Predicting Long-Term Mortality in Patients with Angina across the Spectrum of Dysglycemia: A Machine Learning Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Model Development and Evaluation
2.3. Comparison with GRACE Discharge Score
3. Results
3.1. Characteristics of Enrolled Patients
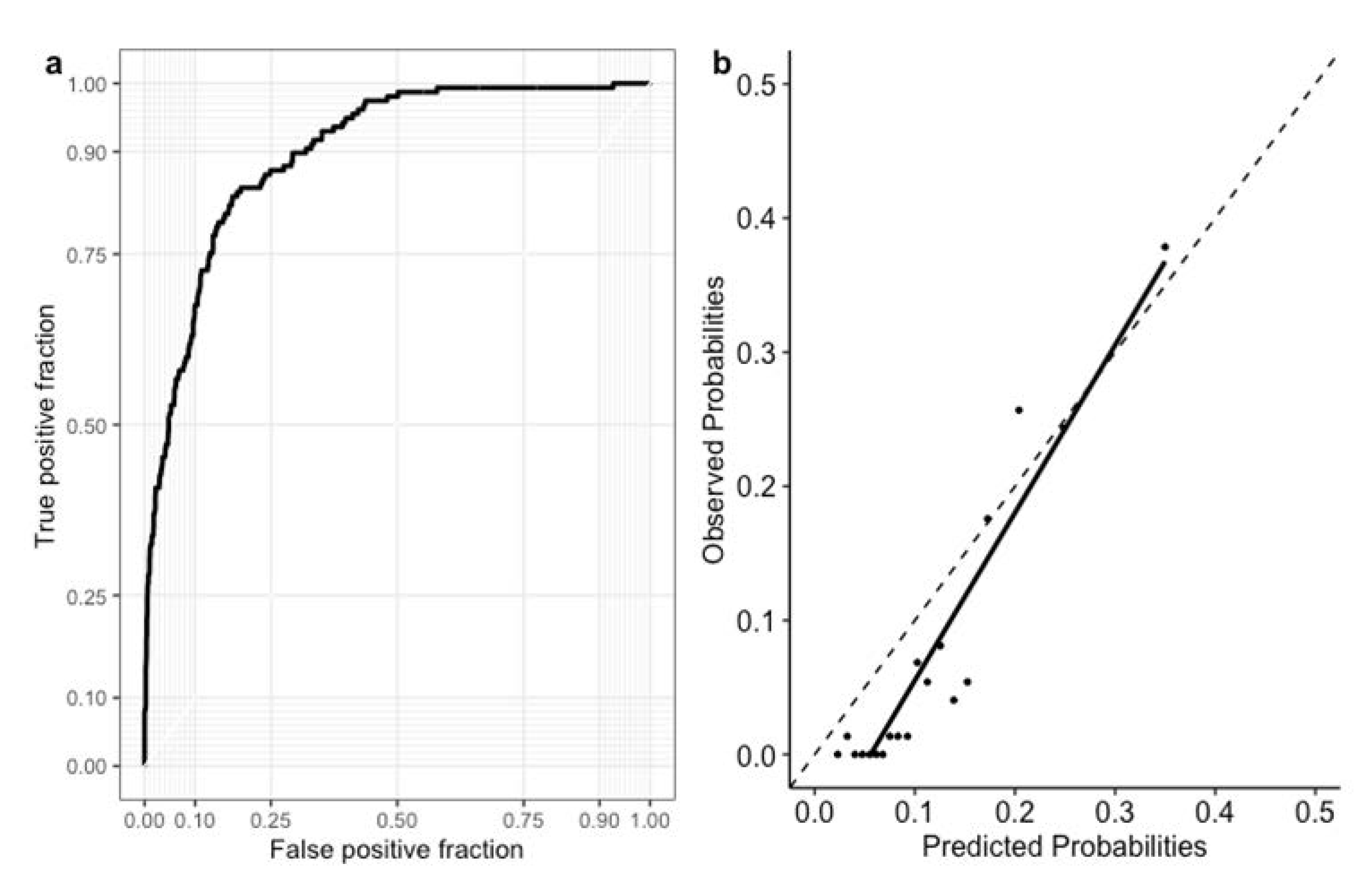
3.2. Feature Importance and Model Performance
3.3. Comparison with GRACE Discharge Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, T.J.; Berry, C. Angina: Contemporary diagnosis and management. Heart 2020, 106, 387–398. [Google Scholar] [CrossRef]
- Ferrari, R.; Camici, P.G.; Crea, F.; Danchin, N.; Fox, K.; Maggioni, A.P.; Manolis, A.J.; Marzilli, M.; Rosano, G.M.C.; Lopez-Sendon, J.L. Expert consensus document: A ‘diamond’ approach to personalized treatment of angina. Nat. Rev. Cardiol. 2018, 15, 120–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaram, V.; Bloom, C.; Zakeri, R.; Halcox, J.; Cohen, A.; Bowrin, K.; Briere, J.B.; Banerjee, A.; Simon, D.I.; Cleland, J.G.F.; et al. Temporal trends in the incidence, treatment patterns, and outcomes of coronary artery disease and peripheral artery disease in the UK, 2006–2015. Eur. Heart J. 2020, 41, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Marzilli, M.; Crea, F.; Morrone, D.; Bonow, R.O.; Brown, D.L.; Camici, P.G.; Chilian, W.M.; DeMaria, A.; Guarini, G.; Huqi, A.; et al. Myocardial ischemia: From disease to syndrome. Int. J. Cardiol. 2020, 314, 32–35. [Google Scholar] [CrossRef]
- Brier, G.W. Verification of Forecasts Expressed in Terms of Probability. Mon. Weather. Rev. 1950, 78, 1–3. [Google Scholar] [CrossRef]
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbæk, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef]
- Schulman-Marcus, J.; Hartaigh, B.; Gransar, H.; Lin, F.; Valenti, V.; Cho, I.; Berman, D.; Callister, T.; DeLago, A.; Hadamitzky, M.; et al. Sex-Specific Associations Between Coronary Artery Plaque Extent and Risk of Major Adverse Cardiovascular Events: The CONFIRM Long-Term Registry. JACC Cardiovasc. Imaging 2016, 9, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 41, 407–477. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar]
- Dweck, M.R.; Newby, D.E. Non-obstructive coronary artery disease can no longer be ignored. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, S.; D’Arrigo, P.; Franchina, G.; Capodanno, D. Risk Stratification in Patients with Coronary Artery Disease: A Practical Walkthrough in the Landscape of Prognostic Risk Models. Interv. Cardiol. 2018, 13, 112–120. [Google Scholar] [CrossRef]
