Experimental Validation of a Microwave System for Brain Stroke 3-D Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Microwave Imaging Prototype Device
2.2. The Head and Stroke Phantoms
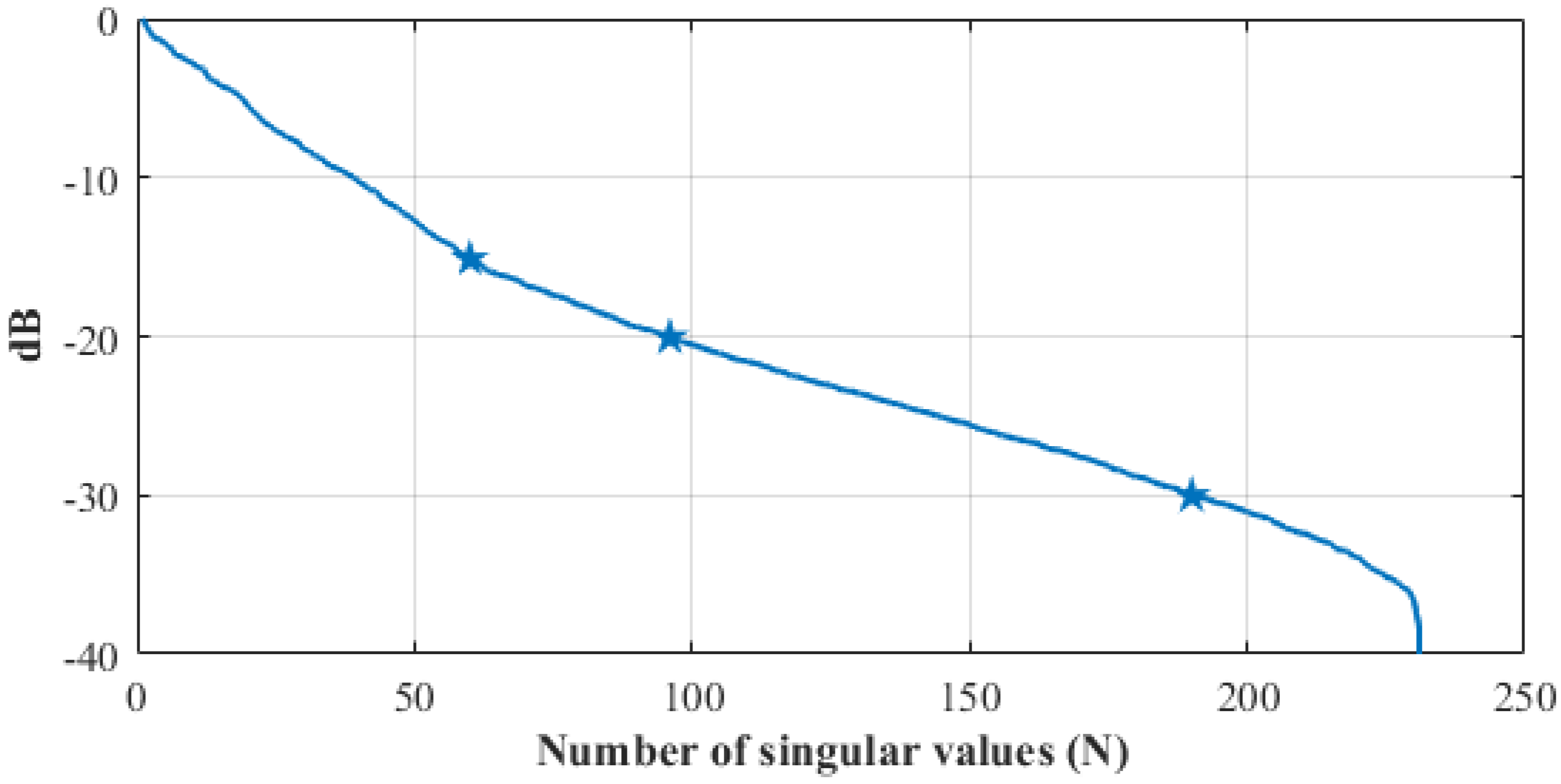
2.3. The Imaging Algorithm
2.4. The Calibration Procedure
3. Experimental Procedure and Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computerized tomography |
| EM | Electromagnetic |
| HP | Homogeneous phantom |
| IF | Intermediate frequency |
| IHC | Intracranial hemorrhagic stroke |
| IS | Ischemic stroke |
| MRI | Magnetic resonance imaging |
| MTP | Multi-tissue phantom |
| MWI | Microwave imaging |
| RX | Receiver |
| SP4T | Single-pole-four-throw |
| SPDT | Single-pole-double-throw |
| TSVD | Truncated singular value decomposition |
| TX | Transmitter |
| VNA | Vector network analyzer |
References
- Benjamin, E.J.; Muntner, P.; Bittencourt, M.S. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Walsh, K.B. Non-invasive sensor technology for prehospital stroke diagnosis: Current status and future directions. Int. J. Stroke 2019, 14, 592–602. [Google Scholar] [CrossRef]
- Semenov, S.Y.; Corfield, D.R. Microwave tomography for brain imaging: Feasibility assessment for stroke detection. Int. J. Antennas Propagat. 2008, 2008, 254830:1–254830:8. [Google Scholar] [CrossRef] [Green Version]
