Results of a Prospective Trial to Compare 68Ga-DOTA-TATE with SiPM-Based PET/CT vs. Conventional PET/CT in Patients with Neuroendocrine Tumors
Abstract
:1. Introduction
2. Materials and Methods
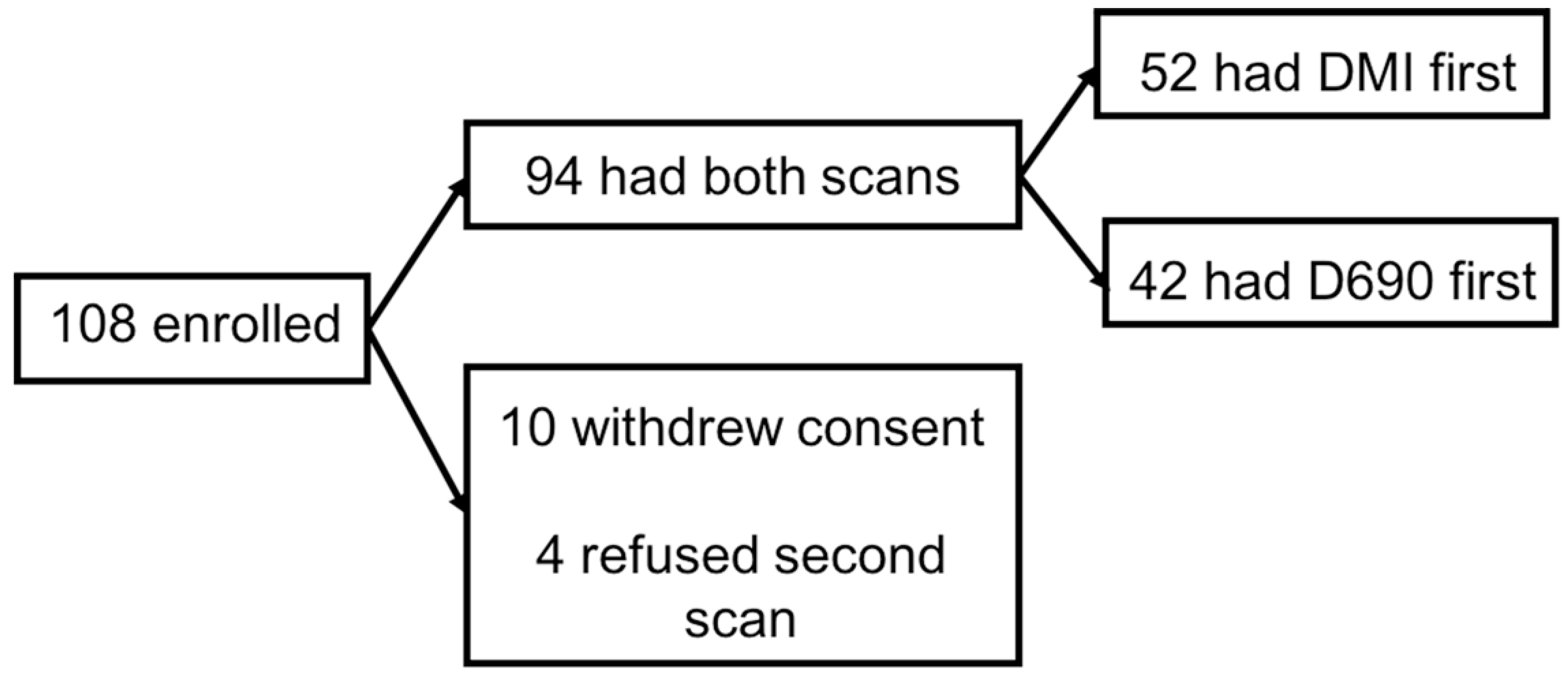
2.1. Participants
2.2. D690 PET/CT Protocol
2.3. DMI PET/CT Protocol
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and PET Findings for the Entire Cohort
3.2. Results When SiPM PET/CT Was Used First Followed by Conventional PET/CT
3.3. Results When Conventional PET/CT Was Used First Followed by SiPM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H.; Colletti, P.M.; Delgado-Bolton, R.; Esposito, G.; Krause, B.J.; Iagaru, A.H.; Nadel, H.; Quinn, D.I.; Rohren, E.; Subramaniam, R.M.; et al. Appropriate Use Criteria for (18)F-FDG PET/CT in Restaging and Treatment Response Assessment of Malignant Disease. J. Nucl. Med. 2017, 58, 2026–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; De Cristoforo, C.; et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Spanoudaki, V.; Levin, C.S. Photo-detectors for time of flight positron emission tomography (ToF-PET). Sensors 2010, 10, 10484–10505. [Google Scholar] [CrossRef] [PubMed]
- Van der Vos, C.S.; Koopman, D.; Rijnsdorp, S.; Arends, A.J.; Boellaard, R.; van Dalen, J.A.; Lubberink, M.; Willemsen, A.T.M.; Visser, E.P. Quantification, improvement, and harmonization of small lesion detection with state-of-the-art PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S.; Maramraju, S.H.; Khalighi, M.M.; Deller, T.W.; Delso, G.; Jansen, F. Design Features and Mutual Compatibility Studies of the Time-of-Flight PET Capable GE SIGNA PET/MR System. IEEE Trans. Med. Imaging 2016, 35, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.F.C.; Ilan, E.; Peterson, W.T.; Uribe, J.; Lubberink, M.; Levin, C.S. Studies of a Next-Generation Silicon-Photomultiplier-Based Time-of-Flight PET/CT System. J. Nucl. Med. 2017, 58, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.; Park, S.Y.; Hatami, N.; Davidzon, G.; Srinivas, S.; Gambhir, S.S.; Iagaru, A. 18F-FDG silicon photomultiplier