- Kuhl, J.; Jorneskog, G.; Wemminger, M.; Bengtsson, M.; Lundman, P.; Kalani, M. Long-term clinical outcome in patients with acute coronary syndrome and dysglycaemia. Cardiovasc. Diabetol. 2015, 14, 120. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, G.; De Bacquer, D.; De Backer, G.; Kotseva, K.; Mellbin, L.; Wood, D.; Rydén, L. Screening for Glucose Perturbations and Risk Factor Management in Dysglycemic Patients with Coronary Artery Disease-A Persistent Challenge in Need of Substantial Improvement: A Report from ESC EORP EUROASPIRE V. Diabetes Care 2020, 43, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Shuvy, M.; Beeri, G.; Klein, E.; Cohen, T.; Shlomo, N.; Minha, S.; Pereg, D. Accuracy of the Global Registry of Acute Coronary Events (GRACE) Risk Score in Contemporary Treatment of Patients with Acute Coronary Syndrome. Can. J. Cardiol. 2018, 34, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Xian, Y.; Chen, J.; Gao, Z.; Qiao, S.; Yang, Y.; Gao, R.; Xu, B.; Yuan, J. Prognostic value of the GRACE discharge score for predicting the mortality of patients with stable coronary artery disease who underwent percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2020, 95, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Wan, Z.F.; Zhao, N.; Zhang, Y.P.; Mi, L.; Wang, X.H.; Zhou, D.; Wu, Y.; Yuan, Z.Y. Adjustment of the GRACE score by HemoglobinA1c enables a more accurate prediction of long-term major adverse cardiac events in acute coronary syndrome without diabetes undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 2015, 14, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple imputation by chained equations: What is it and how does it work? Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. In Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Volume 2016, pp. 785–794. [Google Scholar]
- Gensheimer, M.F.; Narasimhan, B. A Scalable Discrete-Time Survival Model for Neural Networks. PeerJ 2019, 7, e6257. [Google Scholar] [CrossRef] [PubMed]
- Teppola, P.; Taavitsainen, V.M. Parsimonious and robust multivariate calibration with rational function Least Absolute Shrinkage and Selection Operator and rational function Elastic Net. Anal. Chim. Acta 2013, 768, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Cai, T.; Pencina, M.J.; D’Agostino, R.B.; Wei, L.J. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat. Med. 2011, 30, 1105–1117. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. Advances in Neural Information Processing Systems; Long Beach, CA, USA, 2017; Volume 2017, pp. 4765–4774. Available online: https://papers.nips.cc/paper/2017 (accessed on 19 April 2021).
