Diagnostic Performance of a Contrast-Enhanced Ultra-Low-Dose High-Pitch CT Protocol with Reduced Scan Range for Detection of Pulmonary Embolisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Image Acquisition and Reconstruction Parameters
2.3. Radiation Dose
2.4. Analysis of Subjective Image Quality
2.5. Analysis of Objective Image Quality
2.6. Diagnostic Accuracy and Disease Severity
2.7. Statistical Analysis
3. Results
3.1. Patient Population, Scan Range, and Radiation Dose
3.2. Analysis of Subjective Image Quality
3.3. Analysis of Objective Image Quality
3.4. Diagnostic Accuracy and Severity
3.4.1. Patient-Based Accuracy
3.4.2. Segment-Based Accuracy
3.4.3. Disease Severity
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Standard Protocol * ULD Protocol Crosstabulation | Chi-Square Tests | Symmetric Measures | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment | Count | % within Standard | ||||||||||||
| ULD | Total | ULD | Total | Pearson Chi-Square | Fisher’s Exact Test | Nominal Cramer’s V | ||||||||
| 1 | 0 | 1 | 0 | Value | df | Asymptotic Significance (2-sided) | Exact Significance (2-sided) | Approximate Significance | Value | |||||
| 01 RUL anterior segment | Standard | 1 | 20 | 0 | 20 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 22 | 22 | 0.0% | 100.0% | 100.0% | ||||||||
| 02 RUL apical segment | Standard | 1 | 20 | 0 | 20 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 22 | 22 | 0.0% | 100.0% | 100.0% | ||||||||
| 03 RUL posterior segment | Standard | 1 | 19 | 0 | 19 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 23 | 23 | 0.0% | 100.0% | 100.0% | ||||||||
| 04 RML apical segment | Standard | 1 | 22 | 0 | 22 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 20 | 20 | 0.0% | 100.0% | 100.0% | ||||||||
| 05 RML basal segment | Standard | 1 | 22 | 0 | 22 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 20 | 20 | 0.0% | 100.0% | 100.0% | ||||||||
| 06 RLL lateral basal segment | Standard | 1 | 21 | 0 | 21 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 21 | 21 | 0.0% | 100.0% | 100.0% | ||||||||
| 07 RLL anterior basal segment | Standard | 1 | 18 | 0 | 18 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 24 | 24 | 0.0% | 100.0% | 100.0% | ||||||||
| 08 RLL medial basal segment | Standard | 1 | 20 | 0 | 20 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 22 | 22 | 0.0% | 100.0% | 100.0% | ||||||||
| 09 RLL posterior basal segment | Standard | 1 | 26 | 0 | 26 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 16 | 16 | 0.0% | 100.0% | 100.0% | ||||||||
| 10 RLL superior segment | Standard | 1 | 19 | 0 | 19 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 23 | 23 | 0.0% | 100.0% | 100.0% | ||||||||
| 11 LUL anterior segment | Standard | 1 | 13 | 0 | 13 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 29 | 29 | 0.0% | 100.0% | 100.0% | ||||||||
| 12 LUL anterior apical segment | Standard | 1 | 13 | 0 | 13 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 29 | 29 | 0.0% | 100.0% | 100.0% | ||||||||
| 13 LUL apical posterior segment | Standard | 1 | 16 | 2 | 18 | 88.9% | 11.1% | 100.0% | 34.462 | 1 | 0.000 | 0.000 | 0.000 | 0.906 |
| 0 | 0 | 24 | 24 | 0.0% | 100.0% | 100.0% | (95% CI 0.751–1.000) | |||||||
| 14 LUL apical lingula segment | Standard | 1 | 12 | 0 | 12 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 30 | 30 | 0.0% | 100.0% | 100.0% | ||||||||
| 15 LUL basal lingula segment | Standard | 1 | 12 | 0 | 12 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 30 | 30 | 0.0% | 100.0% | 100.0% | ||||||||
| 16 LLL lateral segment | Standard | 1 | 16 | 0 | 16 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 26 | 26 | 0.0% | 100.0% | 100.0% | ||||||||
| 17 LLL anteromedial segment | Standard | 1 | 15 | 0 | 15 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 27 | 27 | 0.0% | 100.0% | 100.0% | ||||||||
