Abstract
Randomized control trials and meta-analyses comparing colonoscopies with and without computer-aided detection (CADe) assistance showed significant increases in adenoma detection rates (ADRs) with CADe. A major limitation of CADe is its false positives (FPs), ranked 3rd in importance among 59 research questions in a modified Delphi consensus review. The definition of FPs varies. One commonly used definition defines an FP as an activation of the CADe system, irrespective of the number of frames or duration of time, not due to any polypoid or nonpolypoid lesions. Although only 0.07 to 0.2 FPs were observed per colonoscopy, video analysis studies using FPs as the primary outcome showed much higher numbers of 26 to 27 per colonoscopy. Most FPs were of short duration (91% < 0.5 s). A higher number of FPs was also associated with suboptimal bowel preparation. The appearance of FPs can lead to user fatigue. The polypectomy of FPs results in increased procedure time and added use of resources. Re-training the CADe algorithms is one way to reduce FPs but is not practical in the clinical setting during colonoscopy. Water exchange (WE) is an emerging method that the colonoscopist can use to provide salvage cleaning during insertion. We discuss the potential of WE for reducing FPs as well as the augmentation of ADRs through CADe.
1. Introduction
Missed lesions account for 57.8% of interval colorectal cancers (i.e., cancers that occur within 3–5 years after a negative colonoscopy) [1]. To reduce incidences of missed lesions and interval cancers, measures were proposed to improve the quality of colonoscopies. One of the most important quality metrics is the adenoma detection rate (ADR), defined as the proportion of patients with at least one adenoma [2].
Artificial intelligence (AI) is being used in the computer-aided detection (CADe) and diagnosis (CADx) of polyps [3]. Randomized controlled trials (RCTs) showed CADe-assisted colonoscopy significantly increased the ADR [4,5,6,7,8]. A meta-analysis confirmed that the ADR was significantly higher in the CADe group than in the conventional group (36.6% vs. 25.2%; RR, 1.44; 95% confidence interval, 1.27–1.62; p < 0.01; I2 = 42%) [9].
An accompanying limitation of the CADe is false positives (FPs), which occur when the algorithm identifies a “polyp” that the endoscopist would disagree with. FPs were ranked 3rd in importance among 59 future research questions related to CADe [10]. Therefore, we conducted this systemic review on the definitions, causes, and adverse effects of the CADe FPs. We assessed CADe-overlaid video analyses, RCTs using real-time CADe to enhance polyp detection during colonoscopies, and studies that used FPs as the primary outcome. We also reviewed water exchange (WE) colonoscopy, a novel insertion method that may help to decrease FPs. We test the hypothesis that the systematic review of the literature on FPs will yield insight into methods of managing and limiting the adverse effects of this drawback of CADe.
2. Method
We performed a systematic review of the literature by searching PubMed with the following string: (automatic polyp detection OR computer-aided detection OR deep learning OR artificial intelligence) AND colonoscopy in the past four years (Jan. 2017 to Apr. 2021). The search was performed for titles, abstracts, and keywords. We included full-text articles in English. The exclusion criteria were non-research reports (i.e., systematic reviews, editorials, or case reports), research not related to artificial intelligence, not focusing on colonoscopy (e.g., computed tomography colonography, capsule endoscopy, chatbot, etc.), not related to polyp detection (e.g., CADx, regulatory issues, robotic colonoscopy, quality optimization, etc.), not applying real-time video analysis, or not reporting FPs. For clinical studies, non-RCTs were excluded. We identified 9 articles on the applications of CADe based on deep learning for the real-time detection of polyps on colonoscopy videos, 6 articles on RCTs comparing colonoscopies with or without CADe to assist in polyp detection, as well as 2 CADe-overlaid video analysis studies using FPs as the primary outcome (Figure 1). We also turned to one of our most recent reviews on WE colonoscopies and tabulated 3 articles on RCTs comparing WE with air insufflation that reported FP-related procedural data [11].
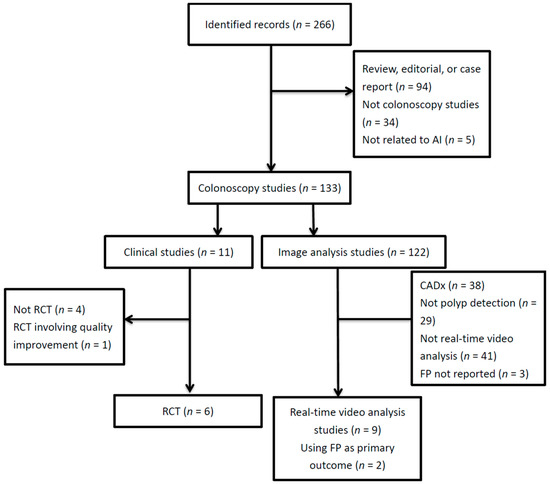
Figure 1.
