Upregulation of sICAM-1 and sVCAM-1 Levels in the Cerebrospinal Fluid of Patients with Schizophrenia Spectrum Disorders
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Sample
2.2. Cerebrospinal Fluid Analysis and Instrumental Diagnostics
2.3. Measurement of Cell Adhesion Markers
2.4. Data Handling and Statistical Analyses
3. Results
3.1. Sociodemographic Data
3.2. Cell Adhesion Molecules in the Cerebrospinal Fluid
3.3. Basic Cerebrospinal Fluid Findings and Instrumental Diagnostics
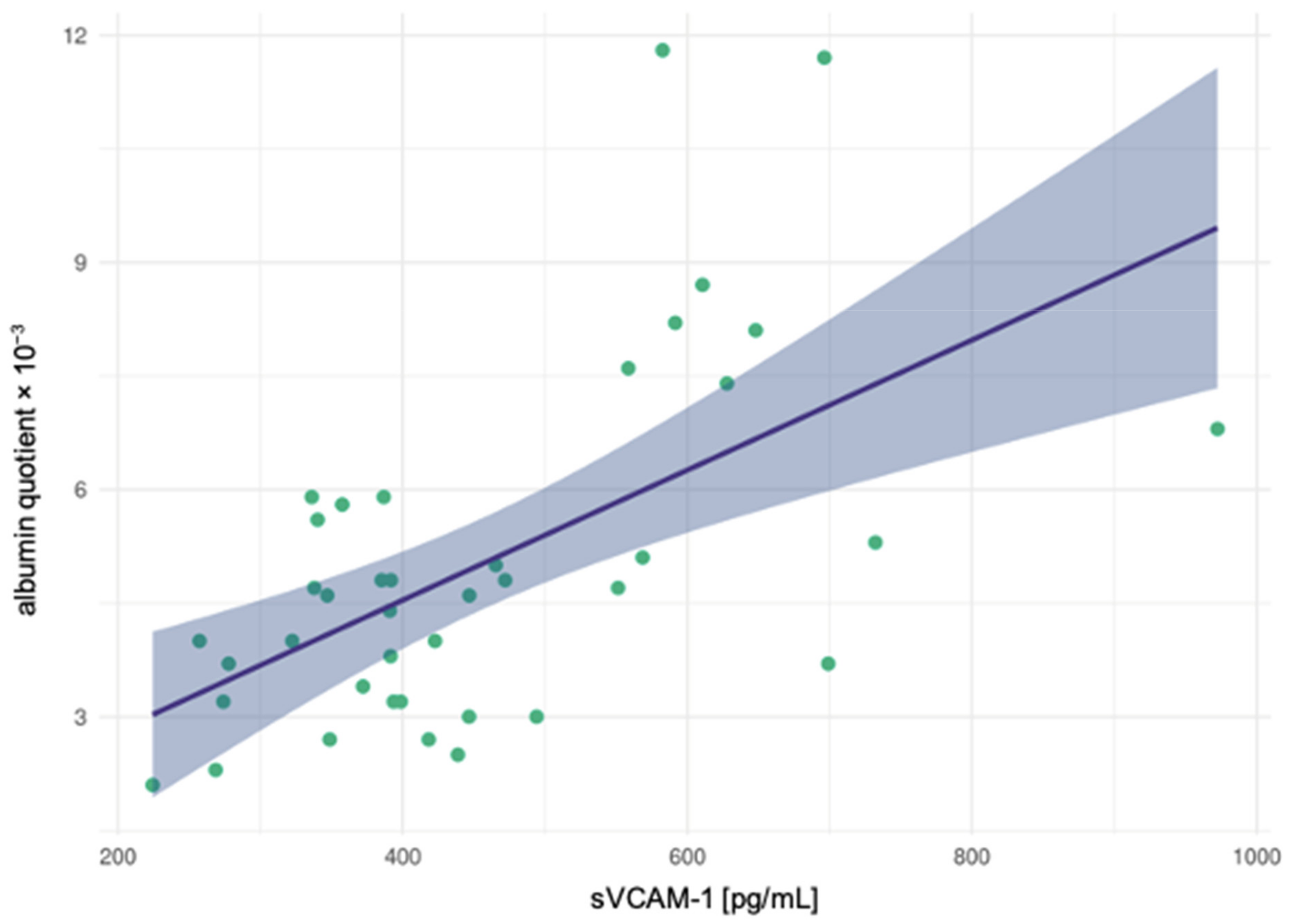
3.4. Correlation Analyses
4. Discussion
4.1. Integration of Our Findings into the Context of the Current Research
4.2. Pathophysiological and Clinical Considerations
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollak, T.A.; Lennox, B.R.; Müller, S.; Benros, M.E.; Prüss, H.; Tebartz van Elst, L.; Klein, H.; Steiner, J.; Frodl, T.; Bogerts, B.; et al. Autoimmune psychosis: An international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry 2020, 7, 93–108. [Google Scholar] [CrossRef]
- Bechter, K. Updating the mild encephalitis hypothesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. The Role of Intercellular Adhesion Molecule-1 in the Pathogenesis of Psychiatric Disorders. Front. Pharmacol. 2019, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Dev, S.I.; Chen, G.; Liou, S.C.; Martin, A.S.; Irwin, M.R.; Carroll, J.E.; Tu, X.; Jeste, D.V.; Eyler, L.T. Abnormal levels of vascular endothelial biomarkers in schizophrenia. Eur Arch. Psychiatry Clin. Neurosci. 2018, 268, 849–860. [Google Scholar] [CrossRef] [Green Version]
- Stefanović, M.P.; Petronijević, N.; Dunjić-Kostić, B.; Velimirović, M.; Nikolić, T.; Jurišić, V.; Lačković, M.; Damjanović, A.; Totić-Poznanović, S.; Jovanović, A.A.; et al. Role of sICAM-1 and sVCAM-1 as biomarkers in early and late stages of schizophrenia. J. Psychiatr. Res. 2016, 73, 45–52. [Google Scholar] [CrossRef]
- Pollak, T.A.; Drndarski, S.; Stone, J.M.; David, A.S.; McGuire, P.; Abbott, N.J. The blood-brain barrier in psychosis. Lancet Psychiatry 2018, 5, 79–92. [Google Scholar] [CrossRef]
- Endres, D.; Meixensberger, S.; Dersch, R.; Feige, B.; Stich, O.; Venhoff, N.; Matysik, M.; Maier, S.J.; Michel, M.; Runge, K.; et al. Cerebrospinal fluid, antineuronal autoantibody, EEG, and MRI findings from 992 patients with schizophreniform and affective psychosis. Transl. Psychiatry 2020, 10, 279. [Google Scholar] [CrossRef]
