The Association of Lumbar Disc Herniation with Lumbar Volumetric Bone Mineral Density in a Cross-Sectional Chinese Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Baseline Data Collection
2.3. Lumbar Vertebra Scanning by QCT
2.4. Lumbar Vertebral Trabecular Volumetric Bone Mineral Density (Trab.vBMD) Measurement
2.5. Lumbar Scanning by MRI
2.6. Definition of Lumbar Intervertebral Disc Herniation
2.7. Statistical Analysis
3. Results
3.1. Characteristics of Subjects
3.2. Stratification Analysis Relationship of LDH with Characteristics Data and Lumbar Trab.vBMD
3.3. Stratification Analysis after Covariate Adjusting
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | Bone mineral density |
| Trab.vBMD | Lumbar vertebral trabecular volumetric BMD |
| LBP | Low back pain |
| LDH | Lumbar intervertebral disc herniation |
| QCT | Quantitative computed tomography |
| MRI | Magnetic resonance images |
| DXA | Dual-energy X-ray absorptiometry |
| BMI | Body mass index |
| TSE | Turbo spin-echo |
| FOV | Field of view |
| HIZ | High-intensity zone |
| ANOVA | Variance analysis |
| LDD | Lumbar intervertebral disc degeneration |
| SN | Schmorl’s node |
References
- Heikkinen, J.; Honkanen, R.; Williams, L.; Leung, J.; Rauma, P.; Quirk, S.; Koivumaa-Honkanen, H. Depressive disorders, anxiety disorders and subjective mental health in common musculoskeletal diseases: A review. Maturitas 2019, 127, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D. Acupuncture for chronic low back pain. N. Engl. J. Med. 2010, 363, 1777–1778. [Google Scholar] [CrossRef]
- Deyo, R.A.; Mirza, S.K. CLINICAL PRACTICE. Herniated Lumbar Intervertebral Disk. N. Engl. J. Med. 2016, 374, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- el Barzouhi, A.; Verwoerd, A.J.H.; Peul, W.C.; Verhagen, A.P.; Lycklama à Nijeholt, G.J.; Van der Kallen, B.F.; Koes, B.W.; Vleggeert-Lankamp, C.L.A.M. Prognostic value of magnetic resonance imaging findings in patients with sciatica. J. Neurosurg. Spine 2016, 24, 978–985. [Google Scholar] [CrossRef]
- Del Grande, F.; Maus, T.P.; Carrino, J.A. Imaging the intervertebral disk: Age-related changes, herniations, and radicular pain. Radiol. Clin. N. Am. 2012, 50, 629–649. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.H.; Zafonte, R.D. Sciatica. N. Engl. J. Med. 2015, 372, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Li, N.; Li, K.; Li, X.Y.; Zhang, P.; Xuan, Y.J.; Cheng, X.G. Discordance in diagnosis of osteoporosis by quantitative computed tomography and dual-energy X-ray absorptiometry in Chinese elderly men. J. Orthop Transl. 2019, 18, 59–64. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Curtis, E.M.; Ward, K.A.; Harvey, N.C.; Dennison, E.M.; Cooper, C. Fracture prediction, imaging and screening in osteoporosis. Nat. Rev. Endocrinol. 2019, 15, 535–547. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Lee, S.K.; Moon, B.J.; Lee, J.K. Traumatic lumbar disc herniation mimicking epidural hematoma: A case report and literature review. Medicine 2019, 98, e15438. [Google Scholar] [CrossRef]
- Simpson, A.; Biswas, D.; Emerson, J.; Lawrence, B.; Grauer, J. Quantifying the effects of age, gender, degeneration, and adjacent level degeneration on cervical spine range of motion using multivariate analyses. Spine 2008, 33, 183–186. [Google Scholar] [CrossRef]
- Ravindra, V.M.; Senglaub, S.S.; Rattani, A.; Dewan, M.C.; Hartl, R.; Bisson, E.; Park, K.B.; Shrime, M.G. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Global Spine J. 2018, 8, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, L.; Zhao, Y.; Su, Y.; Sun, W.; Chen, S.; Liu, Y.; Yang, M.; Yu, A.; Guglielmi, G.; et al. Muscle Density, but Not Size, Correlates Well With Muscle Strength and Physical Performance. J. Am. Med. Dir. Assoc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, J.; Duanmu, Y.; Zhang, C.; Zhao, W.; Wang, L.; Cheng, X.; Veronese, N.; Cafarelli, F.P.; Guglielmi, G. Quantitative analysis of modified functional muscle-bone unit and back muscle density in patients with lumbar vertebral fracture in Chinese elderly men: A case-control study. Aging Clin. Exp. Res. 2019, 31, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Xu, L.; Cheng, X.; Ma, Y.; Liu, D.; Guo, Z.; Su, Y.; Wang, Q. Relation of visceral and subcutaneous adipose tissue to bone mineral density in chinese women. Int. J. Endocrinol. 2013, 2013, 378632. [Google Scholar] [CrossRef]
- Cheng, X.; Yuan, H.; Cheng, J.; Weng, X.; Xu, H.; Gao, J.; Huang, M.; Wang, Y.X.J.; Wu, Y.; Xu, W.; et al. Chinese expert consensus on the diagnosis of osteoporosis by imaging and bone mineral density. Quant. Imaging Med. Surg. 2020, 10, 2066–2077. [Google Scholar] [CrossRef]
- Dachena, C.; Casu, S.; Fanti, A.; Lodi, M.B.; Mazzarella, G. Combined Use of MRI, fMRIand Cognitive Data for Alzheimer’s Disease: Preliminary Results. Appl. Sci. 2019, 9, 3156. [Google Scholar] [CrossRef]
- Li, Y.; Fredrickson, V.; Resnick, D.K. How should we grade lumbar disc herniation and nerve root compression? A systematic review. Clin. Orthop. Relat. Res. 2015, 473, 1896–1902. [Google Scholar] [CrossRef]
- Fardon, D. Nomenclature and classification of lumbar disc pathology. Spine 2001, 26, 461–462. [Google Scholar] [CrossRef]