Literature flow diagram. AI, artificial intelligence; CADx, computer-aided diagnosis; FP, false positive; RCT, randomized controlled trial.
3. Definition of False Positives
False positives have various definitions across different studies (Table 1). In general, the term refers to computer prompts indicating polyps that the endoscopist does not consider to be polyps [12,13]. Some investigators have used false alarms to define tracking boxes on non-polyp structures that were continuously tracked [7,8], while other authors ignored brief false positives [4,12]. Variations in practices have led to inconsistent reports on the frequency of FPs. The CADe-assisted detection of colon polyps has reported an average of 0.071 to 0.201 FPs per colonoscopy [4,5,6,7,8,14]. However, studies using FPs as the primary outcome reported an average of 26.3 to 27.3 FPs per colonoscopy, despite having extracted videos from the same RCT [5] or having used a similar CADe model to that of a previous RCT [7].

Table 1.
Various definitions of false positives and false positive rates across studies.
Defining FPs based on the duration of time is an objective way of classifying FPs. However, the threshold required for reporting FPs is unsettled. One report suggested that only FPs > 2 s be reported [13], and another only reported FPs > 1 s [15], while the majority of FPs (i.e., more than 90%) lasted <0.5 s [13]. It is unknown whether ignoring the transient FPs (i.e., those lasting for <1 or 2 s) would increase the risk of missing a real polyp. A recent report on colonoscopic video analysis with CADe showed that missed polyps had a shorter appearance time (defined as the interval on the video between appearance and disappearance of a polyp) than detected polyps had [16]. Future prospective studies that explore every flash of a prompt, including those lasting <0.5 s, would be necessary to answer the question of whether those FPs could be discarded or not. Defining FPs based on time can also be influenced by the endoscopist’s technique, such as the speed of withdrawal.
The definition of the presence of an FP is dependent on the judgment of the endoscopist. For studies analyzing CADe-overlaid videos, FPs were judged by a single expert reader or through the consensus of 2–3 reviewers [12,15,18]. Thus, there is room for errors due to subjectivity in these definitions.
Although the frequency of FPs was reported as per-colonoscopy, RCTs evaluating CADe systems did not employ the algorithm during insertion [4,6,7,8,14], and the post hoc analysis of videos for FPs invariably involved only the withdrawal phase [12]. It is conceivable that more FPs would be observed during insertion, where the bowel lumen is supposed to be kept minimally distended and the bowel contents have not yet been removed through cleaning. Thus, the impacts of using CADe, and its associated increase in FPs during the insertion phase, are unknown.
Some studies added those FPs that were detected by the CADe, considered to be a polyp, and removed by the endoscopist—but that were later determined to be normal tissue through pathological examination [14].
4. Studies That Report False Positives
4.1. Using CADe Based on Deep Learning for Real-Time Polyp Detection in Colonoscopy Videos
The details of these reports are summarized in Table 2. From the perspective of the clinical endoscopist, we only included studies evaluating CADe with the real-time capability to detect polyps. All CADe algorithms used in these studies were based on deep learning, which offered better sensitivity and specificity than the outdated hand-crafted-feature method [23,24]. Most of these studies were reporting on the development and validation of CADe systems with per-frame sensitivity between 56.8–98.8% and specificity between 63.3–98%.

Table 2.
Recent studies using CADe-overlaid videos for real-time detection of polyps.
In these reports, the false-positive rate (FPR) was reported either as a per-frame analysis [16,18] or a per-polyp analysis [15,17]. To determine the per-frame FPR, the videos were transformed into frames of still images. The per-frame FPR was typically calculated as the number of FP frames divided by the number of frames without polyps; However, a minority of the studies reported the per-frame FPR as the number of FP frames divided by the total number of frames [20,26]. Because of the minor difference in these two definitions, we categorized both as per-frame FPRs in the current review. When only the specificity of a CADe system was reported, the per-frame FPR was calculated as 1-per-frame specificity (i.e., the number of true negative frames divided by the number of frames without polyps). To calculate the per-polyp FPR, the number of polyps identified by the CADe but judged to not be polyps by the endoscopist was divided by the total number of polyps that were identified by the CADe. Reporting the per-polyp FPR is more realistic and clinically relevant. The FPR varied widely (per-frame, 0.9–37%). Most studies validated algorithms under the conditions of ideal bowel preparation for polyp detection. In a study with variable bowel preparation [15], the CADe model still performed well, with a per-polyp sensitivity of 98.8%; however, the per-polyp FPR was as high as 60%.