PET/CT: A pilot study comparing semi-quantitative measurements with standard PET/CT. PLoS ONE 2017, 12, e0178936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lantos, J.; Mittra, E.S.; Levin, C.S.; Iagaru, A. Standard OSEM vs. regularized PET image reconstruction: Qualitative and quantitative comparison using phantom data and various clinical radiopharmaceuticals. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 110–118. [Google Scholar] [PubMed]
- Kitajima, K.; Suzuki, K.; Nakamoto, Y.; Onishi, Y.; Sakamoto, S.; Senda, M.; Kita, M.; Sugimura, K. Low-dose non-enhanced CT versus full-dose contrast-enhanced CT in integrated PET/CT studies for the diagnosis of uterine cancer recurrence. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Ocampo, F.; Lopez-Mora, D.A.; Flotats, A.; Paillahueque, G.; Camacho, V.; Duch, J.; Fernandez, A.; Domenech, A.; Estorch, M.; Carrio, I. Digital vs. analog PET/CT: Intra-subject comparison of the SUVmax in target lesions and reference regions. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mora, D.A.; Flotats, A.; Fuentes-Ocampo, F.; Camacho, V.; Fernandez, A.; Ruiz, A.; Duch, J.; Sizova, M.; Domenech, A.; Estorch, M.; et al. Comparison of image quality and lesion detection between digital and analog PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Torabi, M.; Aquino, S.L.; Harisinghani, M.G. Current concepts in lymph node imaging. J. Nucl. Med. 2004, 45, 1509–1518. [Google Scholar] [PubMed]
- Baratto, L.; Duan, H.; Ferri, V.; Khalighi, M.; Iagaru, A. The Effect of Various beta Values on Image Quality and Semiquantitative Measurements in 68Ga-RM2 and 68Ga-PSMA-11 PET/MRI Images Reconstructed with a Block Sequential Regularized Expectation Maximization Algorithm. Clin. Nucl. Med. 2020, 45, 506–513. [Google Scholar] [CrossRef] [PubMed]

| Organs | SiPM PET/CT SUVmean (Mean ± SD) | Conventional PET/CT SUVmean (Mean ± SD) | Difference of SUVmean (mean ± SD) | 95% CI | ICC |
|---|---|---|---|---|---|
| Pituitary | 7.87 ± 3.91 | 5.80 ± 3.13 | 2.07 ± 2.03 | 0.075–0.823 | 0.379 |