- Pencina, M.J.; D’Agostino, R.B., Sr.; Steyerberg, E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 2011, 30, 11–21. [Google Scholar] [CrossRef]
- Lindholm, D.; Lindbäck, J.; Armstrong, P.W.; Budaj, A.; Cannon, C.P.; Granger, C.B.; Hagström, E.; Held, C.; Koenig, W.; Östlund, O.; et al. Biomarker-Based Risk Model to Predict Cardiovascular Mortality in Patients with Stable Coronary Disease. J. Am. Coll. Cardiol. 2017, 70, 813–826. [Google Scholar] [CrossRef]
- Arnold, S.V.; Grodzinsky, A.; Gosch, K.L.; Kosiborod, M.; Jones, P.G.; Breeding, T.; Towheed, A.; Beltrame, J.; Alexander, K.P.; Spertus, J.A. Predictors of Physician Under-Recognition of Angina in Outpatients with Stable Coronary Artery Disease. Circ. Cardiovasc. Qual Outcomes 2016, 9, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Lanza, G.A.; Crea, F. Primary coronary microvascular dysfunction: Clinical presentation, pathophysiology, and management. Circulation 2010, 121, 2317–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepine, C.J.; Ferdinand, K.C.; Shaw, L.J.; Light-McGroary, K.A.; Shah, R.U.; Gulati, M.; Duvernoy, C.; Walsh, M.N.; Bairey Merz, C.N. Emergence of Nonobstructive Coronary Artery Disease: A Woman’s Problem and Need for Change in Definition on Angiography. J. Am. Coll. Cardiol. 2015, 66, 1918–1933. [Google Scholar] [CrossRef]
- Shahim, B.; De Bacquer, D.; De Backer, G.; Gyberg, V.; Kotseva, K.; Mellbin, L.; Schnell, O.; Tuomilehto, J.; Wood, D.; Rydén, L. The Prognostic Value of Fasting Plasma Glucose, Two-Hour Postload Glucose, and HbA(1c) in Patients with Coronary Artery Disease: A Report from EUROASPIRE IV: A Survey From the European Society of Cardiology. Diabetes Care 2017, 40, 1233–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, S.; George, A.; John, J.; Sathyapalan, T. Adjustment of the GRACE score by 2-h post-load glucose improves prediction of long-term major adverse cardiac events in acute coronary syndrome in patients without known diabetes. Eur. Heart J. 2018, 39, 2740–2745. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.S.; Tu, S.T.; Lee, I.T.; Lin, S.D.; Lin, S.Y.; Su, S.L.; Lee, W.J.; Sheu, W.H. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab. Res. Rev. 2011, 27, 79–84. [Google Scholar] [CrossRef]
- Henry, C.J.; Lightowler, H.J.; Newens, K.; Sudha, V.; Radhika, G.; Sathya, R.M.; Mohan, V. Glycaemic index of common foods tested in the UK and India. Br. J. Nutr. 2008, 99, 840–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.Y.; Pan, C.Y.; Yu, J.M.; China Heart Survey Group. The relationship between coronary artery disease and abnormal glucose regulation in China: The China Heart Survey. Eur. Heart J. 2006, 27, 2573–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Zou, X.; Yao, J.; Jiang, Q.; Zhang, Y.; Tu, M.; Yang, S.; Xu, S.; Lin, W.; Huang, H.; et al. The correlation between the oral glucose tolerance test 30-minutes plasma glucose and risk factors for diabetes and cardiovascular diseases: A cross-sectional epidemiological study of diabetes in Fujian Province in the South-East of China. J. Endocrinol. Investig. 2011, 34, e115–e120. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, A.; Minato, S.; Yano, M.; Takeuchi, M.; Kitaoka, K.; Kurata, M.; Yoshino, G.; Wu, B.; Kazumi, T.; Fukuo, K. Association of serum orosomucoid with 30-min plasma glucose and glucose excursion during oral glucose tolerance tests in non-obese young Japanese women. BMJ Open Diabetes Res. Care 2018, 6, e000508. [Google Scholar] [CrossRef] [Green Version]