The FPR can be varied by adjusting its confidence level in the CADe algorithm. An image with a polyp detection probability greater than the confidence level is recognized by the CADe to be a polyp [16]. The CADe algorithm achieved a higher sensitivity of 94% for polyp detection and a higher per-frame FPR of 7.8% with a confidence level of ≥10%. When the confidence level was adjusted to ≥30%, the FPR decreased to 2.7% at the expense of reducing the sensitivity to 88%. Whenever possible, an ideal CADe system should strike a balance between high sensitivity and a low FPR [16].
4.2. RCTs Comparing Real-Time CADe with Control
The details of these reports are summarized in Table 3. All of the systems were based on deep learning convolutional neural networks. Out of these 6 studies, 5 were conducted in China, where the reported ADR was usually lower than those in the Western countries. All 6 studies consistently showed that the CADe increased the ADR [4,5,6,7,8,14], and most of the studies did not report missing any polyps [4,6,7,8]. All 6 studies saw an increase in the number of diminutive (<5 mm) adenomas, and some also saw an increase in the number of small (<10 mm) adenomas [4,5,6]. The withdrawal time, excluding biopsy, was comparable between the CADe and the control groups [4,5,7,8,14]. A false positive was defined in some reports as an area that was traced continuously but deemed not to be a polyp by the endoscopist [4,7,8] or was not defined in others [5,6,14]. False positives mainly consisted of bubbles, feces, and crumpled colon walls [4,7,14].

Table 3.
Recent RCTs comparing real-time CADe with control on adenoma detection during colonoscopy.
4.3. Video Analysis Studies Using FPs as the Primary Uutcome
The details of these reports are summarized in Table 4. False positives were identified as artifacts from the bowel wall and bowel content [12]. The clinical relevance of FPs was determined by the time required to explore the causes of FPs (in post hoc analysis). Most (94.3%) were bowel wall images with short exposure times and were determined to have no clinical impact, as no additional exploratory time was needed.

Table 4.
Video analysis studies using FPs as the primary outcome.
Holzwanger et al. defined FPs according to time thresholds (0.5 s, 1 s, and 2 s) [13]. The different threshold definitions for FPs resulted in different reported diagnostic performances of the CADe, and the data suggested that using the same benchmarks to define FPs is the prerequisite for comparing the performances among different CADe algorithms.
5. The Causes of False Positives
The reported causes of false positives included feces, bubbles, wrinkled walls, normal structures (such as ileocecal valve), local inflammation, local bleeding, suction marks, polypectomy sites, and round drug capsules [4,7,15]. Of all these causes, the majority originated from rumpled colon folds, feces, debris, and bubbles (Table 1). The proportion of these elements depends on the CADe system used. Even with the same CADe algorithm, different settings (such as the confidence level) would result in different FPRs [16].
6. Adverse Effects of FPs
6.1. Increased Withdrawal Time
The time expended to differentiate an FP from a true lesion can potentially increase the withdrawal time. Although most RCTs on the real-time application of CADe found a longer withdrawal time in the CADe group compared to the control group [4,6,7,8], the withdrawal time without biopsy was not significantly different. Nonetheless, the withdrawal time without biopsy was numerically longer in all 6 RCTs (Table 3). In a post hoc analysis of a small fraction (40/342 or <11.7%) of the original CADe groups in the RCT studies, Hassan et al. found that 94% of FPs were discarded by the endoscopist immediately without further exploration, and the time wasted on the remaining FPs only contributed to about 1% of the withdrawal time. This was extrapolated to the original RCT data to suggest that FPs were insignificant, even though not all of the original FPs were assessed. Nevertheless, the mean withdrawal time was moderately correlated with the number of FP prompts (p = 0.0003; r = 0.5, 95% CI: 0.2–0.7) [12]. This positive correlation raised questions about the conclusion that FPs had no clinical impact [12]. Another study analyzing FPs also found that a higher number of FPs was associated with longer withdrawal time (Table 4) [13]. It appears that FPs did contribute to a longer withdrawal time, but that the impact might be quite limited by the commercially available system and experienced endoscopists. In a real-life situation, where the bowel preparation is usually less than optimal and endoscopists are less experienced, the impacts of bowel preparation on FPs and withdrawal time require more objective studies.
