Image Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Agreement between Readers
3.3. Radiological Pattern and Lesions’ Distribution
3.4. Standard vs. Short Protocols
3.5. Acquisition Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.V.; Lonial, S.; Joao, C.; Anderson, K.C.; García-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently pre-cedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, H.; Miguel, J.S.; Dimopoulos, M.A.; Palumbo, A.; Garcia Sanz, R.; Powles, R.; Lentzsch, S.; Ming Chen, W.; Hou, J.; Jurczyszyn, A.; et al. International Myeloma Working Group reccomendation for global myeloma care. Leukemia 2014, 28, 981–992. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 Patients With Newly Diagnosed Multiple Myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- Hillengass, J.; Landgren, O. Challenges and opportunities of novel imaging techniques in monoclonal plasma cell disorders: Imaging “early myeloma”. Leuk. Lymphoma 2013, 54, 1355–1363. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Hillengass, J.; Usmani, S.; Zamagni, E.; Lentzsch, S.; Davies, F.E.; Raje, N.; Sezer, O.; Zweegman, S.; Shah, J.; et al. Role of magnetic resonance imaging in the management of patients with mul-tiple myeloma: A consensus statement. J. Clin. Oncol. 2015, 33, 657–664. [Google Scholar] [CrossRef]
- Messiou, C.; Hillengass, J.; Delorme, S.; Lecouvet, F.E.; Moulopoulos, L.A.; Collins, D.J.; Blackledge, M.D.; Abildgaard, N.; Østergaard, B.; Schlemmer, H.P.; et al. Guidelines for acquisition, interpretation and reporting of whole-body MRI in mye-loma: Myeloma response assessment and diagnosis system (MY-RADS). Radiology 2019, 291, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Kröpil, P.; Fenk, R.; Fritz, L.B.; Blondin, D.; Kobbe, G.; Mödder, U.; Cohnen, M. Comparison of whole-body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma. Eur. Radiol. 2007, 18, 51–58. [Google Scholar] [CrossRef]
- Wolf, M.B.; Murray, F.; Kilk, K.; Hillengass, J.; Delorme, S.; Heiss, C.; Neben, K.; Goldschmidt, H.; Kauczor, H.U.; Weber, M.A. Sensitivity of whole body CT and MRI versus projection radiography in the detection of os-teolysis in patients with monoclonal plasma cell disease. Eur. J. Radiol. 2014, 83, 1222–1230. [Google Scholar] [CrossRef]
- Ormond Filho, A.G.; Carneiro, B.C.; Pastore, D.; Silva, I.P.; Yamashita, S.R.; Consolo, F.D.; Hungria, V.T.M.; Sandes, A.F.; Rizzatti, E.G.; Nico, M.A.C. Whole body imaging of Multiple myeloma: Diagnostic criteria. Radiographics 2019, 39, 1077–1097. [Google Scholar] [CrossRef]
- Dutoit, J.C.; Verstraete, K.L. MRI in multiple myeloma: A pictorial rieview of diagnostic and post-treatment findings. Insights Imaging 2016, 7, 553–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, S.L.; Messiou, C.; Collins, D.J.; Morgan, V.A.; Simpkin, C.J.; West, S.; Davies, F.E.; Morgan, G.; DeSouza, N.M. Whole-Body Diffusion-weighted MR Imaging for Assessment of Treatment Response in Myeloma. Radiology 2014, 271, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Bray, T.J.P.; Singh, S.; Latifoltojar, A.; Rajesparan, K.; Rahman, F.; Narayanan, P.; Naaseri, S.; Lopes, A.; Bainbridge, A.; Punwani, S.; et al. Diagnostic utility of whole body Dixon MRI in multiple myeloma: A multi-reader study. PLoS ONE 2017, 12, e0180562. [Google Scholar] [CrossRef] [Green Version]
- Koutoulidis, V.; Papanikolaou, N.; Moulopoulos, L. Functional and molecular MRI of the bone marrow in multiple myeloma. Br. J. Radiol. 2018, 91, 20170389. [Google Scholar] [CrossRef]
- Myeloma: Diagnosis and Management. NICE (NG35) and Appendices. Available online: https://www.nice.org.uk/guidance/ng35 (accessed on 31 March 2017).
