Forensic Post-Mortem Investigation in AAS Abusers: Investigative Diagnostic Protocol. A Systematic Review
Abstract
1. Introduction
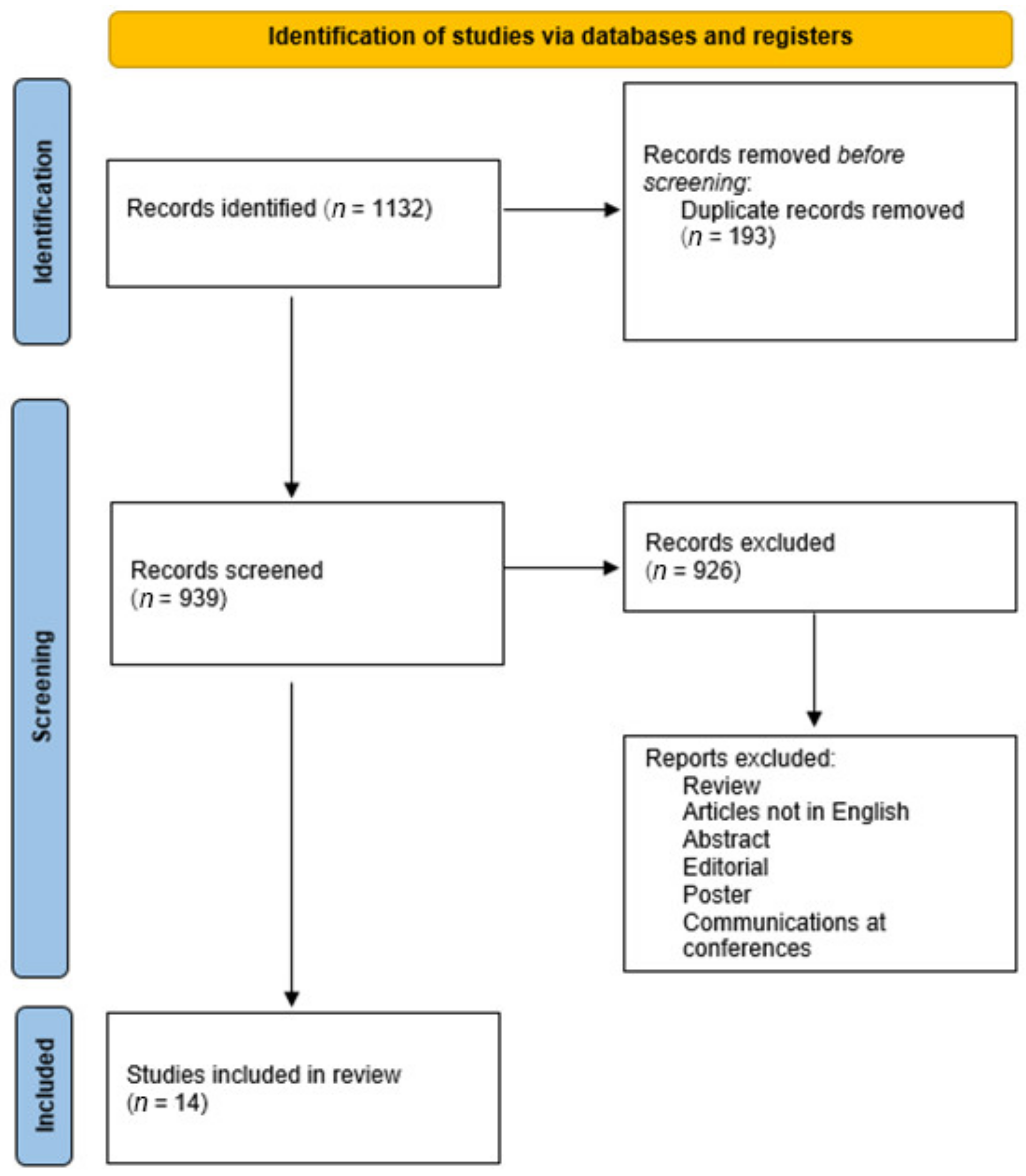
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Quality Assessment and Data Extraction
2.3. Characteristics of Eligible Studies
3. Results
4. Discussion
- A complete autopsy with special regard to AAS target organs and apparatus (the cardiovascular system above all);
- Histological analysis of AAS target organs, with a focus on concentric cardiac hypertrophy, coronary thrombosis, left ventricle hypertrophy (LVH), fibroblasts cardiac proliferation, and myocytolysis;
- A broad toxicological investigation, preceded by a careful evaluation of clinical-anamnestic data, to confirm AAS consumption (including the type of AAS, concentration, and interval of exposure) and possible detection of other substances that could have contributed to the fatal outcome. For this purpose, different matrices can be used; urine is the most common because it provides a prolonged detection window, but several other matrices, such as blood, serum, plasma, hair, oral fluid, and nails can also be used; in addition, gonad samples could be useful to detect early adverse effects, such as hypogonadism or azoospermia [74,88].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-Guerra, A.I.; Tapia, J.; Menéndez-Quintanal, L.M.; Lucena, J.S. Sudden cardiac death in anabolic androgenic steroids abuse: Case report and literature review. Forensic Sci. Res. 2019, 4, 267–273. [Google Scholar] [CrossRef]
- Piacentino, D.; Kotzalidis, G.D.; Del Casale, A.; Aromatario, M.R.; Pomara, C.; Girardi, P.; Sani, G. Anabolic-androgenic steroid use and psychopathology in athletes. A systematic review. Curr. Neuropharmacol. 2015, 13, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.F.; Gamaleddin, I.; Alsubaie, E.G.; Al-Surimi, K.M. Prevalence and risk factors associated with anabolic-androgenic steroid use: A cross-sectional study among gym users in Riyadh, Saudi Arabia. Oman Med. J. 2020, 35, e110. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.; Schoenfeld, B.J.; Nakazato, K. The role of hormones in muscle hypertrophy. Phys. Sportsmed. 2018, 46, 129–134. [Google Scholar] [CrossRef]
- Pomara, C.; Neri, M.; Bello, S.; Fiore, C.; Riezzo, I.; Turillazzi, E. Neurotoxicity by synthetic androgen steroids: Oxidative stress, apoptosis, and neuropathology: A review. Curr. Neuropharmacol. 2015, 13, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, G.; Sessa, F.; Maglietta, F. Immunodeficiency as a side effect of anabolic androgenic steroid abuse: A case of necrotizing myofasciitis. Forensic Sci. Med. Pathol. 2019, 15, 616–621. [Google Scholar] [CrossRef]