6.2. Unnecessary Polypectomies of Non-Neoplastic Lesions
The presence of FPs might lead to unnecessary biopsies of non-neoplastic tissues. Of the 6 total, 4 RCTs [4,7,8,14] (with another 1 unreported [6] and 1 showing no difference [5]) listed in Table 3 showed a significant increase in the biopsy of non-neoplastic polyps in the CADe group, which was typically double the number reported for the control group. Hyperplastic polyps and inflammatory polyps were lumped together in these studies. The removal of hyperplastic polyps—other than the diminutive ones at the distal rectosigmoid colon—is justified, as these polyps contribute to the serrated pathway of colorectal carcinogenesis [27]. Therefore, it is unknown how many of the biopsies were really unnecessary. If these biopsies were, in fact, unwarranted, then there exists an avoidable non-indicated use of medical resources. Unnecessary biopsies could also add to the cost of pathology processing.
The application of the CADx to characterize the polyps following their detection with the CADe might help reduce the number of unnecessary polypectomies of non-neoplastic polyps. Preliminary results showed promise for simultaneously classifying polyps with endocytoscopic images [28], or even with white light images [29] after using the CADe to detect the polyps in white light.
6.3. Increased User Fatigue, Distractions, and Decreased Enthusiasm
The recurrent appearance of FPs on the screen may lead to increased fatigue and decreased vigilance on the part of the endoscopist [30]. Vigilance is a limited resource and depletes with repetitive stimulus [31]. Hassan et al. reported the number of FPs far outnumbered that of true positives—a 25-fold difference [12]. Inundating the endoscopist with such a large amount of prompts on the screen, even if only very transient attention is demanded for each prompt, engenders the risk of the fatigue of the endoscopist. However, a study showed that a real-time CADe system, integrated on one primary endoscopy monitor instead of the two monitors used in most RCTs (Table 3), improved the ADR without an increase in the subjective fatigue level reported by the endoscopists during the colonoscopy [14]. The unblinded report, developed by proponents of the CADe algorithm under study, raised questions regarding the objectivity of the results.
False positives cause distractions and the need for refocusing, potentially resulting in adverse effects during the search for real polyps. To illustrate how difficult it is to refocus after distraction, a study on mobile phone use while driving showed that the risk of a rear-end accident occurring increased by 2.34–3.56 times, despite increasing their time headway by 0.41–0.59 s to offset the distraction of texting while driving [32].
Too many FPs may hamper the enthusiasm of the endoscopist to apply the CADe in clinical practice. One recent survey on the views of gastroenterologists regarding the potential use of artificial intelligence found that 33.9% of respondents worried about high numbers of FPs [33]. Reports that emphasize the lack of importance of FPs based on subjective assessment need to be re-evaluated by studies with more objective and unbiased designs.
7. How to Address the Occurrence of FPs
There is considerable variability in FPRs in the literature (Table 1). This variability suggests that there are diverse definitions of FPs and various conditions that affect the occurrence of FPs inside the bowel lumen, which indicates that there is an opportunity to minimize FPs through standardizing the definitions of FPs and optimizing the condition of the bowel lumen.
Standardizing the definitions of FPs will require agreement amongst programmers of the CADe system. An example of a simple method that could be used to reduce FPs is re-training the CADe algorithms with scenarios that currently lead to FPs. Another approach could be the adoption of recurrent neural networks, which have memory and can process temporal sequences of frames in a way that is similar to the learning process of human brains [10]. Misawa et al. reported that when they changed their old algorithm [17] to YoloV3 (You Only Look Once, Version 3), a state-of-the-art, real-time object detection algorithm, better specificity was achieved (increasing from 90.9% to 93.7%) [19]. Lee et al. proposed to reduce FPs by using the median filter (which reduced the FPR from 12.5% to 6.3%), a nonlinear spatial filter that is particularly effective for eliminating salt-and-pepper noise [21]. To filter out most short flashes, Podlasek et al. suggested setting a threshold of persistent time for FPs to show up; however, this method might introduce a minor detection lag, depending on the desired sensitivity [22]. These methods are beyond the expertise of the clinical endoscopists.
Optimal bowel preparation is the prerequisite for a high-quality CADe-assisted colonoscopy and is associated with fewer FPs [13]. As the major source of CADe FP alerts is the wrinkled walls, they can be reduced by ensuring adequate luminal insufflation. The use of an anti-spasmodic agent, such as Hyoscine-n-butylbromide, might be helpful in reducing the contraction of the colon wall [34]. Adding simethicone or rinse water to the bowel preparation regimen helps eliminate bubble-induced FPs [35,36]. However, whether the addition of these measures would actually decrease FPs remains to be studied.
Before the FPs can be effectively reduced, proper training of the endoscopist to recognize and ignore FPs is needed to enable the widespread adoption of the CADe for the detection of colon neoplasms [12].