- Endres, D.; Perlov, E.; Baumgartner, A.; Hottenrott, T.; Dersch, R.; Stich, O.; Tebartz van Elst, L. Immunological findings in psychotic syndromes: A tertiary care hospital’s CSF sample of 180 patients. Front. Hum. Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Najjar, S.; Pahlajani, S.; De Sanctis, V.; Stern, J.N.H.; Najjar, A.; Chong, D. Neurovascular Unit Dysfunction and Blood-Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front. Psychiatry 2017, 8, 83. [Google Scholar] [CrossRef]
- Najjar, S.; Pearlman, D.M.; Devinsky, O.; Najjar, A.; Zagzag, D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: A review of clinical and experimental evidence. J. Neuroinflamm. 2013, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Orlovska-Waast, S.; Köhler-Forsberg, O.; Brix, S.W.; Nordentoft, M.; Kondziella, D.; Krogh, J.; Benros, M.E. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: A systematic review and meta-analysis. Mol. Psychiatry 2019, 24, 869–887. [Google Scholar] [CrossRef] [Green Version]
- Banks, W.A.; Erickson, M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Bartos, A.; Egg, R.; Gilhus, N.E.; Giovannoni, G.; Rauer, S.; Sellebjerg, F. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur. J. Neurol. 2006, 13, 913–922. [Google Scholar] [CrossRef]
- Tumani, H.; Huss, A.; Bachhuber, F. The cerebrospinal fluid and barriers—Anatomic and physiologic considerations. Handb. Clin. Neurol. 2017, 146, 21–32. [Google Scholar] [CrossRef]
- Wildemann, B.; Oschman, P.; Reiber, H. Laboratory Diagnosis in Neurology, 1st ed.; Thieme: Stuttgart, Germany, 2010. [Google Scholar]
- Carvey, P.M.; Hendey, B.; Monahan, A.J. The blood-brain barrier in neurodegenerative disease: A rhetorical perspective. J. Neurochem. 2009, 111, 291–314. [Google Scholar] [CrossRef]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and physiology of the blood-brain barrier. Semin. Cell Dev. Biol. 2015, 38, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [Green Version]
- Blank, T.; Detje, C.N.; Spieß, A.; Hagemeyer, N.; Brendecke, S.M.; Wolfart, J.; Staszewski, O.; Zöller, T.; Papageorgiou, I.; Schneider, J.; et al. Brain Endothelial- and Epithelial-Specific Interferon Receptor Chain 1 Drives Virus-Induced Sickness Behavior and Cognitive Impairment. Immunity 2016, 44, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Yousef, H.; Czupalla, C.J.; Lee, D.; Chen, M.B.; Burke, A.N.; Zera, K.A.; Zandstra, J.; Berber, E.; Lehallier, B.; Mathur, V.; et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 2019, 25, 988–1000. [Google Scholar] [CrossRef]
- Schwarz, M.J.; Ackenheil, M.; Riedel, M.; Müller, N. Blood-cerebrospinal fluid barrier impairment as indicator for an immune process in schizophrenia. Neurosci. Lett. 1998, 253, 201–203. [Google Scholar] [CrossRef]