- Torrisi., M.; Pennisi, G.; Russo, I. Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review. Medicina 2020, 56, 587. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; Cipolloni, L. Anabolic-androgenic steroids and brain injury: miRNA evaluation in users compared to cocaine abusers and elderly people. Aging 2020, 12, 15314–15327. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Salerno, M.; Bertozzi, G. miRNAs as Novel Biomarkers of Chronic Kidney Injury in Anabolic-Androgenic Steroid Users: An Experimental Study. Front. Pharmacol. 2020, 11, 563756. [Google Scholar] [CrossRef]
- Reyes-Vallejo, L. Uso y abuso de agentes anabolizantes en la actualidad [Current use and abuse of anabolic steroids]. Actas Urol. Esp. 2020, 44, 309–313. [Google Scholar] [CrossRef]
- Fineschi, V.; Neri, M.; Di Donato, S.; Pomara, C.; Riezzo, I.; Turillazzi, E. An immunohistochemical study in a fatality due to ovarian hyperstimulation syndrome. Int. J. Leg. Med. 2006, 120, 293–299. [Google Scholar] [CrossRef]
- Montisci, M.; Basso, C. Doping e Morte Improvvisa. In Doping Antidoping; Ferrara, S.D., Ed.; Piccin: Padova, Italy, 2004; pp. 385–413. [Google Scholar]
- Bertozzi, G.; Salerno, M.; Pomara, C.; Sessa, F. Neuropsychiatric and Behavioral Involvement in AAS Abusers. A Literature Review. Medicina 2019, 55, 396. [Google Scholar] [CrossRef]
- Hartgens, F.; Kuipers, H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004, 34, 513–554. [Google Scholar] [CrossRef]
- Pomara., C.; Barone, R.; Marino Gammazza, A.; Sangiorgi, C.; Barone, F.; Pitruzzella, A.; Locorotondo, N.; Di Gaudio, F.; Salerno, M.; Maglietta, F.; et al. Effects of Nandrolone Stimulation on Testosterone Biosynthesis in Leydig Cells. J. Cell. Physiol. 2016, 231, 1385–1391. [Google Scholar] [CrossRef]
- Sullivan, M.L.; Martinez, C.M.; Gennis, P.; Gallagher, E.J. The cardiac toxicity of anabolic steroids. Prog. Cardiovasc. Dis. 1998, 41, 1–15. [Google Scholar] [CrossRef]
- Urhausen, A.; Albers, T.; Kindermann, W. Are the cardiac effects of anabolic steroid abuse in strength athletes reversible? Heart 2004, 90, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef]
- Fineschi, V.; Di Paolo, M.; Neri, M. Anabolic steroid- and exercise-induced cardio-depressant cytokines and myocardial β1 receptor expression in CD1 mice. Curr. Pharm. Biotechnol. 2011, 12, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Junior, P.P.; Chaves, E.A.; Costa-E-Sousa, R.H.; Masuda, M.O.; de Carvalho, A.C.; Nascimento, J.H. Cardiac autonomic dysfunction in rats chronically treated with anabolic steroid. Eur. J. Appl. Physiol. 2006, 96, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, M.; Agozzino, M.; Toni, C. Sudden anabolic steroid abuse-related death in athletes. Int. J. Cardiol. 2007, 114, 114–117. [Google Scholar] [CrossRef]