The optimization of the condition of the bowel lumen can be controlled by the colonoscopist using water exchange colonoscopy, which will be discussed in detail below.
8. Water Exchange and Its Potential Beneficial Effect on Reducing FPs
Among the Gastrointestinal (GI) Endoscopy Editorial Board’s top 10 topics in endoscopy in 2019, water exchange (WE) and artificial intelligence (i.e., CADe) were both considered important advances in GI endoscopy [37]. The coincidence brought both to the forefront of the discussion on the improvement of ADR.
Compared with traditional gas (i.e., air or CO2) insufflation for colonoscopes, WE is an effective insertion method that minimizes insertion pain and enhances ADR [38,39,40]. It features infusing water to guide the scope advancement in an airless lumen, while suctioning the infused water at the same time during insertion, thus aiming at the almost complete removal of the infused water when cecal intubation is achieved. A network meta-analysis concluded that WE produced the highest ADR when compared with water immersion and gas insufflation [41]. A modified Delphi review also endorsed WE as having better bowel cleanliness, as well as less insertion pain and higher ADRs, than gas insufflation [42].
Holzwanger et al. reported that a high FPR was associated with fair or poor Aronchick bowel preparation scores [13]. WE can effectively salvage-clean bubbles and fecal debris during insertion, resulting in better bowel cleanliness during withdrawal. Table 5 summarizes 3 key RCTs, including more than 2000 patients in each group, comparing air insufflation and WE in terms of ADR. WE consistently showed better Boston Bowel Preparation Scale (BBPS) scores than air insufflation, both in the whole colon and the right colon, the latter of which was usually the dirtiest colon segment [38,39,40]. WE might also help reduce FPs associated with crumpled folds, as there is less need for suction cleaning, and thus the related spasms, during withdrawal [43]. In an analysis of the CADe-overlaid withdrawal phase videos of colonoscopies from an RCT comparing right colon ADR inserted with WE or air insufflation, Tang et al. found WE was associated with a significantly lower FPR compared with air insufflation (5 [4.1%] vs. 19 [15.6%], p = 0.02) (Dr. CP Tang, personal communication 2021).

Table 5.
BBPS scores in key randomized controlled trials comparing ADRs between WE and air insufflation.
Another potential merit of combining WE with CADe is the possible additive effects on increasing ADR. WE and CADe both increase ADR but through different mechanisms. WE increases ADRs mainly through insertion salvage cleaning, thus revealing otherwise unexposed polyps (Table 5). On the other hand, CADe works as a second observer and points out polyps that are exposed but not recognized due to human error [17]. A single-center study clearly demonstrated that even WE missed polyps in the right colon [44]. In other words, the individual strengths of WE and CADe complement the weakness of one another.
9. Conclusions
False positives have emerged as an important research issue in the application of artificial intelligence for the detection of polyps during colonoscopy. The number of FPs per colonoscopy turned out to be much higher (26 to 27 per colonoscopy) than originally reported among recent RCTs using the CADe to assist with polyp detection (0.07 to 0.2 per colonoscopy). This discrepancy might be a result of the different criteria for FPs that were set up in each study. A refinement of the definition of FPs is urgently needed to minimize variability in and facilitate the comparison of FPs reported in one study with those from another. A recurrent theme in published studies showed that a higher number of FPs was associated with less-than-ideal bowel preparation. The occurrence of FPs might result in the unnecessary biopsy of non-neoplastic lesions, which has been shown to be increased with the use of the CADe compared with the control groups in most RCTs. False positives might also lead to fatigue, distraction, and the need for refocusing for the endoscopists. Aside from re-training the CADe algorithms, adjunct medications (e.g., simethicone and an anti-spasmodic) might be beneficial for decreasing FPs. WE holds the potential for reducing FPs through salvage-cleaning feces and bubbles during insertion, thus avoiding cleaning and suction-induced colon spasming during withdrawal. The simultaneous application of the CADe and the CADx can help avoid unnecessary polypectomies of non-neoplastic polyps. Future studies on standardizing the definition and measurement of FPs are needed. Adding WE to CADe is a double-advantage approach, in that it may not only decrease FPs but may also further boost ADR to the benefit of the patients.
Funding
This research was supported by the research fund from the Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation. Dr. Leung’s research and publication effort is supported by VA Clinical Merit and ASGE Clinical Research Funds.
Institutional Review Board Statement
Ethical review and approval were waived for this review article.
Informed Consent Statement
Patient consent was waived due to only literatures were reviewed in this article.