- Alagona, P., Jr.; Ahmad, T.A. Cardiovascular disease risk assessment and prevention: Current guidelines and limitations. Med. Clin. N. Am. 2015, 99, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Everett, B.M.; Murthy, V.L.; Gaber, M.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Di Carli, M.F. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circle 2015, 131, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Shahim, B.; Gyberg, V.; De Bacquer, D.; Kotseva, K.; De Backer, G.; Schnell, O.; Tuomilehto, J.; Wood, D.; Rydén, L. Undetected dysglycaemia common in primary care patients treated for hypertension and/or dyslipidaemia: On the need for a screening strategy in clinical practice. A report from EUROASPIRE IV a registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc. Diabetol. 2018, 17, 21. [Google Scholar]
- Grant, P.J.; Cosentino, F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: New features and the ‘Ten Commandments’ of the 2019 Guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur. Heart J. 2019, 40, 3215–3217. [Google Scholar] [PubMed]
- Holman, R.R.; Coleman, R.L.; Chan, J.C.N.; Chiasson, J.L.; Feng, H.; Ge, J.; Gerstein, H.C.; Gray, R.; Huo, Y.; Lang, Z.; et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 877–886. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circle 2019, 139, e1082–e1143. [Google Scholar]

| Survival (N = 1322) | Death (N = 157) | p | |
|---|---|---|---|
| Age (years) | 58 ± 10 | 72 ± 12 | <0.001 |
| Female (n, %) | 238 (18%) | 20 (13%) | 0.122 |
| Body height (cm) | 165 ± 8 | 162 ± 8 | <0.001 |
| Body weight (kg) | 71.3 ± 11.7 | 66.4 ± 12.2 | <0.001 |
| BMI (kg/m2) | 26.1 ± 3.4 | 25.2 ± 3.9 | 0.004 |
| Waist (cm) | 90.6 ± 8 | 90.5 ± 9.4 | 0.923 |
| SBP (mmHg) | 126 ± 17 | 128 ± 20 | 0.226 |
| DBP (mmHg) | 74 ± 10 | 69 ± 11 | <0.001 |
| Heart rate (beat/min) | 71 ± 11 | 75 ± 13 | <0.001 |
| UACR (mg/g) | 36 ± 176 | 111 ± 274 | <0.001 |
| Uric acid (mg/dL) | 6.6 ± 1.6 | 7.3 ± 2.0 | <0.001 |
| Triglycerides (mg/dL) | 147 ± 105 | 122 ± 78 | 0.004 |
| Total cholesterol (mg/dL) | 173 ± 39 | 169 ± 35 | 0.253 |
| HDL cholesterol (mg/dL) | 46 ± 12 | 49 ± 15 | 0.007 |
| LDL cholesterol (mg/dL) | 106 ± 34 | 103 ± 31 | 0.501 |
| Creatinine (mg/dL) | 1.06 ± 1.01 | 1.01 ±0.57 | 0.298 |
| eGFR (mL/min/1.73 m2) | 82 ± 22 | 63 ± 21 | <0.001 |
| GPT (U/L) | 32 ± 32 | 26 ± 19 | 0.028 |
| Hemoglobin (g/dL) | 13.9 ± 1.5 | 13.2 ± 1.9 | 0.001 |
| WBC (/μL) | 6898 ± 2772 | 6923 ± 2176 | 0.914 |
| CK (U/L) | 151 ± 314 | 180 ± 370 | 0.373 |
| CKMB (U/L) | 9 ± 16 | 11 ± 16 | 0.241 |
| Troponin-T (ng/L) | 3.9 ± 19.5 | 1.4 ± 3.6 | 0.209 |
| OGTT (mg/dL) | |||
| Glucose 0 min | 95 ± 14 | 100 ± 19 | <0.001 |
| Glucose 30 min | 169 ± 32 | 169 ± 36 | 0.962 |
| Glucose 120 min | 145 ± 50 | 166 ± 59 | <0.001 |
| HbA1c (%) | 5.8 ± 0.6 | 6.1 ± 0.8 | <0.001 |
| Glucose status (n, %) | <0.001 | ||
| Normal glucose regulation | 428 (32.4%) | 43 (27.4%) | |
| Prediabetes | 637 (48.2%) | 51 (32.5%) | |
| Diabetes | 257 (19.4%) | 63 (40.1%) | |
| Smoking status (n, %) | <0.001 | ||
| Non-smoker | 590 (44.6%) | 52 (33.1%) | |
| Smoker | 318 (24.1%) | 18 (11.5%) | |
| Ex-smoker | 414 (31.3%) | 87 (55.4%) | |
| Medication (n, %) | |||
| Antiplatelet | 1226 (92.8%) | 147 (93.6%) | 0.830 |