- Petralia, G.; Padhani, A.R.; Pricolo, P.; Zugni, F.; Martinetti, M.; Summers, P.E.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Bellomi, M.; et al. Whole-body magnetic resonance imaging (WB-MRI) in oncology: Recommendations and key uses. La Radiol. Med. 2019, 124, 218–233. [Google Scholar] [CrossRef]
- Stecco, A.; Buemi, F.; Iannessi, A.; Carriero, A.; Gallamini, A. Current concepts in tumor imaging with whole-body MRI with diffusion imaging (WB-MRI-DWI) in multiple myeloma and lymphoma. Leuk. Lymphoma 2018, 59, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Wennmann, M.; Hielscher, T.; Kintzelé, L.; Menze, B.H.; Langs, G.; Merz, M.; Sauer, S.; Kauczor, H.-U.; Schlemmer, H.-P.; Delorme, S.; et al. Spatial Distribution of Focal Lesions in Whole-Body MRI and Influence of MRI Protocol on Staging in Patients with Smoldering Multiple Myeloma According to the New SLiM-CRAB-Criteria. Cancers 2020, 12, 2537. [Google Scholar] [CrossRef]
- Terpos, E.; Morgan, G.; Dimopoulos, M.; Drake, M.T.; Lentzsch, S.; Raje, N.; Sezer, O.; García-Sanz, R.; Shimizu, K.; Turesson, I.; et al. International Myeloma Working Group Recommendations for the Treatment of Multiple Myeloma–Related Bone Disease. J. Clin. Oncol. 2013, 31, 2347–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pianko, M.J.; Terpos, E.; Roodman, G.D.; Divgi, C.R.; Zweegman, S.; Hillengass, J.; Lentzsch, S. Whole-Body Low-Dose Computed Tomography and Advanced Imaging Tech-niques for Multiple Myeloma Bone Disease. Clin. Cancer Res. 2014, 20, 5888–5897. [Google Scholar] [CrossRef] [Green Version]
- Amos, B.; Agarwal, A.; Kanekar, S. Imaging of Multiple Myeloma. Hematol. Clin. N. Am. 2016, 30, 843–865. [Google Scholar] [CrossRef]
- Chrzan, R.; Jurczyszyn, A.; Urbanik, A. Whole-Body Low-Dose Computed Tomography (WBLDCT) in Assessment of Patients with Multiple Myeloma—Pilot Study and Standard Imaging Protocol Suggestion. Pol. J. Radiol. 2017, 82, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filonzi, G.; Mancuso, K.; Zamagni, E.; Nanni, C.; Spinnato, P.; Cavo, M.; Fanti, S.; Salizzoni, E.; Bazzocchi, A. A comparison of different Staging System for Multiple Myeloma: Can the MRI pattern play a prognostic role? AJR 2017, 209, 152–158. [Google Scholar] [CrossRef] [PubMed]



| Sequence | District | TE (ms) | TR (ms) | NSA | DFOV (mm) | Voxel Size (mm) | Thickness (mm) | Time (s) |
|---|---|---|---|---|---|---|---|---|
| T1 TSE Coronal | Head | 15 | 922 | 1 | 365 | 1.16 × 1.46 | 6 | 50 |
| Thorax | 15 | 922 | 1 | 365 | 1.16 × 1.46 | 6 | 50 | |
| Abdomen | 15 | 922 | 1 | 365 | 1.16 × 1.46 | 6 | 50 | |
| Lower limb | 15 | 922 | 1 | 365 | 1.16 × 1.46 | 6 | 39 | |
| Feet | 15 | 922 | 1 | 365 | 1.16 × 1.46 | 6 | 39 | |
| T2 STIR TSE Coronal | Head | 60 | 8704 | 1 | 365 | 1.25 × 1.82 | 6 | 87 |
| Abdomen | 60 | 8704 | 1 | 365 | 1.25 × 1.82 | 6 | 71 | |
| Upper limb | 60 | 8704 | 1 | 365 | 1.25 × 1.82 | 6 | 87 | |
| Lower limb | 60 | 8704 | 1 | 365 | 1.25 × 1.82 | 6 | 90 | |
| Feet | 60 | 8704 | 1 | 365 | 1.25 × 1.82 | 6 | 90 | |