- Semenov, S.; Huynh, T.; Williams, T.; Nicholson, B.; Vasilenko, A. Dielectric properties of brain tissue in acute ischemic stroke: Experimental study on swine. Bioelectromagnetics 2017, 38, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, A.; Estatico, C.; Pastorino, M.; Randazzo, A. Microwave Detection of Brain Injuries by Means of a Hybrid Imaging Method. IEEE Open J. Antennas Propag. 2020, 1, 513–523. [Google Scholar] [CrossRef]
- Alqadami, A.S.M.; Nguyen-Trong, N.; Mohammed, B.; Stancombe, A.E.; Heitzmann, M.T.; Abbosh, A. Compact Unidirectional Conformal Antenna Based on Flexible High-Permittivity Custom-Made Substrate for Wearable Wideband Electromagnetic Head Imaging System. IEEE Trans. Antennas Propag. 2020, 68, 183–194. [Google Scholar] [CrossRef]
- Karadima, O.; Rahman, M.; Sotiriou, J.; Ghavami, N.; Lu, P.; Ahsan, S.; Kosmas, P. Experimental Validation of Microwave Tomography with the DBIM-TwIST Algorithm for Brain Stroke Detection and Classification. Sensors 2020, 20, 840. [Google Scholar] [CrossRef] [Green Version]
- Merunka, I.; Massa, A.; Vrba, D.; Fiser, O.; Salucci, M.; Vrba, J. Microwave Tomography System for Methodical Testing of Human Brain Stroke Detection Approaches. Int. J. Antennas Propag. 2019, 2019, 4074862:1–4074862:13. [Google Scholar] [CrossRef]