- Pfirrmann, C.; Dora, C.; Schmid, M.; Zanetti, M.; Hodler, J.; Boos, N. MR image-based grading of lumbar nerve root compromise due to disk herniation: Reliability study with surgical correlation. Radiology 2004, 230, 583–588. [Google Scholar] [CrossRef]
- Haughton, V. Medical imaging of intervertebral disc degeneration: Current status of imaging. Spine 2004, 29, 2751–2756. [Google Scholar] [CrossRef]
- Huang, D.Y.; Shen, Y.J.; Wang, F.; Li, F.; Fang, Z.; Liu, J. Correlative analysis of degenerative lumbar scoliosis and osteoporosis. Zhongguo Gu Shang 2019, 32, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zeng, Y.; Chen, Z.; Li, W.; Zhang, X.; Mai, S. Degenerative lumbar scoliosis patients with proximal junctional kyphosis have lower muscularity, fatty degeneration at the lumbar area. Eur. Spine J. 2020. [Google Scholar] [CrossRef]
- Hackinger, S.; Trajanoska, K.; Styrkarsdottir, U.; Zengini, E.; Steinberg, J.; Ritchie, G.R.S.; Hatzikotoulas, K.; Gilly, A.; Evangelou, E.; Kemp, J.P.; et al. Evaluation of shared genetic aetiology between osteoarthritis and bone mineral density identifies SMAD3 as a novel osteoarthritis risk locus. Hum. Mol. Genet. 2017, 26, 3850–3858. [Google Scholar] [CrossRef]
- Kasher, M.; Williams, F.M.K.; Freidin, M.B.; Cherny, S.; Livshits, G. An in-depth study of the associations between osteoarthritis- and osteoporosis-related phenotypes at different skeletal locations. Osteoporos. Int. 2020, 31, 2197–2208. [Google Scholar] [CrossRef]
- Naylor, A. The biophysical and biochemical aspects of intervertebral disc herniation and degeneration. Ann. R. Coll. Surg. Engl. 1962, 31, 91–114. [Google Scholar]
- Harada, Y.; Nakahara, S. A pathologic study of lumbar disc herniation in the elderly. Spine 1989, 14, 1020–1024. [Google Scholar] [CrossRef]
- Yukawa, Y.; Kato, F.; Matsubara, Y.; Kajino, G.; Nakamura, S.; Nitta, H. Serial magnetic resonance imaging follow-up study of lumbar disc herniation conservatively treated for average 30 months: Relation between reduction of herniation and degeneration of disc. J. Spinal Disord. 1996, 9, 251–256. [Google Scholar] [CrossRef]
- Kang, J.D.; Stefanovic-Racic, M.; McIntyre, L.A.; Georgescu, H.I.; Evans, C.H. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine 1997, 22, 1065–1073. [Google Scholar] [CrossRef]
- Kos, N.; Gradisnik, L.; Velnar, T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med. Arch. 2019, 73, 421–424. [Google Scholar] [CrossRef]
- Yang, Z.; Griffith, J.; Leung, P.; Lee, R. Effect of osteoporosis on morphology and mobility of the lumbar spine. Spine 2009, 34, E115–E121. [Google Scholar] [CrossRef]