Data Availability Statement
All literatures reviewed are accessible on PubMed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Le Clercq, C.M.; Bouwens, M.W.; Rondagh, E.J.; Bakker, C.M.; Keulen, E.T.; de Ridder, R.J.; Winkens, B.; Masclee, A.A.; Sanduleanu, S. Postcolonoscopy colorectal cancers are preventable: A population-based study. Gut 2014, 63, 957–963. [Google Scholar] [CrossRef]
- Rex, D.K.; Schoenfeld, P.S.; Cohen, J.; Pike, I.M.; Adler, D.G.; Fennerty, M.B.; Lieb, J.G., 2nd; Park, W.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for colonoscopy. Gastrointest. Endosc. 2015, 81, 31–53. [Google Scholar] [CrossRef]
- Vinsard, D.G.; Mori, Y.; Misawa, M.; Kudo, S.E.; Rastogi, A.; Bagci, U.; Rex, D.K.; Wallace, M.B. Quality assurance of computer-aided detection and diagnosis in colonoscopy. Gastrointest. Endosc. 2019, 90, 55–63. [Google Scholar] [CrossRef]
- Liu, W.N.; Zhang, Y.Y.; Bian, X.Q.; Wang, L.J.; Yang, Q.; Zhang, X.D.; Huang, J. Study on detection rate of polyps and adenomas in artificial-intelligence-aided colonoscopy. Saudi J. Gastroenterol. 2020, 26, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A.; et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020, 159, 512–520.e7. [Google Scholar] [CrossRef] [PubMed]
- Su, J.R.; Li, Z.; Shao, X.J.; Ji, C.R.; Ji, R.; Zhou, R.C.; Li, G.C.; Liu, G.Q.; He, Y.S.; Zuo, X.L.; et al. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: A prospective randomized controlled study (with videos). Gastrointest. Endosc. 2020, 91, 415–424.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Berzin, T.M.; Glissen Brown, J.R.; Bharadwaj, S.; Becq, A.; Xiao, X.; Liu, P.; Li, L.; Song, Y.; Zhang, D.; et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: A prospective randomised controlled study. Gut 2019, 68, 1813–1819. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Berzin, T.M.; Glissen Brown, J.R.; Liu, P.; Zhou, C.; Lei, L.; Li, L.; Guo, Z.; Lei, S.; et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): A double-blind randomised study. Lancet Gastroenterol. Hepatol. 2020, 5, 343–351. [Google Scholar] [CrossRef]
- Hassan, C.; Spadaccini, M.; Iannone, A.; Maselli, R.; Jovani, M.; Chandrasekar, V.T.; Antonelli, G.; Yu, H.; Areia, M.; Dinis-Ribeiro, M.; et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 93, 77–85.e6. [Google Scholar] [CrossRef]
- Ahmad, O.F.; Mori, Y.; Misawa, M.; Kudo, S.E.; Anderson, J.T.; Bernal, J.; Berzin, T.M.; Bisschops, R.; Byrne, M.F.; Chen, P.J.; et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy 2020. [Google Scholar] [CrossRef]
- Tang, C.P.; Shao, P.P.; Hsieh, Y.H.; Leung, F.W. A review of water exchange and artificial intelligence in improving adenoma detection. Tzu. Chi. Med. J. 2021, 33, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Badalamenti, M.; Maselli, R.; Correale, L.; Iannone, A.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; et al. Computer-aided detection-assisted colonoscopy: Classification and relevance of false positives. Gastrointest. Endosc. 2020, 92, 900–904.e4. [Google Scholar] [CrossRef]
- Holzwanger, E.A.; Bilal, M.; Glissen Brown, J.R.; Singh, S.; Becq, A.; Ernest-Suarez, K.; Berzin, T.M. Benchmarking definitions of false-positive alerts during computer-aided polyp detection in colonoscopy. Endoscopy 2020. [Google Scholar] [CrossRef]
- Liu, P.; Wang, P.; Glissen Brown, J.R.; Berzin, T.M.; Zhou, G.; Liu, W.; Xiao, X.; Chen, Z.; Zhang, Z.; Zhou, C.; et al. The single-monitor trial: An embedded CADe system increased adenoma detection during colonoscopy: A prospective randomized study. Therap. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Becq, A.; Chandnani, M.; Bharadwaj, S.; Baran, B.; Ernest-Suarez, K.; Gabr, M.; Glissen-Brown, J.; Sawhney, M.; Pleskow, D.K.; Berzin, T.M. Effectiveness of a Deep-learning Polyp Detection System in Prospectively Collected Colonoscopy Videos With Variable Bowel Preparation Quality. J. Clin. Gastroenterol. 2020, 54, 554–557. [Google Scholar] [CrossRef]