- Tobon Vasquez, J.A.; Scapaticci, R.; Turvani, G.; Bellizzi, G.; Rodriguez-Duarte, D.O.; Joachimowicz, N.; Duchêne, B.; Tedeschi, E.; Casu, M.R.; Crocco, L.; et al. A Prototype Microwave System for 3D Brain Stroke Imaging. Sensors 2020, 20, 2607. [Google Scholar] [CrossRef] [PubMed]
- Candefjord, S.; Winges, J.; Malik, A.; Yu, Y.; Rylander, T.; McKelvey, T.; Fhager, A.; Elam, M.; Persson, M. Microwave technology for detecting traumatic intracranial bleedings: Tests on phantom of subdural hematoma and numerical simulations. Med. Biol. Eng. Comput. 2017, 55, 1177–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fhager, A.; Candefjord, S.; Elam, M.; Persson, M. Microwave Diagnostics Ahead: Saving Time and the Lives of Trauma and Stroke Patients. IEEE Microw. Mag. 2018, 19, 78–90. [Google Scholar] [CrossRef]
- Hopfer, M.; Planas, R.; Hamidipour, A.; Henriksson, T.; Semenov, S. Electromagnetic Tomography for Detection, Differentiation, and Monitoring of Brain Stroke: A Virtual Data and Human Head Phantom Study. IEEE Antennas Propag. Mag. 2017, 59, 86–97. [Google Scholar] [CrossRef]
- Scapaticci, R.; Tobon, J.; Bellizzi, G.; Vipiana, F.; Crocco, L. Design and Numerical Characterization of a Low-Complexity Microwave Device for Brain Stroke Monitoring. IEEE Trans. Antennas Propag. 2018, 66, 7328–7338. [Google Scholar] [CrossRef]