| ACE inhibitor | 278 (21.0%) | 46 (29.3%) | 0.023 |
| ARB | 415 (31.4%) | 69 (43.9%) | 0.002 |
| Alpha blocker | 54 (4.1%) | 16 (10.2%) | 0.001 |
| Beta blocker | 368 (27.8%) | 23 (14.6%) | 0.001 |
| CCB | 689 (52.1%) | 78 (49.7%) | 0.622 |
| Diuretics | 153 (11.6%) | 44 (28.0%) | <0.001 |
| CAD history * (n, %) | 140 (10.6%) | 26 (16.6%) | 0.035 |
| Grace score | 90.2±20.3 | 125.3 ± 31 | <0.001 |
| Left ventricular ejection fraction (%) | 52 ± 11 | 47 ± 13 | <0.001 |
| Number of coronary arteries with significant stenosis † (n, %) | 0.007 | ||
| Non-obstructive CAD | 611 (46.2%) | 51 (32.5%) | |
| 1 | 356 (26.9%) | 48 (30.6%) | |
| 2 | 245 (18.5%) | 42 (26.8%) | |
| 3 | 110 (8.3%) | 16 (10.2%) | |
| Non-invasive studies before angiography (n, %) | |||
| Treadmill exercise test | 575 (43.5%) | 66 (42.0%) | 0.793 |
| Myocardial perfusion imaging | 116 (8.8%) | 16 (10.2%) | 0.660 |
| Echocardiography | 282 (21.3%) | 30 (19.1%) | 0.588 |
| Rest electrocardiography | 349 (26.4%) | 45 (28.7%) | 0.609 |
| Percutaneous coronary intervention (n, %) | |||
| without stent insertion | 146 (11.0%) | 36 (22.9%) | <0.001 |
| with stent insertion | 542 (41.0%) | 67 (42.7%) | 0.640 |
| HR | 95% CI | p | |
|---|---|---|---|
| Age | 1.06 | (1.04–0.08) | <0.001 |
| Heart rate | 1.02 | (1.01–1.04) | <0.001 |
| OGTT 30 min | 0.98 | (0.97–0.99) | <0.001 |
| OGTT 120 min | 1.01 | (1.01–1.02) | <0.001 |
| CAD history | 1.66 | (1.03–2.66) | 0.040 |
| Smoking history | 1.31 | (1.06–1.65) | 0.013 |
| ARB use | 1.74 | (1.18–2.55) | 0.008 |
| Diuretic use | 1.57 | (1.01–2.38) | 0.048 |
| Harrell’s C-Index | Brier Score | |
| With all variables | ||
| Cox regression | 0.774 | 0.069 |
| RSF | 0.804 | 0.082 |
| XGBoost | 0.788 | 0.033 |
| DNN | 0.750 | 0.102 |
| With selected features | ||
| Cox regression | 0.741 | 0.076 |
| RSF | 0.829 | 0.080 |
| XGBoost | 0.794 | 0.056 |
| DNN | 0.796 | 0.106 |
| Model | Harrell’s C-Index (95% CI) | p | Absolute IDI (95% CI) | p | NRI (95% CI) | p |
|---|---|---|---|---|---|---|
| GRACE score | 0.739 (0.683, 0.796) | <0.001 | 0.135 (0.068, 0.203) | 0.007 | 0.328 (0.096, 0.583) | 0.027 |
| GRACE score + OGTT 120 min | 0.740 (0.685, 0.797) | <0.001 | 0.115 (0.033, 0.224) | 0.027 | 0.336 (0.103, 0.646) | 0.027 |
| RSF | 0.829 (0.790, 0.869) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-H.; Sheu, W.H.-H.; Yeh, W.-C.; Chang, Y.-C.; Lee, I.-T. Predicting Long-Term Mortality in Patients with Angina across the Spectrum of Dysglycemia: A Machine Learning Approach. Diagnostics 2021, 11, 1060. https://doi.org/10.3390/diagnostics11061060
Li Y-H, Sheu WH-H, Yeh W-C, Chang Y-C, Lee I-T. Predicting Long-Term Mortality in Patients with Angina across the Spectrum of Dysglycemia: A Machine Learning Approach. Diagnostics. 2021; 11(6):1060. https://doi.org/10.3390/diagnostics11061060
Chicago/Turabian StyleLi, Yu-Hsuan, Wayne Huey-Herng Sheu, Wen-Chao Yeh, Yung-Chun Chang, and I-Te Lee. 2021. "Predicting Long-Term Mortality in Patients with Angina across the Spectrum of Dysglycemia: A Machine Learning Approach" Diagnostics 11, no. 6: 1060. https://doi.org/10.3390/diagnostics11061060
APA StyleLi, Y.-H., Sheu, W. H.-H., Yeh, W.-C., Chang, Y.-C., & Lee, I.-T. (2021). Predicting Long-Term Mortality in Patients with Angina across the Spectrum of Dysglycemia: A Machine Learning Approach. Diagnostics, 11(6), 1060. https://doi.org/10.3390/diagnostics11061060