- Schwarz, M.J.; Riedel, M.; Ackenheil, M.; Müller, N. Decreased levels of soluble intercellular adhesion molecule-1 (sICAM-1) in unmedicated and medicated schizophrenic patients. Biol. Psychiatry 2000, 47, 29–33. [Google Scholar] [CrossRef]
- Cai, H.Q.; Catts, V.S.; Webster, M.J.; Galletly, C.; Liu, D.; O’Donnell, M.; Weickert, T.W.; Weickert, C.S. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol. Psychiatry 2020, 25, 761–775. [Google Scholar] [CrossRef] [Green Version]
- Kronig, H.; Riedel, M.; Schwarz, M.J.; Strassnig, M.; Moller, H.J.; Ackenheil, M.; Muller, N. ICAM G241A polymorphism and soluble ICAM-1 serum levels: Evidence for an active immune process in schizophrenia. Neuroimmunomodulation 2005, 12, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzior, H.; Fiebich, B.L.; Yousif, N.M.; Saliba, S.W.; Ziegler, C.; Nickel, K.; Maier, S.J.; Süß, P.; Runge, K.; Matysik, M.; et al. Increased IL-8 concentrations in the cerebrospinal fluid of patients with unipolar depression. Compr. Psychiatry 2020, 102, 152196. [Google Scholar] [CrossRef]
- Ramos, T.N.; Bullard, D.C.; Barnum, S.R. ICAM-1: Isoforms and phenotypes. J. Immunol. 2014, 192, 4469–4474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiber, H.; Peter, J.B. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J. Neurol. Sci. 2001, 184, 101–122. [Google Scholar] [CrossRef]
- Reiber, H.; Uhr, M. Physiologie des Liquors; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Levy, A.D.; Omar, M.H.; Koleske, A.J. Extracellular matrix control of dendritic spine and synapse structure and plasticity in adulthood. Front. Neuroanat. 2014, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Bellon, A.; Krebs, M.O.; Jay, T.M. Factoring neurotrophins into a neurite-based pathophysiological model of schizophrenia. Prog. Neurobiol. 2011, 94, 77–90. [Google Scholar] [CrossRef]
- Merelo, V.; Durand, D.; Lescallette, A.R.; Vrana, K.E.; Hong, L.E.; Faghihi, M.A.; Bellon, A. Associating schizophrenia, long non-coding RNAs and neurostructural dynamics. Front. Mol. Neurosci. 2015, 8, 57. [Google Scholar] [CrossRef]
- Radu, G.; Luca, C.; Petrescu, L.; Bordejevic, D.A.; Tomescu, M.C.; Andor, M.; Cîtu, I.; Mavrea, A.; Buda, V.; Tomescu, C.; et al. The Predictive Value of Endothelial Inflammatory Markers in the Onset of Schizophrenia. Neuropsychiatr. Dis. Treat. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, C.; Wolf, S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 2009, 61, 22–32. [Google Scholar] [CrossRef]