- Guo, Z.; Nemoto, D.; Zhu, X.; Li, Q.; Aizawa, M.; Utano, K.; Isohata, N.; Endo, S.; Kawarai Lefor, A.; Togashi, K. Polyp detection algorithm can detect small polyps: Ex vivo reading test compared with endoscopists. Dig. Endosc. 2021, 33, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.; Kudo, S.E.; Mori, Y.; Cho, T.; Kataoka, S.; Yamauchi, A.; Ogawa, Y.; Maeda, Y.; Takeda, K.; Ichimasa, K.; et al. Artificial Intelligence-Assisted Polyp Detection for Colonoscopy: Initial Experience. Gastroenterology 2018, 154, 2027–2029.e3. [Google Scholar] [CrossRef]
- Urban, G.; Tripathi, P.; Alkayali, T.; Mittal, M.; Jalali, F.; Karnes, W.; Baldi, P. Deep Learning Localizes and Identifies Polyps in Real Time With 96% Accuracy in Screening Colonoscopy. Gastroenterology 2018, 155, 1069–1078.e8. [Google Scholar] [CrossRef]
- Misawa, M.; Kudo, S.E.; Mori, Y.; Hotta, K.; Ohtsuka, K.; Matsuda, T.; Saito, S.; Kudo, T.; Baba, T.; Ishida, F.; et al. Development of a computer-aided detection system for colonoscopy and a publicly accessible large colonoscopy video database (with video). Gastrointest. Endosc. 2021, 93, 960–967.e3. [Google Scholar] [CrossRef]
- Hassan, C.; Wallace, M.B.; Sharma, P.; Maselli, R.; Craviotto, V.; Spadaccini, M.; Repici, A. New artificial intelligence system: First validation study versus experienced endoscopists for colorectal polyp detection. Gut 2020, 69, 799–800. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jeong, J.; Song, E.M.; Ha, C.; Lee, H.J.; Koo, J.E.; Yang, D.H.; Kim, N.; Byeon, J.S. Real-time detection of colon polyps during colonoscopy using deep learning: Systematic validation with four independent datasets. Sci. Rep. 2020, 10, 8379. [Google Scholar] [CrossRef]
- Podlasek, J.; Heesch, M.; Podlasek, R.; Kilisinski, W.; Filip, R. Real-time deep learning-based colorectal polyp localization on clinical video footage achievable with a wide array of hardware configurations. Endosc. Int. Open 2021, 9, E741–E748. [Google Scholar] [CrossRef]
- Fernandez-Esparrach, G.; Bernal, J.; Lopez-Ceron, M.; Cordova, H.; Sanchez-Montes, C.; Rodriguez de Miguel, C.; Sanchez, F.J. Exploring the clinical potential of an automatic colonic polyp detection method based on the creation of energy maps. Endoscopy 2016, 48, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Younghak, S.; Balasingham, I. Comparison of hand-craft feature based SVM and CNN based deep learning framework for automatic polyp classification. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 8037556. [Google Scholar] [CrossRef]
- Wang, P.; Xiao, X.; Glissen Brown, J.R.; Berzin, T.M.; Tu, M.; Xiong, F.; Hu, X.; Liu, P.; Song, Y.; Zhang, D.; et al. Development and validation of a deep-learning algorithm for the detection of polyps during colonoscopy. Nat. Biomed. Eng. 2018, 2, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Peralta, L.F.; Bote-Curiel, L.; Picon, A.; Sanchez-Margallo, F.M.; Pagador, J.B. Deep learning to find colorectal polyps in colonoscopy: A systematic literature review. Artif. Intell. Med. 2020, 108, 101923. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Thosani, N.; Konda, V.; Wallace, M.B.; Rex, D.K.; Chauhan, S.S.; Hwang, J.H.; Komanduri, S.; Manfredi, M.; Maple, J.T.; et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest. Endosc. 2015, 81, 502.e1–502.e16. [Google Scholar] [CrossRef]
- Mori, Y.; Kudo, S.E.; Misawa, M.; Mori, K. Simultaneous detection and characterization of diminutive polyps with the use of artificial intelligence during colonoscopy. VideoGIE 2019, 4, 7–10. [Google Scholar] [CrossRef]
- Ozawa, T.; Ishihara, S.; Fujishiro, M.; Kumagai, Y.; Shichijo, S.; Tada, T. Automated endoscopic detection and classification of colorectal polyps using convolutional neural networks. Therap. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef]
- Lavine, R.A.; Sibert, J.L.; Gokturk, M.; Dickens, B. Eye-tracking measures and human performance in a vigilance task. Aviat. Space Environ. Med. 2002, 73, 367–372. [Google Scholar]