| 18 LLL posteromedial segment | Standard | 1 | 21 | 1 | 22 | 95.5% | 4.5% | 100.0% | 38.182 | 1 | 0.000 | 0.000 | 0.000 | 0.953 |
| 0 | 0 | 20 | 20 | 0.0% | 100.0% | 100.0% | (95% CI 0.904–1.000) | |||||||
| 19 LLL posterolateral segment | Standard | 1 | 21 | 1 | 22 | 95.5% | 4.5% | 100.0% | 38.182 | 1 | 0.000 | 0.000 | 0.000 | 0.953 |
| 0 | 0 | 20 | 20 | 0.0% | 100.0% | 100.0% | (95% CI 0.898–1.000) | |||||||
| 20 LLL superior segment | Standard | 1 | 9 | 0 | 9 | 100.0% | 0.0% | 100.0% | 42.000 | 1 | 0.000 | 0.000 | 0.000 | 1.000 |
| 0 | 0 | 33 | 33 | 0.0% | 100.0% | 100.0% | ||||||||
References
- Belohlavek, J.; Dytrych, V.; Linhart, A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp. Clin. Cardiol. 2013, 18, 129–138. [Google Scholar]
- Kurcz, J.; Garcarek, J.; Guziński, M.; Czarnecka, A.; Sąsiadek, M.J. Multislice computed tomography angiography as an imaging modality of choice in patients with suspicion of pulmonary embolism-own experiences and modern imaging techniques. Adv. Clin. Exp. Med. 2013, 22, 705–713. [Google Scholar] [PubMed]
- DeMonaco, N.A.; Dang, Q.; Kapoor, W.N.; Ragni, M.V. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am. J. Med. 2008, 121, 611–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, E.A.; Yoon, H.C. Four-year cumulative radiation exposure in patients undergoing computed tomography angiography for suspected pulmonary embolism. Radiol. Res. Pract. 2013, 2013, 482403. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrington de González, A.; Mahesh, M.; Kim, K.-P.; Bhargavan, M.; Lewis, R.; Mettler, F.; Land, C. Projected Cancer Risks From Computed Tomographic Scans Performed in the United States in 2007. Arch. Intern. Med. 2009, 169, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Afat, S.; Brockmann, C.; Nikoubashman, O.; Muller, M.; Thierfelder, K.M.; Brockmann, M.A.; Nikolaou, K.; Wiesmann, M.; Kim, J.H.; Othman, A.E. Diagnostic Accuracy of Simulated Low-Dose Perfusion CT to Detect Cerebral Perfusion Impairment after Aneurysmal Subarachnoid Hemorrhage: A Retrospective Analysis. Radiology 2018, 287, 643–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, A.E.; Bongers, M.N.; Zinsser, D.; Schabel, C.; Wichmann, J.L.; Arshid, R.; Notohamiprodjo, M.; Nikolaou, K.; Bamberg, F. Evaluation of reduced-dose CT for acute non-traumatic abdominal pain: Evaluation of diagnostic accuracy in comparison to standard-dose CT. Acta Radiol. 2018, 59, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.H.; Davis, R.; Cooke, J.; Anderson, W. A comparison of radiation doses to the breast in computed tomographic chest examinations for two scanning protocols. Clin. Radiol. 1989, 40, 45–46. [Google Scholar] [CrossRef]
- Parker, L. Does radiation associated with diagnostic CT scans in children and young people increase their subsequent risk of developing cancer? Arch. Dis. Child. Educ. Pract. 2013, 98, 239. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Walter, S.S.; Stock, E.; Brendlin, A.; Kolb, M.; Othman, A.E.; Afat, S. Effects of radiation dose reduction on diagnostic performance of 3rd generation Dual Source CT pulmonary angiography. Eur. J. Radiol. 2021, 134, 109426. [Google Scholar] [CrossRef]
- Atalay, M.K.; Walle, N.L.; Egglin, T.K. Prevalence and nature of excluded findings at reduced scan length CT angiography for pulmonary embolism. J. Cardiovasc. Comput. 2011, 5, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Atalay, M.K.; Walle, N.L.; Grand, D.J.; Mayo-Smith, W.W.; Cronan, J.J.; Egglin, T.K. Scan length optimization for pulmonary embolism at CT angiography: Analysis based on the three-dimensional spatial distribution of 370 emboli in 100 patients. Clin. Radiol. 2011, 66, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Menzel, H.-G.; Schibilla, H.; Teunen, D. European Guidelines on Quality Criteria for Computed Tomography, EUR 16260. Available online: http://www.drs.dk/guidelines/ct/quality/htmlindex.htm (accessed on 1 February 2021).