- Bechmann, I.; Galea, I.; Perry, V.H. What is the blood-brain barrier (not)? Trends. Immunol. 2007, 28, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Stich, O.; Andres, T.A.; Gross, C.M.; Gerber, S.I.; Rauer, S.; Langosch, J.M. An observational study of inflammation in the central nervous system in patients with bipolar disorder. Bipolar. Disord. 2015, 17, 291–302. [Google Scholar] [CrossRef]
- Mantere, O.; Trontti, K.; García-González, J.; Balcells, I.; Saarnio, S.; Mäntylä, T.; Lindgren, M.; Kieseppä, T.; Raij, T.; Honkanen, J.K.; et al. Immunomodulatory effects of antipsychotic treatment on gene expression in first-episode psychosis. J. Psychiatr. Res. 2019, 109, 18–26. [Google Scholar] [CrossRef]
| Sample Material | Method | Research Group | Schizophrenia-Spectrum Disorder Group | Control Group | Results |
|---|---|---|---|---|---|
| CSF | ELISA | Schwarz et al., 1998 [21] | n = 40 | Ø | Significant association of sICAM-1 and BCSFB |
| CSF | ELISA | Schwarz et al., 2000 [22] | n = 18 | Ø | Significant positive correlation of sICAM-1 with negative symptomatology and disease duration |
| Cortex tissue | PCR | Cai et al., 2018 [23] | n = 37 | n = 37 | ↑ expression of ICAM-1 mRNA |
| Plasma | Multiplexing immunoassay (Luminex®) | Cai et al., 2018 [23] | n = 78 | n = 73 | ↑ levels of sICAM-1 |
| Plasma | Multiplexing immunoassay (Meso Scale Discovery MULTI-SPOT®) | Nguyen et al., 2018 [4] | n = 134 | n = 113 | ↑ levels of ‘vascular endothelial index’ including VEGF, sICAM-1, sVCAM-1 |
| Serum | ELISA | Schwarz et al., 2000 [22] | n = 72 | n = 38 | ↓ levels of sICAM-1 and increase of sICAM-1 during treatment |
| Serum | ELISA | Kronig et al., 2005 [24] | n = 70 | n = 128 | ↓ levels of sICAM-1 and relationship to ICAM-1 G214A polymorphism |
| Serum | ELISA | Stefanović et al., 2016 [5] | n = 80 | n = 80 | = levels of sICAM-1 in early-stage, levels of sICAM-1 in late-stage and associations with severity and disease duration |
| Schizophrenia-Spectrum Disorder (n = 40) | Depressive Disorder (n = 39) | |
|---|---|---|
| Sex | 16 M: 24 F | 14 M: 25 F |
| Age (Mean ± SD, range) | 33.63 ± 13.38 | 32.54 ± 7.65 |
| (18–65 years) | (18–44 years) | |
| Clinical Syndrome and Characteristics | ||
| Severe depressive episode | 39 (100%) | |
| With psychotic symptoms | 7 (18%) | |
| Without psychotic symptoms | 32 (82%) | |
| Schizophrenia spectrum disorder | 40 (100%) | |
| Paranoid-hallucinatory | 25 (63%) | |
| Hebephrenic | 1 (3%) | |
| Catatonic | 1 (3%) | |
| Delusional disorders | 1 (3%) | |
| Schizoaffective | 11 (28%) | |
| - Depressive | 6 (15%) | |
| - Manic | 3 (8%) | |
| - Mixed | 2 (5%) | |