- Gruber, H.; Gordon, B.; Williams, C.; James Norton, H.; Hanley, E. Bone mineral density of lumbar vertebral end plates in the aging male sand rat spine. Spine 2003, 28, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Haughton, V. Imaging intervertebral disc degeneration. J. Bone Jt. Surg. Am. 2006, 15–20. [Google Scholar] [CrossRef]
- Janssen, M.; Bertrand, S.; Joe, C.; Levine, M. Lumbar herniated disk disease: Comparison of MRI, myelography, and post-myelographic CT scan with surgical findings. Orthopedics 1994, 17, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Jarvik, J.; Deyo, R. Diagnostic evaluation of low back pain with emphasis on imaging. Ann. Intern. Med. 2002, 137, 586–597. [Google Scholar] [CrossRef]
- Gungor, O.; Gezer, N.S.; Ozdamarlar, U.; Balci, A. The effect of bone mineral density on development of Schmorl’s nodes in young patients. Acta Orthop. Traumatol. Turc. 2020, 54, 287–292. [Google Scholar] [CrossRef]
- Atalay, A.; Kozakcioglu, M.; Cubuk, R.; Tasali, N.; Guney, S. Degeneration of the lumbar spine and dual-energy X-ray absorptiometry measurements in patients without osteoporosis. Clin. Imaging 2009, 33, 374–378. [Google Scholar] [CrossRef]
- Castano-Betancourt, M.C.; Oei, L.; Rivadeneira, F.; de Schepper, E.I.; Hofman, A.; Bierma-Zeinstra, S.; Pols, H.A.; Uitterlinden, A.G.; Van Meurs, J.B. Association of lumbar disc degeneration with osteoporotic fractures; the Rotterdam study and meta-analysis from systematic review. Bone 2013, 57, 284–289. [Google Scholar] [CrossRef]
- Homminga, J.; Aquarius, R.; Bulsink, V.E.; Jansen, C.T.J.; Verdonschot, N. Can vertebral density changes be explained by intervertebral disc degeneration? Med. Eng. Phys. 2012, 34, 453–458. [Google Scholar] [CrossRef]
- Keser, N.; Atici, A.; Celikoglu, E.; Akpinar, P.; Ramazanoglu, A.F.; Aktas, I. Effect of bone mineral density on lumbar discs in young adults: A case-control study. Medicine 2017, 96, e7906. [Google Scholar] [CrossRef]
- Wang, L.; Su, Y.; Wang, Q.; Duanmu, Y.; Yang, M.; Yi, C.; Cheng, X. Validation of asynchronous quantitative bone densitometry of the spine: Accuracy, short-term reproducibility, and a comparison with conventional quantitative computed tomography. Sci. Rep. 2017, 7, 6284. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kang, D.; Lee, I.; Kim, S.Y. Differences in the Incidence of Symptomatic Cervical and Lumbar Disc Herniation According to Age, Sex and National Health Insurance Eligibility: A Pilot Study on the Disease’s Association with Work. Int. J. Environ. Res. Public Health 2018, 15, 2094. [Google Scholar] [CrossRef]