- Qanadli, S.D.; El Hajjam, M.; Vieillard-Baron, A.; Joseph, T.; Mesurolle, B.; Oliva, V.L.; Barré, O.; Bruckert, F.; Dubourg, O.; Lacombe, P. New CT Index to Quantify Arterial Obstruction in Pulmonary Embolism. Am. J. Roentgenol. 2001, 176, 1415–1420. [Google Scholar] [CrossRef]
- Bobak, C.A.; Barr, P.J.; O’Malley, A.J. Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Med. Res. Methodol. 2018, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Koehler, T.; Fingerle, A.A.; Brendel, B.; Richter, V.; Rasper, M.; Rummeny, E.J.; Noel, P.B.; Munzel, D. Ultra Low Dose CT Pulmonary Angiography with Iterative Reconstruction. PLoS ONE 2016, 11, e0162716. [Google Scholar] [CrossRef] [Green Version]
- Laqmani, A.; Regier, M.; Veldhoen, S.; Backhaus, A.; Wassenberg, F.; Sehner, S.; Groth, M.; Nagel, H.-D.; Adam, G.; Henes, F.O. Improved image quality and low radiation dose with hybrid iterative reconstruction with 80 kV CT pulmonary angiography. Eur. J. Radiol. 2014, 83, 1962–1969. [Google Scholar] [CrossRef]
- Kaul, D.; Grupp, U.; Kahn, J.; Ghadjar, P.; Wiener, E.; Hamm, B.; Streitparth, F. Reducing radiation dose in the diagnosis of pulmonary embolism using adaptive statistical iterative reconstruction and lower tube potential in computed tomography. Eur. Radiol. 2014, 24, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Gordic, S.; Desbiolles, L.; Stolzmann, P.; Gantner, L.; Leschka, S.; Husarik, D.B.; Alkadhi, H. Advanced modelled iterative reconstruction for abdominal CT: Qualitative and quantitative evaluation. Clin. Radiol. 2014, 69, e497–e504. [Google Scholar] [CrossRef]
- Uehara, M.; Tanabe, N.; Funabashi, N.; Takaoka, H.; Ikari, J.; Toyama, S.; Shimizu, H.; Hoshino, S.; Sugiura, T.; Saito, M. Detailed distribution of acute pulmonary thromboemboli: Direct evidence for reduction of acquisition length and radiation dose for triple rule-out CT angiography. Int. J. Cardiol. 2011, 147, 234–238. [Google Scholar] [CrossRef]
- Kallen, J.A.; Coughlin, B.F.; O’Loughlin, M.T.; Stein, B. Reduced Z-axis coverage multidetector CT angiography for suspected acute pulmonary embolism could decrease dose and maintain diagnostic accuracy. Emerg. Radiol. 2010, 17, 31. [Google Scholar] [CrossRef]