| Acute polymorphic psychotic | 1 (3%) | |
| Course of Disease | ||
| Recurrent/chronic | 27 (68%) | 27 (69%) |
| First episode | 13 (33%) | 12 (31%) |
| Neurologic Comorbidity | ||
| Seizures/Attacks | 2 (5%) | 0 (0%) |
| Traumatic | 3 (8%) | 0 (0%) |
| Polyneuropathy | 0 (0%) | 0 (0%) |
| Migraine/Headache | 1 (3%) | 1 (3%) |
| Overall | 6 (15%) | 1 (3%) |
| Psychotropic Medication at the Time of Sampling | ||
| SSRI | 4 (10%) | 9 (23%) |
| SSNRI | 1 (3%) | 21 (54%) |
| Tricyclic antidepressants | 0 (0%) | 8 (21%) |
| Bupropion | 0 (0%) | 4 (10%) |
| Mirtazapine | 1 (3%) | 6 (15%) |
| Typical neuroleptics | 9 (23%) | 4 (10%) |
| Atypical neuroleptics | 40 (100%) | 21 (54%) |
| Lithium | 7 (18%) | 9 (23%) |
| Anticonvulsant | 7 (18%) | 1 (3%) |
| Benzodiazepine | 9 (23%) | 3 (8%) |
| Unmedicated | 0 (0%) | 2 (5%) |
| Schizophrenia-Spectrum Disorder (n = 40) | Depressive Disorder (n = 39) | Statistics | |
|---|---|---|---|
| Marital status | n.s. | ||
| Single | 30 (77%) | 31 (79%) | |
| Married | 6 (15%) | 6 (15%) | |
| Divorced | 1 (3%) | 2 (5%) | |
| Widowed | 1 (3%) | 0 (0%) | |
| Unknown | 2 (5%) | 0 (0%) | |
| Level of education | p = 0.013 | ||
| Low | 11 (28%) | 2 (5%) | |
| Middle | 7 (18%) | 8 (21%) | |
| High | 19 (48%) | 28 (72%) | |
| Unknown | 3 (8%) | 1 (3%) | |
| Work situation | n.s. | ||
| Unemployed | 7 (18%) | 6 (15%) | |
| Working | 13 (33%) | 20 (51%) | |
| In training | 11 (28%) | 11 (28%) | |
| Retired | 6 (15%) | 1 (3%) | |
| Housewife/-man | 2 (5%) | 1 (3%) | |
| Unknown | 1 (3%) | 0 (0%) | |
| Housing situation | n.s. | ||
| Alone | 13 (33%) | 18 (47%) | |
| With partner/family | 11 (28%) | 10 (26%) | |
| With parents/guardian | 12 (30%) | 10 (26%) | |
| Other | 4 (10%) | 0 (0%) | |
| Unknown | 0 (0%) | 1 (3%) | |
| Suicide attempts | n.s. | ||
| None | 28 (70%) | 34 (87%) | |
| One | 2 (5%) | 2 (5%) | |
| Two | 4 (10%) | 2 (5%) | |
| Three | 1 (3%) | 0 (0%) | |
| Four | 1 (3%) | 0 (0%) | |
| Five | 1 (3%) | 0 (0%) | |
| Six | 0 (0%) | 1 (3%) | |
| Unclear | 3 (8%) | 0 (0%) | |
| Number of earlier inpatient treatments | n.s. | ||
| None | 12 (30%) | 15 (38%) | |
| One | 6 (15%) | 12 (31%) | |
| Two | 3 (8%) | 6 (15%) | |
| Three | 3 (8%) | 2 (5%) | |
| Four | 2 (5%) | 1 (3%) | |
| Five | 5 (13%) | 2 (5%) | |
| >Five | 7 (18%) | 1 (3%) | |
| Unclear | 2 (5%) | 0 (0%) |
| Schizophrenia-Spectrum Disorder (n = 40) | Depressive Disorder (n = 39) | Statistics | |
|---|---|---|---|
| PAI-1 (pg/mL) (Mean ± SD) | 72.006 ± 46.810 | 30.756 ± 23.397 | z = −4.266 p < 0.001 |
| sICAM-1 (pg/mL) (Mean ± SD) | 1196.252 ± 768.714 | 466.205 ± 277.053 | z = −5.753 p < 0.001 |