- Sarwar, I.; Turvani, G.; Casu, M.R.; Tobon Vasquez, J.A.; Vipiana, F.; Scapaticci, R.; Crocco, L. Low-Cost Low-Power Acceleration of a Microwave Imaging Algorithm for Brain Stroke Monitoring. J. Low Power Electron. Appl. 2018, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Duarte, D.O.; Vasquez, J.A.T.; Scapaticci, R.; Crocco, L.; Vipiana, F. Brick-Shaped Antenna Module for Microwave Brain Imaging Systems. IEEE Antennas Wirel. Propag. Lett. 2020, 19, 2057–2061. [Google Scholar] [CrossRef]
- Garrett, J.; Fear, E. Stable and Flexible Materials to Mimic the Dielectric Properties of Human Soft Tissues. IEEE Antennas Wirel. Propag. Lett. 2014, 19, 599–602. [Google Scholar] [CrossRef]
- Joachimowicz, N.; Duchêne, B.; Conessa, C.; Meyer, O. Anthropomorphic Breast and Head Phantoms for Microwave Imaging. Diagnostics 2018, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Keysight Technologies. Keysight Streamline Series USB Vector Network Analyzer P937XA 2-port, up to 26.5 GHz. Data Sheet Tech. Specif. 2018, 85, 1–24. [Google Scholar]
- Tobon Vasquez, J.A.; Scapaticci, R.; Turvani, G.; Bellizzi, G.; Joachimowicz, N.; Duchêne, B.; Tedeschi, E.; Casu, M.R.; Crocco, L.; Vipiana, F. Design and Experimental Assessment of a 2D Microwave Imaging System for Brain Stroke Monitoring. Int. J. Antennas Propag. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Joachimowicz, N.; Vasquez, J.T.; Turvani, G.; Dassano, G.; Casu, M.R.; Vipiana, F.; Duchêne, B.; Scapaticci, R.; Crocco, L. Head Phantoms for a Microwave Imaging System Dedicated to Cerebrovascular Disease Monitoring. In Proceedings of the 2018 IEEE Conference on Antenna Measurements Applications (CAMA), Vasteras, Sweden, 3–6 September 2018; pp. 1–3. [Google Scholar] [CrossRef]
- Laredo, C.; Zhao, Y.; Rudilosso, S.; Renu, A.; Pariente, J.C.; Chamorro, A.; Urra, X. Prognostic Significance of Infarct Size and Location: The Case of Insular Stroke. Sci. Rep. 2018, 8, 9498. [Google Scholar] [CrossRef]
- Bruno, A.; Shah, N.; Akinwuntan, A.E.; Close, B.; Switzer, J.A. Stroke size correlates with functional outcome on the simplified modified Rankin Scale questionnaire. J. Stroke Cerebrovasc. Dis. 2013, 22, 781–783. [Google Scholar] [CrossRef]
- Saver, J.L. Time Is Brain-Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saver, J.L.; Johnston, K.C.; Homer, D.; Wityk, R.; Koroshetz, W.; Truskowski, L.L.; Haley, E.C. Infarct Volume as a Surrogate or Auxiliary Outcome Measure in Ischemic Stroke Clinical Trials. Stroke 1999, 30, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Stuchly, M.; Stuchly, S. Coaxial line reflection method for measuring dielectric properties of biological substances at radio and microwave frequencies. IEEE Trans. Instrum. Meas. 1980, 29, 176–182. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Ruvio, G. Numerical Sensitivity Analysis for Dielectric Characterization of Biological Samples by Open-Ended Probe Technique. Sensors 2020, 20, 3756. [Google Scholar] [CrossRef]
- Kraszewski, A.; Stuchly, M.; Stuchly, S. VNA calibration method for measurements of dielectric properties. IEEE Trans. Instrum. Meas. 1983, 32, 385–387. [Google Scholar] [CrossRef]
- Tajik, D.; Foroutan, F.; Shumakov, D.S.; Pitcher, A.D.; Nikolova, N.K. Real-Time Microwave Imaging of a Compressed Breast Phantom with Planar Scanning. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 154–162. [Google Scholar] [CrossRef]
- Beaverstone, A.S.; Shumakov, D.S.; Nikolova, N.K. Frequency-Domain Integral Equations of Scattering for Complex Scalar Responses. IEEE Trans. Microw. Theory Technoques 2017, 65, 1120–1132. [Google Scholar] [CrossRef]
- Bertero, M.; Boccacci, P. Introduction to Inverse Problems in Imaging; Inst. Phys.: Bristol, UK, 1998. [Google Scholar]
- Rodriguez-Duarte, D.O.; Tobon Vasquez, J.A.; Scapaticci, R.; Crocco, L.; Vipiana, F. Assessing a Microwave Imaging System for Brain Stroke Monitoring via High Fidelity Numerical Modelling. IEEE J. Electromagn. Microw. Med. Biol. 2021. [Google Scholar] [CrossRef]