- Warm, J.S.; Parasuraman, R.; Matthews, G. Vigilance requires hard mental work and is stressful. Hum. Factors 2008, 50, 433–441. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, R.; Xu, Q.; Yuan, W. Mobile Phone Use in a Car-Following Situation: Impact on Time Headway and Effectiveness of Driver’s Rear-End Risk Compensation Behavior via a Driving Simulator Study. Int. J. Environ. Res. Public Health 2020, 17, 1328. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, V.; Alagappan, M.; Gonzalez, A.; Gupta, K.; Brown, J.R.G.; Cohen, J.; Sawhney, M.; Pleskow, D.; Berzin, T.M. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: A survey of US gastroenterologists. Endosc. Int. Open 2020, 8, E1379–E1384. [Google Scholar] [CrossRef] [PubMed]
- Chaptini, L.A.; Janec, E.M.; Seltzer, G.; Peikin, S.; Elfant, A.B. Sublingual hyoscyamine spray as premedication for colonoscopy: A randomized double-blinded placebo-controlled trial. Am. J. Surg. 2008, 196, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kutyla, M.; O’Connor, S.; Gurusamy, S.R.; Gururatsakul, M.; Gould, K.; Whaley, A.; Kendall, B.J.; Hourigan, L.; Holtmann, G.J. Influence of Simethicone Added to the Rinse Water during Colonoscopies on Polyp Detection Rates: Results of an Unintended Cohort Study. Digestion 2018, 98, 217–221. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, D.; Wang, J.; Wu, J.; Lei, P.; Luo, Q.; Wang, L.; Zhang, B.; Wang, H.; Cui, Y.; et al. Simethicone improves bowel cleansing with low-volume polyethylene glycol: A multicenter randomized trial. Endoscopy 2018, 50, 412–422. [Google Scholar] [CrossRef]
- Hwang, J.H.; Jamidar, P.; Kyanam Kabir Baig, K.R.; Leung, F.W.; Lightdale, J.R.; Maranki, J.L.; Okolo, P.I., 3rd; Swanstrom, L.L.; Chak, A. GIE Editorial Board top 10 topics: Advances in GI endoscopy in 2019. Gastrointest. Endosc. 2020, 92, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, S.; Falt, P.; Rondonotti, E.; Radaelli, F.; Fojtik, P.; Gallittu, P.; Liggi, M.; Amato, A.; Paggi, S.; Smajstrla, V.; et al. Water exchange for screening colonoscopy increases adenoma detection rate: A multicenter, double-blinded, randomized controlled trial. Endoscopy 2017, 49, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Tseng, C.W.; Hu, C.T.; Koo, M.; Leung, F.W. Prospective multicenter randomized controlled trial comparing adenoma detection rate in colonoscopy using water exchange, water immersion, and air insufflation. Gastrointest. Endosc. 2017, 86, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Pan, Y.; Guo, X.; Zhao, L.; Wang, X.; Zhang, L.; Dong, T.; Luo, H.; Ge, Z.; Liu, J.; et al. Water Exchange Method Significantly Improves Adenoma Detection Rate: A Multicenter, Randomized Controlled Trial. Am. J. Gastroenterol. 2017, 112, 568–576. [Google Scholar] [CrossRef]
- Fuccio, L.; Frazzoni, L.; Hassan, C.; La Marca, M.; Paci, V.; Smania, V.; De Bortoli, N.; Bazzoli, F.; Repici, A.; Rex, D.; et al. Water exchange colonoscopy increases adenoma detection rate: A systematic review with network meta-analysis of randomized controlled studies. Gastrointest. Endosc. 2018, 88, 589–597.e11. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, S.; Ishaq, S.; Hassan, C.; Falt, P.; Fuccio, L.; Siau, K.; Leung, J.W.; Anderson, J.; Binmoeller, K.F.; Radaelli, F.; et al. Water-assisted colonoscopy: An international modified Delphi review on definitions and practice recommendations. Gastrointest. Endosc. 2020, 93, 1411–1420.e18. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Koo, M.; Tseng, C.W.; Yang, H.W.; Leung, F.W. Reduction of multitasking distractions underlies the higher adenoma detection rate of water exchange compared to air insufflation—Blinded analysis of withdrawal phase videos. United Eur. Gastroenterol. J. 2019, 7, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Kuo, Y.L.; Hsieh, Y.H.; Tang, J.H.; Leung, F.W. Comparison of Right Colon Adenoma Miss Rates Between Water Exchange and Carbon Dioxide Insufflation: A Prospective Randomized Controlled Trial. J. Clin. Gastroenterol. 2020. ahead of print. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).