- Fei, H.; Li, W.S.; Sun, Z.R.; Ma, Q.W.; Chen, Z.Q. Analysis of Spino-pelvic Sagittal Alignment in Young Chinese Patients with Lumbar Disc Herniation. Orthop. Surg. 2017, 9, 271–276. [Google Scholar] [CrossRef]
- Wang, Y.X.; Griffith, J.F.; Ma, H.T.; Kwok, A.W.; Leung, J.C.; Yeung, D.K.; Ahuja, A.T.; Leung, P.C. Relationship between gender, bone mineral density, and disc degeneration in the lumbar spine: A study in elderly subjects using an eight-level MRI-based disc degeneration grading system. Osteoporos. Int. 2011, 22, 91–96. [Google Scholar] [CrossRef]
- Schwarz-Nemec, U.; Friedrich, K.M.; Prayer, D.; Trattnig, S.; Schwarz, F.K.; Weber, M.; Bettelheim, D.; Grohs, J.G.; Nemec, S.F. Lumbar Intervertebral Disc Degeneration as a Common Incidental Finding in Young Pregnant Women as Observed on Prenatal Magnetic Resonance Imaging. J. Womens Health 2020, 29, 713–720. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, F.; Ma, J.; Ding, W. Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen. Ageing Res. Rev. 2020, 57, 100978. [Google Scholar] [CrossRef]
- Olmarker, K.; Rydevik, B.; Nordborg, C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine 1993, 18, 1425–1432. [Google Scholar] [CrossRef]
- Shambrook, J.; McNee, P.; Harris, E.C.; Kim, M.; Sampson, M.; Palmer, K.T.; Coggon, D. Clinical presentation of low back pain and association with risk factors according to findings on magnetic resonance imaging. Pain 2011, 152, 1659–1665. [Google Scholar] [CrossRef]
- Ghosh, S.; Alomari, R.S.; Chaudhary, V.; Dhillon, G. Composite features for automatic diagnosis of intervertebral disc herniation from lumbar MRI. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar] [CrossRef]
- Karssemeijer, N.; Alomari, R.S.; Summers, R.M.; Corso, J.J.; Chaudhary, V.; Dhillon, G. Automatic diagnosis of lumbar disc herniation with shape and appearance features from MRI. In Proceedings of the Medical Imaging 2010: Computer-Aided Diagnosis, San Diego, CA, USA, 9 March 2010. [Google Scholar]
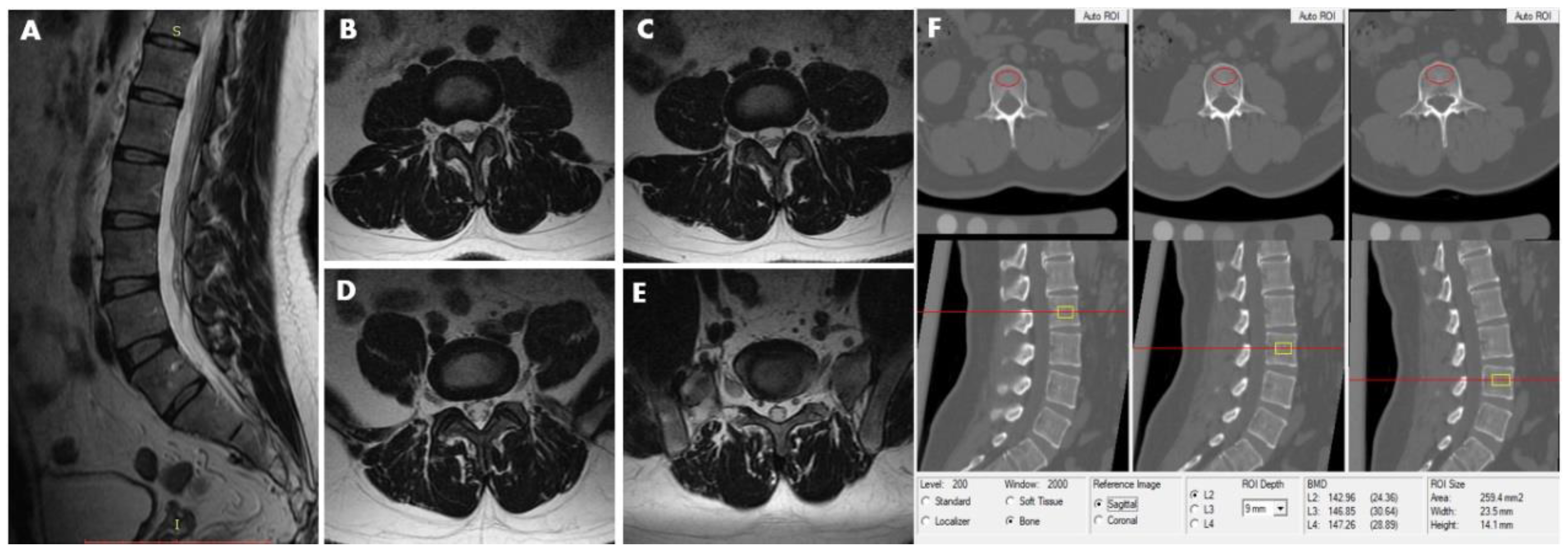
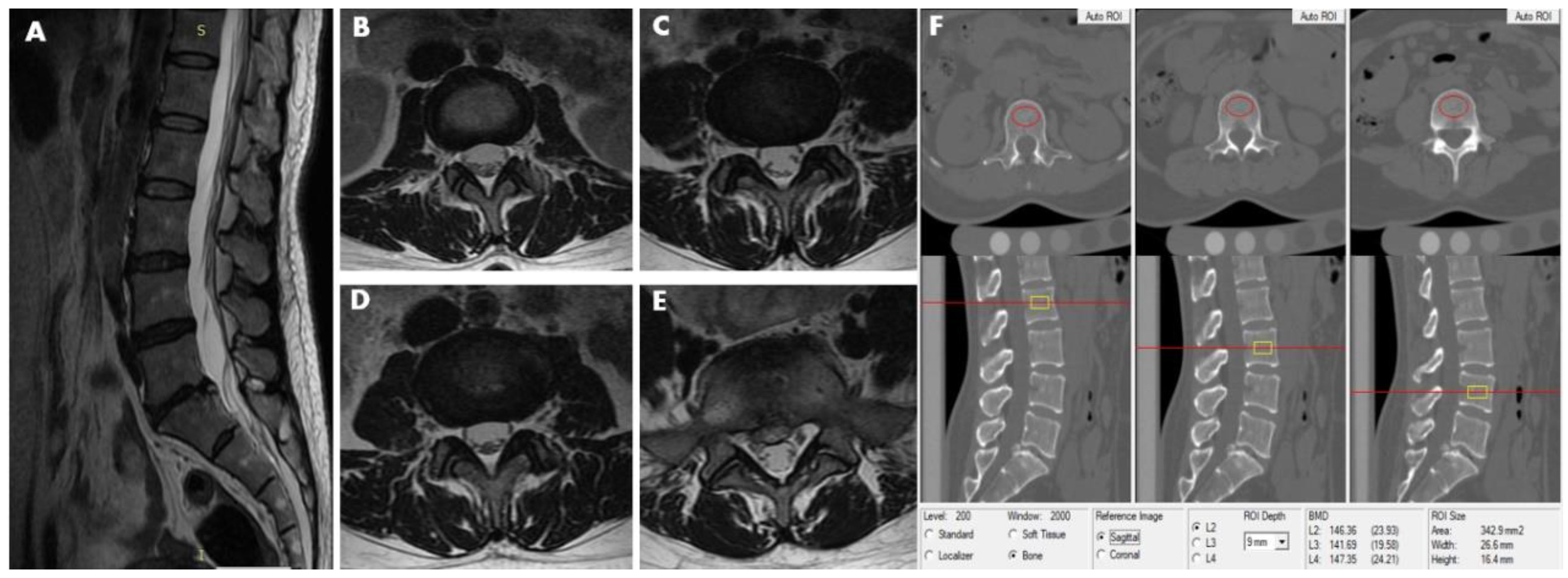

| Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD or Median Value | Mean ± SD or Median Value | ||||||||||
| Parameters | total | Non-LDH | LDH | P1 | P2 | total | Non-LDH | LDH | P1 | P2 | P3 |
| Sample size | 322 | 222 (68.9%) | 100 (31.1%) | 423 | 297 (70.2%) | 126 (29.8%) | |||||
| Age (years) | 39 | 39 | 41 | 0.02 | <0.001 | 40 | 38 | 43 | <0.001 | <0.001 | 0.558 |
| Height (cm) | 172.2 ± 5.9 | 172.0 ± 6.2 | 172.8 ± 5.3 | 0.265 | 160.5 ± 5.6 | 160.4 ± 5.7 | 160.7 ± 5.2 | 0.59 | <0.001 | ||
| Weight (kg) | 77 | 76 | 78 | 0.017 | 59.5 | 59 | 61 | 0.002 | <0.001 | ||