- Gilmore, C.; Mojabi, P.; Zakaria, A.; Ostadrahimi, M.; Kaye, C.; Noghanian, S.; Shafai, L.; Pistorius, S.; LoVetri, J. A Wideband Microwave Tomography System with a Novel Frequency Selection Procedure. IEEE Trans. Biomed. Eng. 2010, 57, 894–904. [Google Scholar] [CrossRef]
- Rodriguez-Duarte, D.O.; Tobon Vasquez, J.A.; Vipiana, F. Hybrid Simulation-Measurement Calibration Technique for Microwave Imaging Systems. In Proceedings of the 2021 European Conference on Antennas and Propagation (EuCAP), Düsseldorf, Germany, 22–26 March 2021; pp. 1–5. [Google Scholar]




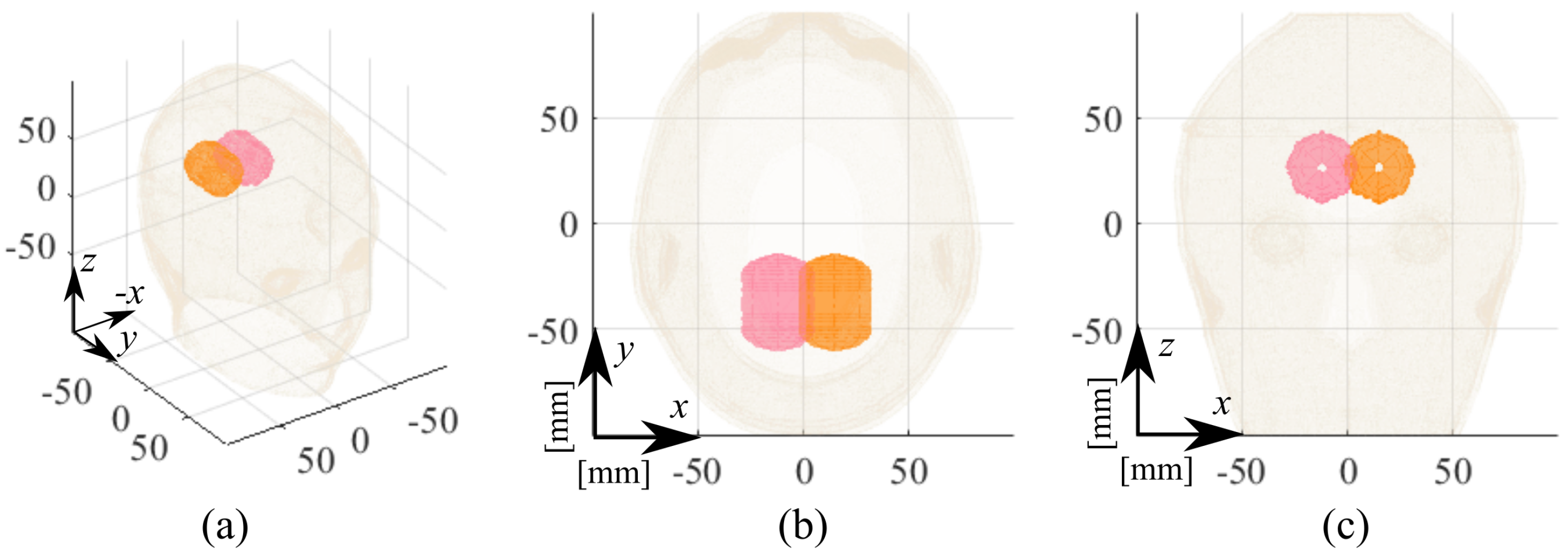



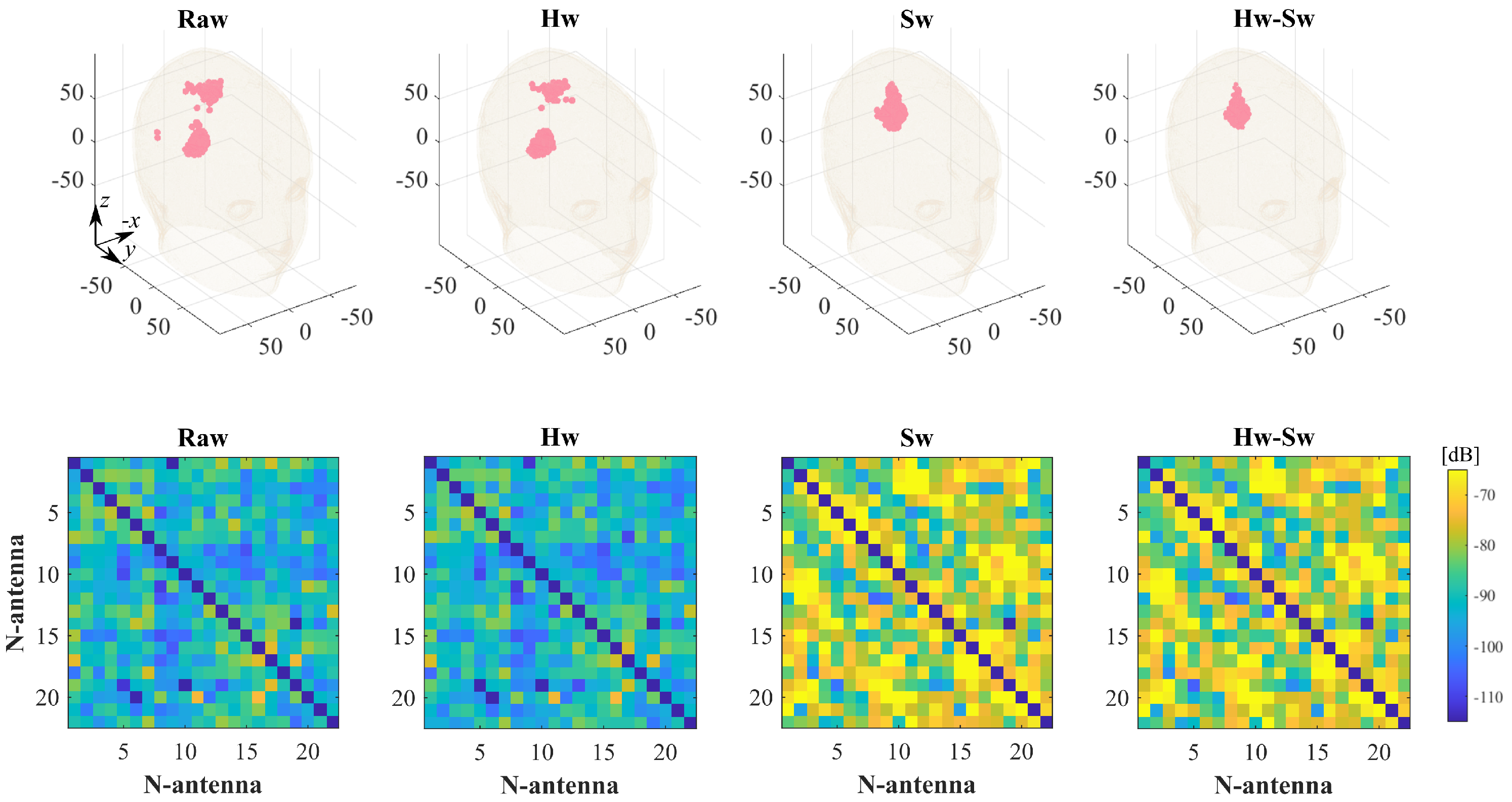
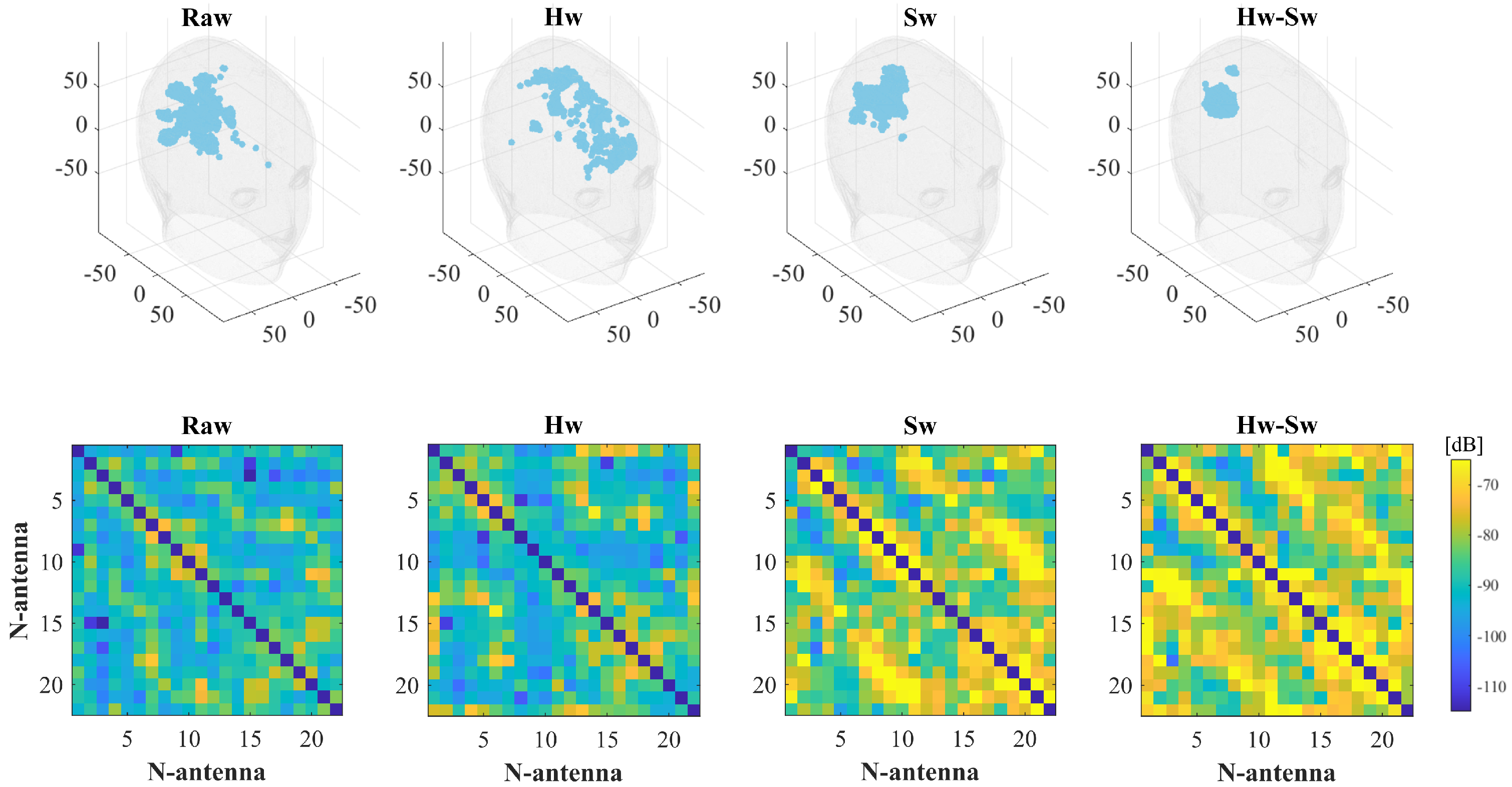
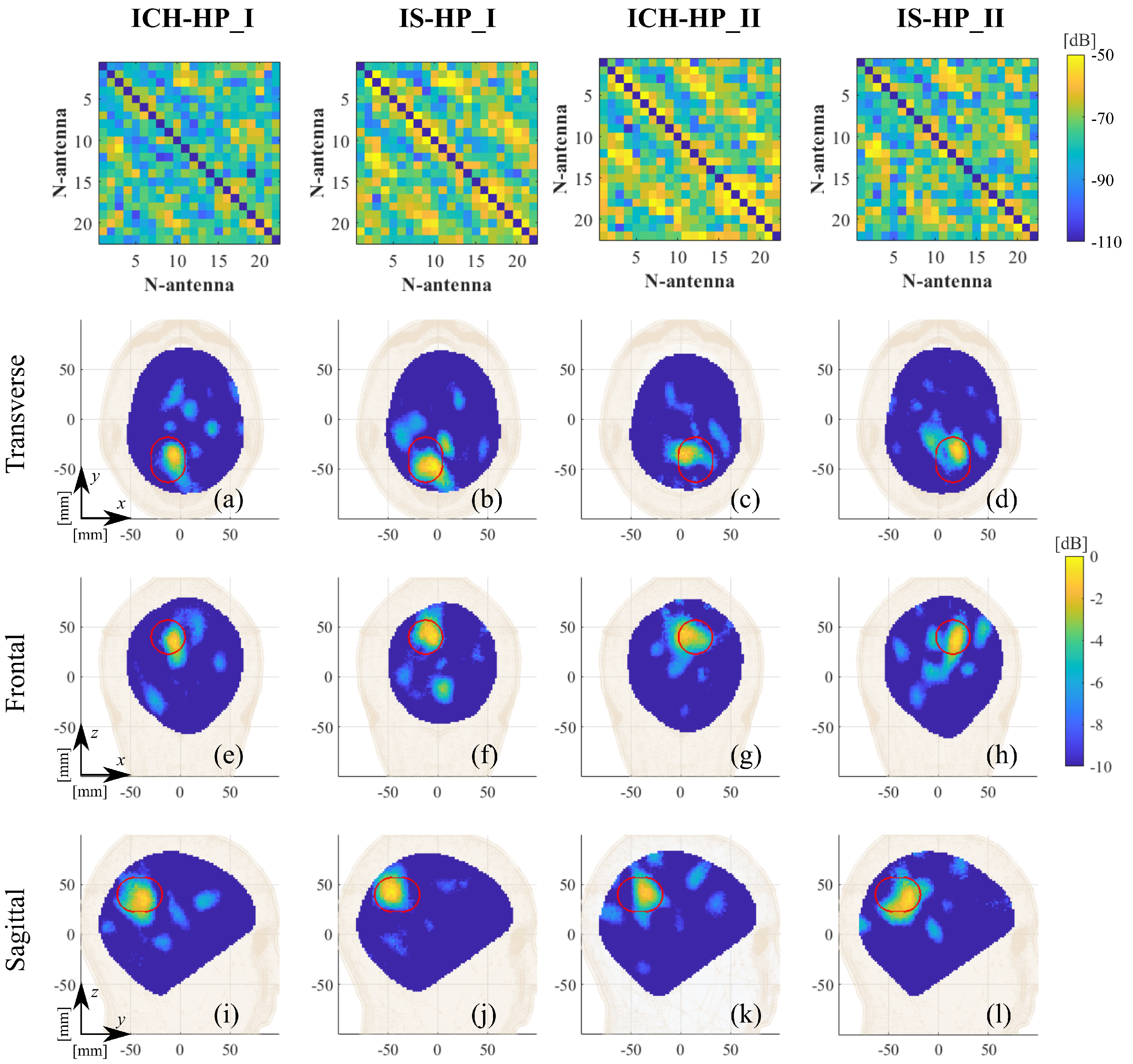
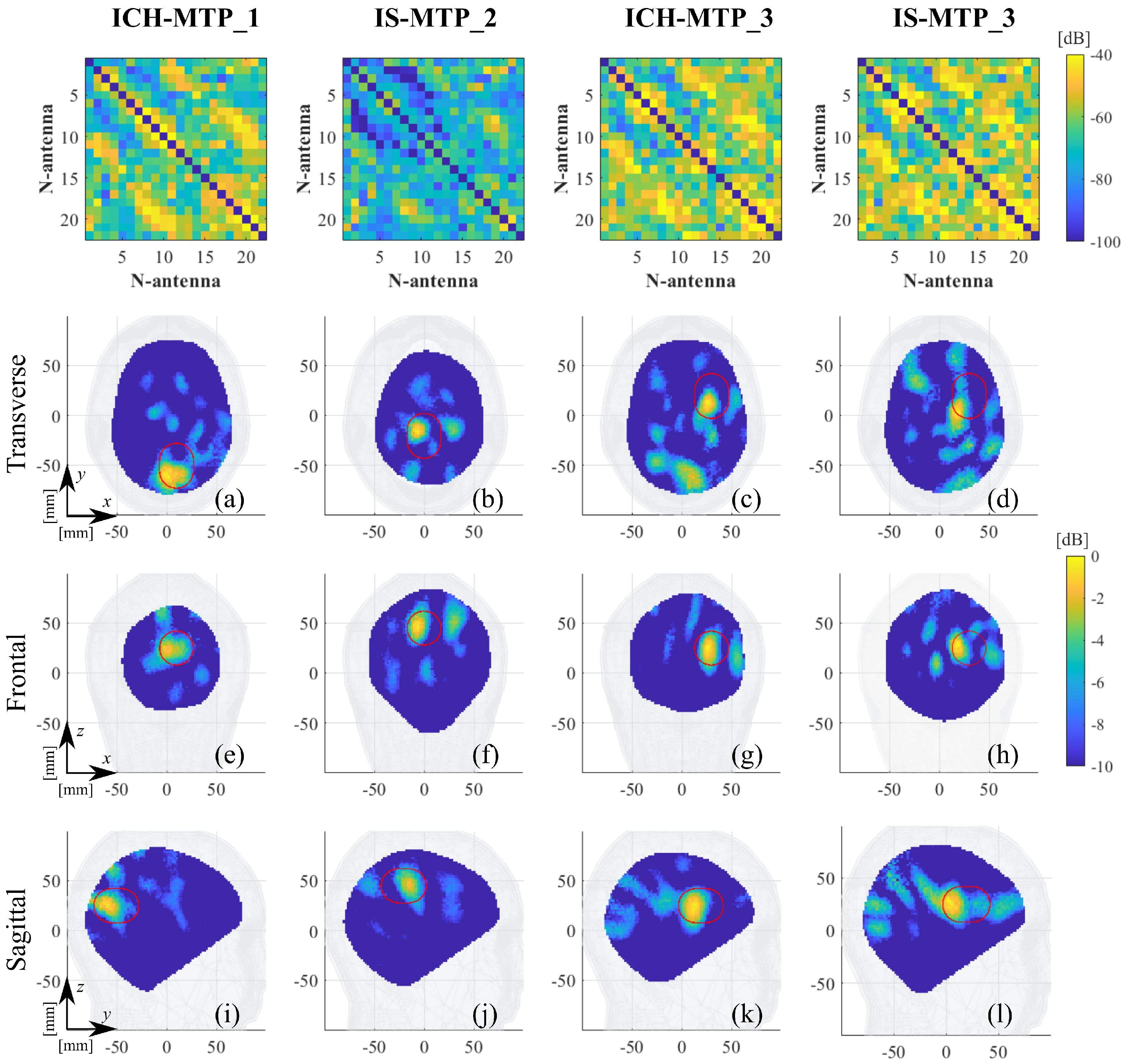
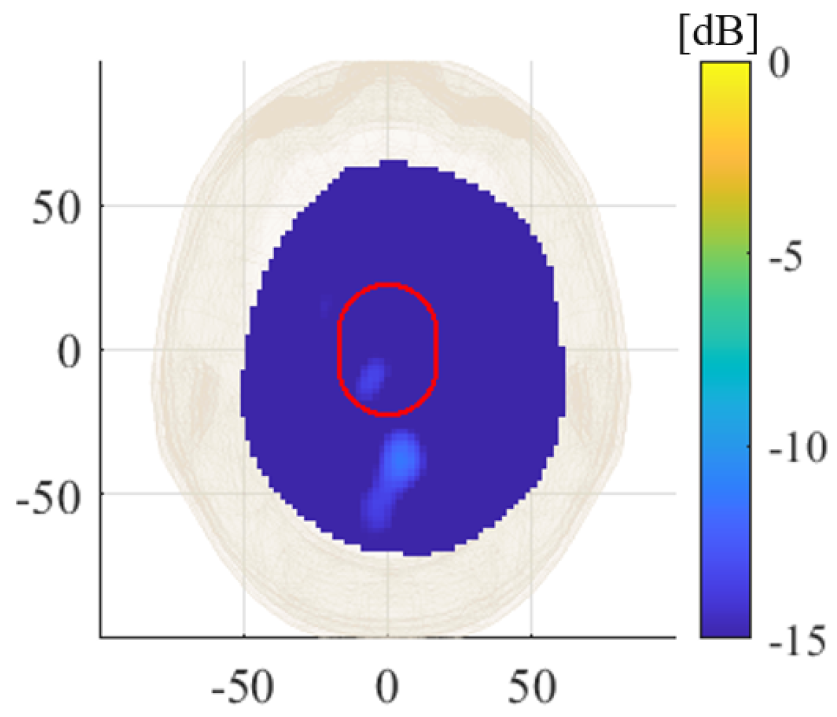


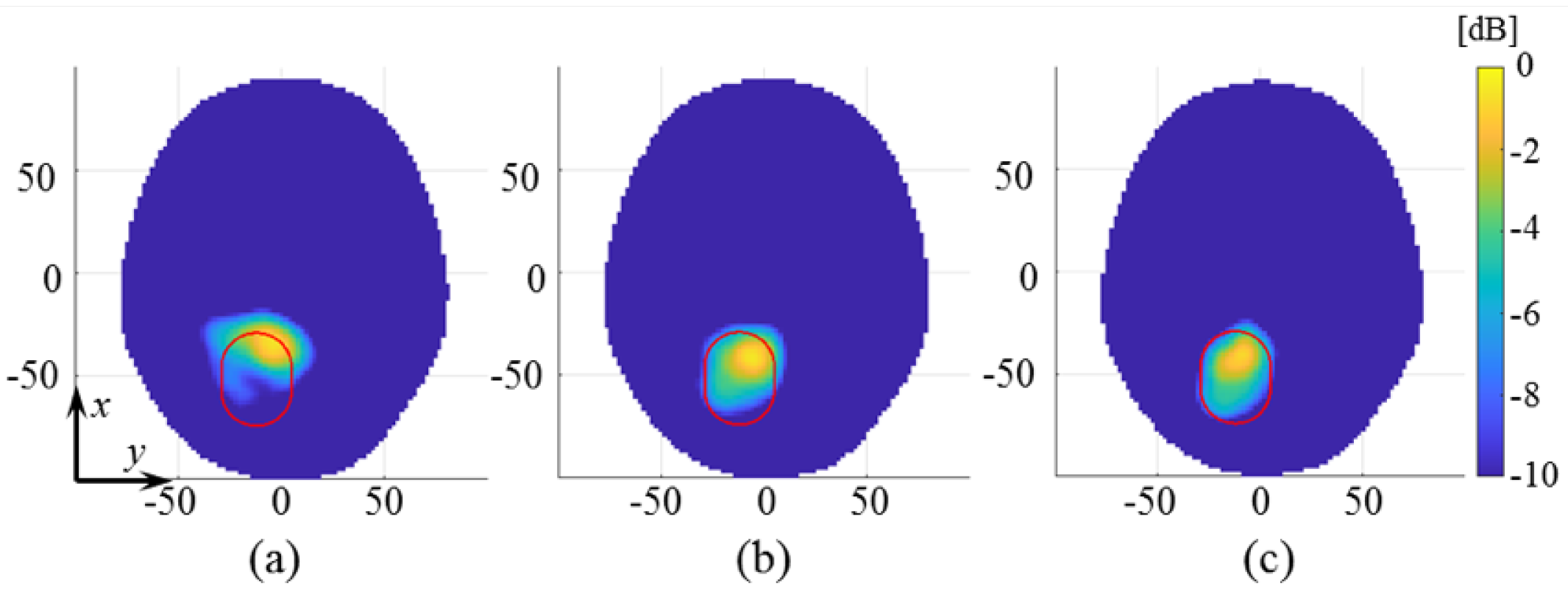
| Label | Stroke | Phantom | Position | RMSE |
|---|---|---|---|---|
| ICH_HP-I | Hemorrhagic | Homogeneous | I, pink, Figure 5 | 0.17 |
| ICH_HP-II | Hemorrhagic | Homogeneous | II, orange, Figure 5 | 0.19 |
| IS_HP-I | Ischemic | Homogeneous | I, pink, Figure 5 | 0.22 |
| IS_HP-II | Ischemic | Homogeneous | II, orange, Figure 5 | 0.21 |
| ICH_MTP-1 | Hemorrhagic | Multi-tissue | 1, blue, Figure 6 | 0.20 |
| ICH_MTP-3 | Hemorrhagic | Multi-tissue | 3, red, Figure 6 | 0.22 |