| T1 TSE Sagittal | Cervical | 7.4 | 408 | 3 | 270 | 0.90 × 1.15 | 3.5 | 300 |
| Thorax | 7.4 | 408 | 2 | 270 | 0.90 × 1.15 | 3.5 | 188 | |
| T2 STIR TSE Sagittal | Cervical | 60 | 2533 | 2 | 270 | 0.90 × 1.25 | 3.5 | 225 |
| Thorax | 60 | 2533 | 2 | 270 | 0.90 × 1.25 | 3.5 | 167 | |
| DWIBS Axial | Head | 66 | 6421 | 2 | 520 | 5.00 × 4.98 | 6 | 135 |
| Abdomen | 66 | 6421 | 2 | 520 | 5.00 × 4.98 | 6 | 135 | |
| Upper limb | 66 | 6421 | 2 | 520 | 5.00 × 4.98 | 6 | 135 | |
| Lower limb | 66 | 6421 | 2 | 520 | 5.00 × 4.98 | 6 | 135 | |
| Feet | 66 | 6421 | 2 | 520 | 5.00 × 4.98 | 6 | 135 | |
| Cervical | 66 | 6421 | 2 | 520 | 5.00 × 4.98 | 6 | 135 | |
| Thorax | 66 | 6421 | 2 | 520 | 5.00 × 4.98 | 6 | 135 |
| N = 64 | Agreement (κ; 95% CIs) | p-Value | τ-Value (95% CIs) | p-Value |
|---|---|---|---|---|
| Pattern * | 0.954 (0.885–1) | <0.0001 | 0.958 (0.894–1) | <0.0001 |
| Skull ^ | 0.750 (0.329–1) | <0.0001 | 0.745 (0.368–1) | 0.014 |
| Sternum and ribs ^ | 0.717 (0.541–0.861) | <0.0001 | 0.820 (0.688–0.935) | <0.0001 |
| Spine ^ | 0.754 (0.621–0.878) | <0.0001 | 0.881 (0.810–0.942) | |
| Upper limbs ^ | 0.860 (0.662–1) | <0.0001 | 0.856 (0.670–1) | <0.0001 |
| Pelvis ^ | 0.727 (0.577–0.862) | <0.0001 | 0.855 (0.757–0.935) | <0.0001 |
| Lower limbs ^ | 1 (1–1) | <0.0001 | 1 (1–1) | 0.004 |
| N = 64 | Negative Findings | Positive Findings | ||
|---|---|---|---|---|
| <5 Lesions | 5–20 Lesions | >20 Lesions | ||
| Skull (n, %) | 58/64 (90.6) | 4 (6.3) | 2 (3.1) | 0 (0) |
| Sternum and ribs (n, %) | 41/64 (64.0) | 12 (18.8) | 9 (14.1) | 2 (3.1) |
| Spine (n, %) | 24/64 (37.5) | 20 (31.2) | 15 (23.5) | 5 (7.8) |
| Upper limbs (n, %) | 52/64 (81.2) | 7 (10.9) | 4 (6.3) | 1 (1.6) |
| Pelvis (n, %) | 31/64 (48.4) | 20 (31.3) | 11 (17.2) | 2 (3.1) |
| Lower limbs (n, %) | 58/64 (90.6) | 6 (9.4) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ippolito, D.; Giandola, T.; Maino, C.; Gandola, D.; Ragusi, M.; Brambilla, P.; Bonaffini, P.A.; Sironi, S. Diagnostic Value of Whole-Body MRI Short Protocols in Bone Lesion Detection in Multiple Myeloma Patients. Diagnostics 2021, 11, 1053. https://doi.org/10.3390/diagnostics11061053
Ippolito D, Giandola T, Maino C, Gandola D, Ragusi M, Brambilla P, Bonaffini PA, Sironi S. Diagnostic Value of Whole-Body MRI Short Protocols in Bone Lesion Detection in Multiple Myeloma Patients. Diagnostics. 2021; 11(6):1053. https://doi.org/10.3390/diagnostics11061053
Chicago/Turabian StyleIppolito, Davide, Teresa Giandola, Cesare Maino, Davide Gandola, Maria Ragusi, Paolo Brambilla, Pietro Andrea Bonaffini, and Sandro Sironi. 2021. "Diagnostic Value of Whole-Body MRI Short Protocols in Bone Lesion Detection in Multiple Myeloma Patients" Diagnostics 11, no. 6: 1053. https://doi.org/10.3390/diagnostics11061053
APA StyleIppolito, D., Giandola, T., Maino, C., Gandola, D., Ragusi, M., Brambilla, P., Bonaffini, P. A., & Sironi, S. (2021). Diagnostic Value of Whole-Body MRI Short Protocols in Bone Lesion Detection in Multiple Myeloma Patients. Diagnostics, 11(6), 1053. https://doi.org/10.3390/diagnostics11061053