- Remy-Jardin, M.; Pistolesi, M.; Goodman, L.R.; Gefter, W.B.; Gottschalk, A.; Mayo, J.R.; Sostman, H.D. Management of suspected acute pulmonary embolism in the era of CT angiography: A statement from the Fleischner Society. Radiology 2007, 245, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Prologo, J.D.; Gilkeson, R.C.; Diaz, M.; Cummings, M. The effect of single-detector CT versus MDCT on clinical outcomes in patients with suspected acute pulmonary embolism and negative results on CT pulmonary angiography. Am. J. Roentgenol. 2005, 184, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.; Kucher, N.; Nguyen, P.L.; Fanikos, J.; Marks, P.W.; Goldhaber, S.Z. The effect of excessive anticoagulation on mortality and morbidity in hospitalized patients with anticoagulant-related major hemorrhage. Arch. Intern. Med. 2004, 164, 1557–1560. [Google Scholar] [CrossRef] [Green Version]
- Suh, J.M.; Cronan, J.J.; Healey, T.T. Dots are not clots: The over-diagnosis and over-treatment of PE. Emerg. Radiol. 2010, 17, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Oden, A.; Fahlen, M. Oral anticoagulation and risk of death: A medical record linkage study. Br. Med. J. 2002, 325, 1073–1075. [Google Scholar] [CrossRef] [Green Version]

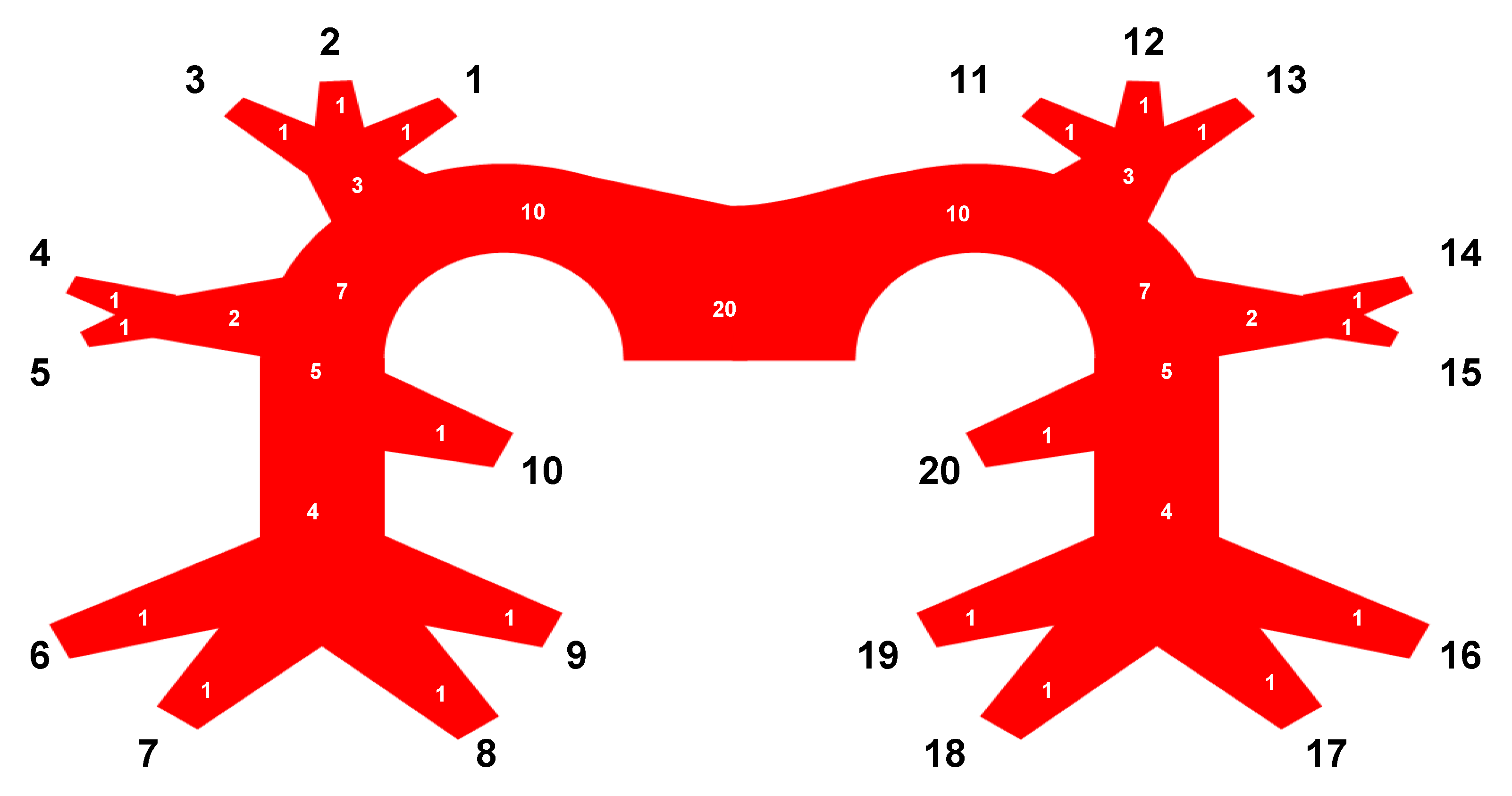




| # | Lobe | Vessel Segment | # | Lobe | Vessel Segment |
|---|---|---|---|---|---|
| 1 | RUL | Anterior segment | 11 | LUL | Anterior segment |
| 2 | RUL | Apical segment | 12 | LUL | Anterior apical segment |
| 3 | RUL | Posterior segment | 13 | LUL | Apical posterior segment |
| 4 | RML | Apical segment | 14 | LUL | Apical lingula segment |
| 5 | RML | Basal segment | 15 | LUL | Basal lingula segment |
| 6 | RLL | Lateral basal segment | 16 | LLL | Lateral segment |
| 7 | RLL | Anterior basal segment | 17 | LLL | Anteromedial segment |
| 8 | RLL | Medial basal segment | 18 | LLL | Posteromedial segment |
| 9 | RLL | Posterior basal segment | 19 | LLL | Posterolateral segment |