| Parotid | 2.22 ± 1.30 | 2.18 ± 1.32 | 0.04 ± 0.38 | 0.000–0.990 | 0.010 |
| Aortic arch | 1.05 ± 0.37 | 1.07 ± 0.45 | −0.02 ± 0.40 | 0.548–0.977 | 0.878 |
| Lung | 0.46 ± 0.41 | 0.47 ± 0.42 | −0.012 ± 0.15 | 0.899–0.997 | 0.982 |
| Liver | 4.70 ± 2.26 | 5.17 ± 2.64 | −0.47 ± 1.17 | 0.753–0.956 | 0.891 |
| Spleen | 17.14 ± 6.63 | 17.64 ± 7.54 | −0.50 ± 2.85 | 0.851–0.941 | 0.905 |
| Adrenals | 9.52 ± 4.88 | 9.24 ± 4.90 | 0.27 ± 2.46 | 0.828–0.912 | 0.876 |
| Gluteal muscle | 0.58 ± 0.18 | 0.60 ± 0.27 | −0.01 ± 0.21 | 0.805–0.995 | 0.966 |
| Gluteal fat | 0.35 ± 0.14 | 0.35 ± 0.19 | 0.005 ± 1.10 | 0.948–0.999 | 0.992 |
| Organs | SiPM (Mean ± SD) | Conventional PET (Mean ± SD) | p Value | n |
|---|---|---|---|---|
| All | 28.7 ± 19.6 (range: 3.2–118.8) | 27.6 ± 19.1 (range: 2.5–109.0) | 0.049 | 162 |
| Lymph nodes | 28.6 ± 14.9 (range: 3.2–77.5) | 27.6 ± 16.5 (range: 3.6–68.4) | 0.052 | 40 |
| Liver | 26.9 ± 14.2 (range: 4.8–59.6) | 26.2 ± 14.5 (range: 5–71) | 0.619 | 67 |
| Bone | 34.2 ± 29.4 (range: 3.8–88.3) | 27.1 ± 23.1 (range: 2.5–69.6) | 0.034 | 16 |
| Pancreas | 34.7 ± 18.9 (range: 8.1–67) | 33.9 ± 22.8 (range: 3.6–83.4) | 0.286 | 11 |
| Other | 27.6 ± 28.6 (range: 3.6–118.8) | 28.8 ± 27.7 (range: 34.1–109) | 0.419 | 28 |
| Organs | Conventional PET (Mean ± SD) | SiPM (Mean ± SD) | p Value | n |
|---|---|---|---|---|
| All | 32 ± 40.4 (range: 0.9–234.3) | 35.4 ± 43.7 (range: 1.2–225) | <0.001 | 108 |
| Lymph nodes | 46.6 ± 62.1 (range: 2.3–234.3) | 49 ± 62.4 (range: 2.4–215.7) | 0.163 | 17 |
| Liver | 30.6 ± 23.8 (range: 7.19–45.6) | 34.4 ± 27.8 (range: 6.81–42.8) | <0.001 | 55 |
| Bone | 64.1 ± 82.2 (range: 2.1–209.1) | 72.2 ± 92.6 (range: 2.2–225) | 0.123 | 8 |
| Pancreas | 26.2 ± 33.3 (range: 5–124.9) | 29 ± 34.2 (range: 6.5–126.5) | 0.003 | 13 |
| Other | 8.5 ± 7.3 (range: 0.9–21.7) | 9.3 ± 8.2 (range: 1.2–22.4) | 0.280 | 15 |
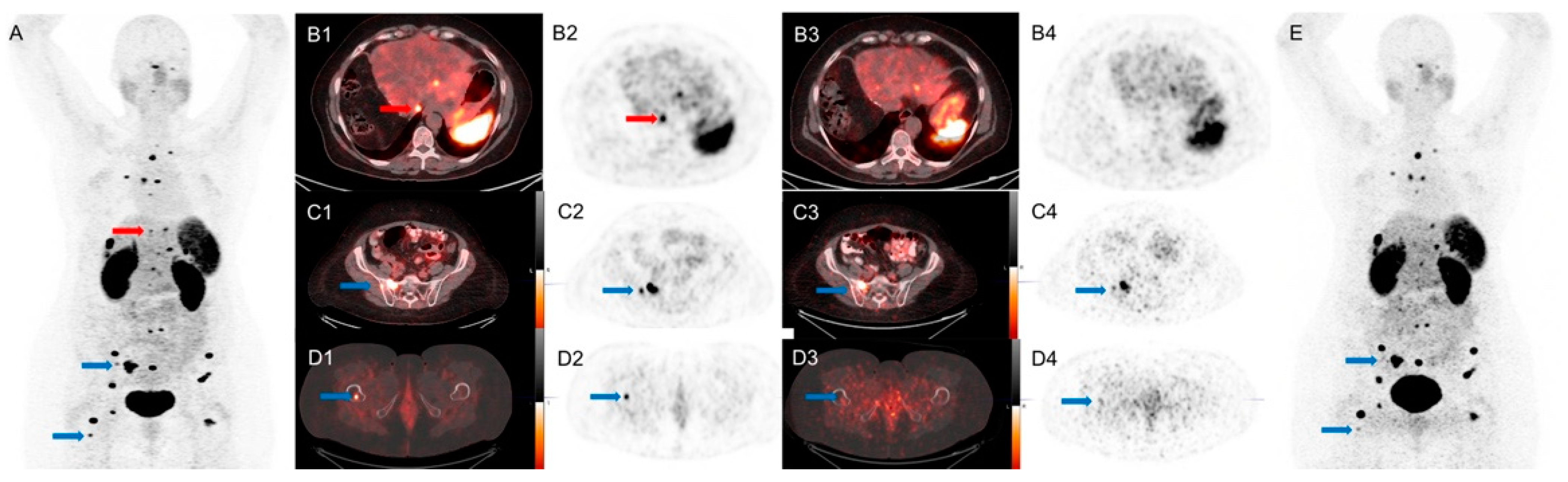
| Age | Sex | Referral Category | First Scan | Delay Time (min) | Lesions Detected by SiPM PET/CT | Lesions Detected by Conventional PET/CT | Lesion Location |
|---|---|---|---|---|---|---|---|