| BMI (kg/m2) | 25.9 | 25.6 | 26.4 | 0.027 | 0.093 | 23.2 | 22.9 | 24.0 | 0.003 | <0.001 | <0.001 |
| Waistline (cm) | 90 | 90 | 92 | 0.034 | 0.145 | 79 | 77.5 | 81 | 0.000 | 0.005 | <0.001 |
| Hipline (cm) | 101 | 100 | 101.25 | 0.015 | 0.235 | 95 | 95 | 97 | 0.008 | 0.014 | <0.001 |
| Lumbar BMD (mg/cm3) | 148.0 ± 31.1 | 149.6 ± 31.3 | 144.6 ± 30.6 | 0.188 | 163.8 ± 35.4 | 166.1 ± 33.7 | 158.5 ± 38.6 | 0.059 | <0.001 | ||
| Adjusted vBMD (mg/cm3) | 148.0 (144.4 to 151.6) | 148.0 (142.6 to 153.4) | 0.997 | 163.8 (160.4 to 167.2) | 163.9 (158.5 to 169.2) | 0.986 | |||||
| Age Group 1 (20–39) | p0 | p1 ≤ 0.05 for within Group | p2 for Covariance Analysis | Age Group 2 (40–60) | p0 | p1 ≤ 0.05 for within Group | p2 for Covariance Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-LDH | 1 Segment | ≥2 Segments | Total | Non-LDH | 1 Segment | ≥2 Segments | Total | |||||||
| Sample size Rate (%) | 123 38.2% | 32 9.9% | 9 2.8% | 164 50.9% | 99 30.7% | 35 10.9% | 24 7.5% | 158 49.1% | ||||||
| Age | 33 | 31.5 | 32 | 33 | 0.638 † | - | 0.216 | 45 | 45 | 48 | 45 | 0.309 | - | <0.001 |
| Height (cm) | 172.0 ± 6.5 | 174.4 ± 5.1 | 174.1 ± 4.8 | 172.6 ± 6.2 | 0.114 ‡ | - | - | 171.9 ± 5.8 | 171.7 ± 5.0 | 171.6 ± 5.8 | 171.8± 5.6 | 0.961 | - | - |
| Weight (kg) | 75 | 79 | 81 | 77 | 0.017 † | 0.005 b | - | 78 | 75 | 76.5 | 77 | 0.753 | - | - |
| BMI (kg/m2) | 25.2 | 26.6 | 26.2 | 25.5 | 0.078 † | - | 0.004 | 26.0 | 25.6 | 27.0 | 26.0 | 0.546 | - | 0.473 |
| Waistline (cm) | 89 | 91.5 | 88 | 89.75 | 0.088 † | - | 0.038 | 91 | 92 | 92.5 | 92 | 0.770 | - | 0.785 |
| Hipline (cm) | 100 | 101 | 103 | 101 | 0.031 † | 0.001 b | 0.135 | 100 | 101 | 100.5 | 100.5 | 0.399 | - | 0.523 |
| L vBMD (mg/cm3) | 161.2 ± 26.2 | 167.0 ± 27.3 | 143.5 ± 12.1 | 161.4 ± 26.2 | 0.057 ‡ | 0.045 c | - | 135.1 ± 31.2 | 140.1 ± 28.2 | 121.8 ± 23.1 | 134.2 ± 29.8 | 0.061 | - | - |
| Herniation segments | 0.052 | 0.09 | ||||||||||||
| Age Group 1 (20–39) | p0 | p1 ≤ 0.05 for within Group | p2 for Covariance Analysis | Age Group 2 (40–60) | p0 | p1 ≤ 0.05 for within Group | p2 for Covariance Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-LDH | 1-Level | ≥2-Levels | Total | Non-LDH | 1-Level | ≥2-Levels | Total | |||||||
| Sample size Rate (%) | 158 37.4% | 38 9.0% | 8 1.9% | 204 48.3% | 139 32.9% | 49 11.6% | 31 7.3% | 220 51.7% | ||||||
| Age | 32 | 32 | 30.5 | 32 | 0.675 † | - | 0.293 | 45 | 46 | 50 | 46 | 0.027 † | 0.007 b | <0.001 |
| Height (cm) | 161.0 ± 5.8 | 161.9 ± 5.0 | 161.1 ± 3.0 | 161.2 ± 5.6 | 0.654 ‡ | - | 159.7 ± 5.6 | 160.2 ± 5.7 | 159.8 ± 5.1 | 159.8 ± 5.5 | 0.825 ‡ | - | ||
| Weight (kg) | 58 | 59 | 69 | 58 | 0.018 † | 0.004 b | 60 | 61 | 64 | 60.5 | 0.053 † | - | ||