| IS_MTP-2 | Ischemic | Multi-tissue | 2, green, Figure 6 | 0.21 |
| IS_MTP-3 | Ischemic | Multi-tissue | 3, green, Figure 6 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Duarte, D.O.; Tobon Vasquez, J.A.; Scapaticci, R.; Turvani, G.; Cavagnaro, M.; Casu, M.R.; Crocco, L.; Vipiana, F. Experimental Validation of a Microwave System for Brain Stroke 3-D Imaging. Diagnostics 2021, 11, 1232. https://doi.org/10.3390/diagnostics11071232
Rodriguez-Duarte DO, Tobon Vasquez JA, Scapaticci R, Turvani G, Cavagnaro M, Casu MR, Crocco L, Vipiana F. Experimental Validation of a Microwave System for Brain Stroke 3-D Imaging. Diagnostics. 2021; 11(7):1232. https://doi.org/10.3390/diagnostics11071232
Chicago/Turabian StyleRodriguez-Duarte, David O., Jorge A. Tobon Vasquez, Rosa Scapaticci, Giovanna Turvani, Marta Cavagnaro, Mario R. Casu, Lorenzo Crocco, and Francesca Vipiana. 2021. "Experimental Validation of a Microwave System for Brain Stroke 3-D Imaging" Diagnostics 11, no. 7: 1232. https://doi.org/10.3390/diagnostics11071232
APA StyleRodriguez-Duarte, D. O., Tobon Vasquez, J. A., Scapaticci, R., Turvani, G., Cavagnaro, M., Casu, M. R., Crocco, L., & Vipiana, F. (2021). Experimental Validation of a Microwave System for Brain Stroke 3-D Imaging. Diagnostics, 11(7), 1232. https://doi.org/10.3390/diagnostics11071232