| 50 | F | Restaging | SiPM | 19.1 | 6 | 4 | Lymph nodes |
| 76 | F | Restaging | SiPM | 19.2 | 7 | 6 | Liver |
| 64 | M | Staging | SiPM | 20.3 | 8 | 6 | Liver |
| 58 | M | Surveillance | SiPM | 22.9 | 7 | 6 | Liver |
| 47 | F | Restaging | SiPM | 18.9 | 9 | 6 | Liver |
| 82 | M | Staging | SiPM | 22.1 | 2 | 1 | Liver, bone |
| 67 | M | Staging | SiPM | 23.4 | 3 | 2 | Lymph nodes |
| 74 | F | Staging | SiPM | 21.3 | 4 | 3 | Lymph nodes |
| 49 | F | Staging | Conventional | 43.8 | 8 | 6 | Bone, Lymph nodes |
| 52 | M | Surveillance | Conventional | 35.7 | 2 | 1 | Lymph nodes |
| 54 | F | Staging | Conventional | 19.6 | 7 | 6 | Liver |
| 41 | M | Restaging | Conventional | 46.8 | 7 | 6 | Liver |
| 72 | M | Restaging | Conventional | 25.5 | 8 | 6 | Lymph nodes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratto, L.; Toriihara, A.; Hatami, N.; Aparici, C.M.; Davidzon, G.; Levin, C.S.; Iagaru, A. Results of a Prospective Trial to Compare 68Ga-DOTA-TATE with SiPM-Based PET/CT vs. Conventional PET/CT in Patients with Neuroendocrine Tumors. Diagnostics 2021, 11, 992. https://doi.org/10.3390/diagnostics11060992
Baratto L, Toriihara A, Hatami N, Aparici CM, Davidzon G, Levin CS, Iagaru A. Results of a Prospective Trial to Compare 68Ga-DOTA-TATE with SiPM-Based PET/CT vs. Conventional PET/CT in Patients with Neuroendocrine Tumors. Diagnostics. 2021; 11(6):992. https://doi.org/10.3390/diagnostics11060992
Chicago/Turabian StyleBaratto, Lucia, Akira Toriihara, Negin Hatami, Carina M. Aparici, Guido Davidzon, Craig S. Levin, and Andrei Iagaru. 2021. "Results of a Prospective Trial to Compare 68Ga-DOTA-TATE with SiPM-Based PET/CT vs. Conventional PET/CT in Patients with Neuroendocrine Tumors" Diagnostics 11, no. 6: 992. https://doi.org/10.3390/diagnostics11060992
APA StyleBaratto, L., Toriihara, A., Hatami, N., Aparici, C. M., Davidzon, G., Levin, C. S., & Iagaru, A. (2021). Results of a Prospective Trial to Compare 68Ga-DOTA-TATE with SiPM-Based PET/CT vs. Conventional PET/CT in Patients with Neuroendocrine Tumors. Diagnostics, 11(6), 992. https://doi.org/10.3390/diagnostics11060992