| BMI (kg/m2) | 22.6 | 22.3 | 27.1 | 22.6 | 0.040 † | 0.015 b 0.013 c | <0.001 | 23.4 | 24.3 | 25.0 | 23.8 | 0.036 † | 0.031 b | 0.012 |
| Waistline (cm) | 76 | 77.5 | 84.5 | 76 | 0.018 † | 0.006 b 0.028 c | 0.069 | 80 | 83 | 84 | 81 | 0.041 † | 0.030 b | 0.020 |
| Hipline (cm) | 95 | 94 | 101.5 | 95 | 0.211 † | 0.010 b | 0.001 | 96 | 98 | 99 | 97 | 0.039 † | 0.042 b | 0.876 |
| BMD (mg/cm3) | 177.9 ± 28.3 | 180.8 ± 34.5 | 172.9 ± 23.2 | 178.3 ± 29.3 | 0.753 ‡ | - | 152.6 ± 34.3 | 148.8 ± 37.5 | 143.0 ± 35.8 | 150.4 ± 35.3 | 0.369 ‡ | 0.359 | ||
| Herniation segments | 0.496 | 0.965 | ||||||||||||
| Gender | Age Group | LDH Levels | Adjusted Mean Value (SD) | 95% Confidence Interval | Paired Comparison, p > 0.05 | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Limit | Limit | |||||
| Men | 1 | 0 | 160.8 (2.3) | 156.2 | 165.2 | ≥0.05 (0.052–0.433) * |
| 1 | 168.3 (4.6) | 159.3 | 177.4 | |||
| ≥2 | 145.3 (8.4) | 128.7 | 162.0 | |||
| 2 | 0 | 134.1 (2.8) | 128.6 | 139.6 | ≥0.05 (0.086–0.649) * | |
| 1 | 140.9 (4.7) | 131.6 | 150.2 | |||
| ≥2 | 124.6 (5.7) | 113.3 | 135.8 | |||
| Women | 1 | 0 | 177.6 (2.3) | 173.1 | 182.0 | ≥0.05 (0.961–1) * |
| 1 | 182.5 (4.6) | 173.4 | 191.7 | |||
| ≥2 | 171.3 (10.3) | 150.9 | 191.6 | |||
| 2 | 0 | 150.7 (2.5) | 145.8 | 155.7 | ≥0.05 (1.0) * | |
| 1 | 149.4 (4.2) | 141.1 | 157.7 | |||
| ≥2 | 150.3 (5.4) | 139.8 | 160.9 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, J.; Wang, L.; Li, Q.; Huang, P.; Liu, Y.; Blake, G.M.; Tian, W.; Cheng, X. The Association of Lumbar Disc Herniation with Lumbar Volumetric Bone Mineral Density in a Cross-Sectional Chinese Study. Diagnostics 2021, 11, 938. https://doi.org/10.3390/diagnostics11060938
Geng J, Wang L, Li Q, Huang P, Liu Y, Blake GM, Tian W, Cheng X. The Association of Lumbar Disc Herniation with Lumbar Volumetric Bone Mineral Density in a Cross-Sectional Chinese Study. Diagnostics. 2021; 11(6):938. https://doi.org/10.3390/diagnostics11060938
Chicago/Turabian StyleGeng, Jian, Ling Wang, Qing Li, Pengju Huang, Yandong Liu, Glen M. Blake, Wei Tian, and Xiaoguang Cheng. 2021. "The Association of Lumbar Disc Herniation with Lumbar Volumetric Bone Mineral Density in a Cross-Sectional Chinese Study" Diagnostics 11, no. 6: 938. https://doi.org/10.3390/diagnostics11060938
APA StyleGeng, J., Wang, L., Li, Q., Huang, P., Liu, Y., Blake, G. M., Tian, W., & Cheng, X. (2021). The Association of Lumbar Disc Herniation with Lumbar Volumetric Bone Mineral Density in a Cross-Sectional Chinese Study. Diagnostics, 11(6), 938. https://doi.org/10.3390/diagnostics11060938