| sVCAM-1 (pg/mL) (Mean ± SD) | 456.197 ± 155.549 | 234.195 ± 151.553 | z = −5.354 p < 0.001 |
| Reference | Schizophrenia-Spectrum Disorder (n = 40) | Depressive Disorder (n = 39) | Statistics | |
|---|---|---|---|---|
| WBC counts (Mean ± SD) | in/µL | 1.85 ± 1.46 | 1.82 ± 1.23 | Z = −0.189 p = 0.850 |
| Number of increased WBC counts | <5/µL | ↑: 3 (8%) | ↑: 2 (5%) | p = 1.00 |
| Protein concentration (Mean ± SD) | in mg/L | 406.45 ± 196.15 | 418.87 ± 153.68 | z = −1.098 p = 0.272 |
| Number of increased protein concentration | <450 mg/L | ↑: 12 (30%) | ↑: 14 (36%) | Chi2 = 0.311 p = 0.577 |
| Albumin quotient (Mean ± SD) | 5.02 ± 2.29 | 5.12 ± 2.05 | z = −0.608 p = 0.543 | |
| Number of increased albumin quotients | <40 y.: <6.5 × 10−3 40–60 y.: <8 × 10−3 >60 y.: <9.3 × 10−3 | ↑: 6 (15%) | ↑: 8 (21%) | Chi2 = 0.412 p = 0.521 |
| IgG-Index (Mean ± SD) | in mg/L | 0.49 ± 0.04 | 0.49 ± 0.09 | z = −0.005 p = 0.996 |
| Number of increased IgG indices | <0.7 mg/L | ↑: 0 (0%) | ↑: 1 (3%) | p = 0.494 |
| OCBs in CSF | negative | 1* (3%) | 2 (5%) | Chi2 = 0.556 p = 0.346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meixensberger, S.; Kuzior, H.; Fiebich, B.L.; Süß, P.; Runge, K.; Berger, B.; Nickel, K.; Denzel, D.; Schiele, M.A.; Michel, M.; et al. Upregulation of sICAM-1 and sVCAM-1 Levels in the Cerebrospinal Fluid of Patients with Schizophrenia Spectrum Disorders. Diagnostics 2021, 11, 1134. https://doi.org/10.3390/diagnostics11071134
Meixensberger S, Kuzior H, Fiebich BL, Süß P, Runge K, Berger B, Nickel K, Denzel D, Schiele MA, Michel M, et al. Upregulation of sICAM-1 and sVCAM-1 Levels in the Cerebrospinal Fluid of Patients with Schizophrenia Spectrum Disorders. Diagnostics. 2021; 11(7):1134. https://doi.org/10.3390/diagnostics11071134
Chicago/Turabian StyleMeixensberger, Sophie, Hanna Kuzior, Bernd L. Fiebich, Patrick Süß, Kimon Runge, Benjamin Berger, Kathrin Nickel, Dominik Denzel, Miriam A. Schiele, Maike Michel, and et al. 2021. "Upregulation of sICAM-1 and sVCAM-1 Levels in the Cerebrospinal Fluid of Patients with Schizophrenia Spectrum Disorders" Diagnostics 11, no. 7: 1134. https://doi.org/10.3390/diagnostics11071134
APA StyleMeixensberger, S., Kuzior, H., Fiebich, B. L., Süß, P., Runge, K., Berger, B., Nickel, K., Denzel, D., Schiele, M. A., Michel, M., Maier, S., Bechter, K., Domschke, K., Tebartz van Elst, L., & Endres, D. (2021). Upregulation of sICAM-1 and sVCAM-1 Levels in the Cerebrospinal Fluid of Patients with Schizophrenia Spectrum Disorders. Diagnostics, 11(7), 1134. https://doi.org/10.3390/diagnostics11071134