| 10 | RLL | Superior segment | 20 | LLL | Superior segment |
| Parameter | Male | Female | Total |
|---|---|---|---|
| Patient population | |||
| Absolute (n) | 61 | 69 | 130 |
| Reconstructions (n) | 122 | 138 | 260 |
| Mean age (y) | 65 ± 16 | 63 ± 18 | 64 ± 16 |
| Mean BMI | 27.7 ± 2.4 | 27.6 ± 1.7 | 27.7 ± 2.1 |
| Contrast medium (mL) | 62 ± 21 | 54 ± 20 | 58 ± 20 |
| Scan range | |||
| Mean scan range in standard protocol (cm) | 29.7 ± 4.1 | 28.7 ± 4.1 | 29.2 ± 4.1 |
| Mean scan range in ULD protocol (cm) | 18.7 ± 2.7 | 17.9 ± 2.7 | 18.3 ± 2.7 |
| Diagnosis (n) | |||
| Pulmonary embolism | 20 | 22 | 42 |
| Other findings causative of acute thoracic symptoms | |||
| Pleural effusion | 22 | 24 | 46 |
| Pulmonary xongestion | 14 | 9 | 23 |
| Pulmonary infiltrate | 7 | 3 | 10 |
| Pneumothorax | 4 | 2 | 6 |
| No causative findings | 1 | 2 | 3 |
| kV | 108 (80–120) | 104 (80–120) | 106 (80–120) |
| mAs | 197 | 190 | 193 |
| mAs ref | 180 | 180 | 180 |
| CTDI | 6.93 ± 2.76 | 7.07 ± 3.5 | 7 ± 3.17 |
| DLP (mGy) | 243.4 ± 96.31 | 236.26 ± 91.86 | 239.61 ± 95.82 |
| Mean effective radiation dose (ED) | |||
| Standard protocol (100% mAs, ADMIRE 3, full scan range) | 4.0 ± 1.6 | 4.6 ± 1.8 | 4.3 ± 1.7 |
| ULD protocol (25% mAs, ADMIRE 5, reduced scan range) | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.7 ± 0.3 |
| Protocol | ED | Rating | ICC | ICC: 95% CI | Standard vs. ULD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mSv) | Median | IQR | Av. Measure | Lower Bound | Upper Bound | p | r | ||||
| Image quality | Standard | 4.3 ± 1.7 | 5 | 4 | 5 | 0.971 | 0.961 | 0.979 | 0.486 | >0.5 | |
| ULD | 0.7 ± 0.3 | 4 | 3 | 5 | 0.967 | 0.956 | 0.976 | ||||
| Diagnostic Confidence | Standard | 4.3 ± 1.7 | 5 | 4 | 5 | 0.979 | 0.955 | 0.975 | 0.28 | >0.5 | |
| ULD | 0.7 ± 0.3 | 4 | 4 | 5 | 0.953 | 0.937 | 0.965 | ||||
| Protocol | ED | Value | ICC: 95% CI | ||
|---|---|---|---|---|---|
| (mSv) | Patient-Based Analysis | Lower Bound | Upper Bound | ||
| ULD | 0.7 ± 0.3 | Sensitivity | 92.9% | 80.5 | 98.5 |
| Specificity | 100% | 95.9 | 100 | ||
| Positive likelihood ratio | |||||
| Negative likelihood ratio | 0.1 | 0 | 0.2 | ||
| Disease prevalence | 32.3% | 24.4 | 41.1 | ||
| Positive predictive value | 100% | ||||
| Negative predictive value | 96.7% | 90.8 | 98.9 | ||
| Accuracy | 97.7% | 93.4 | 99.5 | ||
| Protocol | ED | Value | ICC: 95% CI | ||
|---|---|---|---|---|---|
| (mSv) | Segment-Based Analysis | Lower Bound | Upper Bound | ||
| ULD | 0.7 ± 0.3 | Sensitivity | 98.9% | 97.2 | 99.7 |
| Specificity | 100% | 99.8 | 100 | ||
| Positive likelihood ratio | |||||
| Negative likelihood ratio | 0.01 | 0 | 0.3 | ||
| Disease prevalence | 13.81% | 12.5 | 15.2 | ||
| Positive predictive value | 100% | ||||
| Negative predictive value | 99.8% | 99.5 | 99.9 | ||
| Accuracy | 99.9% | 98.6 | 100 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brendlin, A.S.; Winkelmann, M.T.; Peisen, F.; Artzner, C.P.; Nikolaou, K.; Othman, A.E.; Afat, S. Diagnostic Performance of a Contrast-Enhanced Ultra-Low-Dose High-Pitch CT Protocol with Reduced Scan Range for Detection of Pulmonary Embolisms. Diagnostics 2021, 11, 1251. https://doi.org/10.3390/diagnostics11071251
Brendlin AS, Winkelmann MT, Peisen F, Artzner CP, Nikolaou K, Othman AE, Afat S. Diagnostic Performance of a Contrast-Enhanced Ultra-Low-Dose High-Pitch CT Protocol with Reduced Scan Range for Detection of Pulmonary Embolisms. Diagnostics. 2021; 11(7):1251. https://doi.org/10.3390/diagnostics11071251
Chicago/Turabian StyleBrendlin, Andreas S., Moritz T. Winkelmann, Felix Peisen, Christoph P. Artzner, Konstantin Nikolaou, Ahmed E. Othman, and Saif Afat. 2021. "Diagnostic Performance of a Contrast-Enhanced Ultra-Low-Dose High-Pitch CT Protocol with Reduced Scan Range for Detection of Pulmonary Embolisms" Diagnostics 11, no. 7: 1251. https://doi.org/10.3390/diagnostics11071251
APA StyleBrendlin, A. S., Winkelmann, M. T., Peisen, F., Artzner, C. P., Nikolaou, K., Othman, A. E., & Afat, S. (2021). Diagnostic Performance of a Contrast-Enhanced Ultra-Low-Dose High-Pitch CT Protocol with Reduced Scan Range for Detection of Pulmonary Embolisms. Diagnostics, 11(7), 1251. https://doi.org/10.3390/diagnostics11071251