- Furlanello, F.; Serdoz, L.V.; Cappato, R.; de Ambroggi, L. Illicit drugs and cardiac arrhythmias in athletes. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Salerno, M.; Di Mizio, G. Anabolic Androgenic Steroids: Searching New Molecular Biomarkers. Front. Pharmacol. 2018, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T.; Bergeron, N.; Chiu, S.; Krauss, R.M. A randomized, controlled trial on the effects of almonds on lipoprotein response to a higher carbohydrate, lower fat diet in men and women with abdominal adiposity. Lipids Health Dis. 2019, 18, 83. [Google Scholar] [CrossRef]
- Parker, E.D.; Schmitz, K.H.; Jacobs, D.R., Jr.; Dengel, D.R.; Schreiner, P.J. Physical activity in young adults and incident hypertension over 15 years of follow-up: The CARDIA study. Am. J. Public Health 2007, 97, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, D.E.; Lakka, H.M.; Salonen, J.T.; Niskanen, L.K.; Rauramaa, R.; Lakka, T.A. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002, 25, 1612–1618. [Google Scholar] [CrossRef]
- Frati, P.; Busardò, F.P.; Cipolloni, L.; Dominicis, E.D.; Fineschi, V. Anabolic Androgenic Steroid (AAS) related deaths: Autoptic, histopathological and toxicological findings. Curr. Neuropharmacol. 2015, 13, 146–159. [Google Scholar] [CrossRef]
- Petersson, A.; Garle, M.; Holmgren, P.; Druid, H.; Krantz, P.; Thiblin, I. Toxicological findings and manner of death in autopsied users of anabolic androgenic steroids. Drug Alcohol Depend. 2006, 81, 241–249. [Google Scholar] [CrossRef]
- Hausmann, R.; Hammer, S.; Betz, P. Performance enhancing drugs (doping agents) and sudden death—A case report and review of the literature. Int. J. Leg. Med. 1998, 111, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Casavant, M.J.; Blake, K.; Griffith, J.; Yates, A.; Copley, L.M. Consequences of use of anabolic androgenic steroids. Pediatr. Clin. N. Am. 2007, 54, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Strano-Rossi, S.; Fiore, C.; Chiarotti, M.; Centini, F. Analytical techniques in androgen anabolic steroids (AASs) analysis for antidoping and forensic purposes. Mini Rev. Med. Chem. 2011, 11, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Polito, R.; Monda, V. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int. J. Mol. Sci. 2020, 21, 3104. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Fanton, L.; Belhani, D.; Vaillant, F.; Tabib, A.; Gomez, L.; Descotes, J.; Dehina, L.; Bui-Xuan, B.; Malicier, D.; Timour, Q. Heart lesions associated with anabolic steroid abuse: Comparison of post-mortem findings in athletes and norethandrolone-induced lesions in rabbits. Exp. Toxicol. Pathol. 2009, 61, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, V.; Riezzo, I.; Centini, F.; Silingardi, E.; Licata, M.; Beduschi, G.; Karch, S.B. Sudden cardiac death during anabolic steroid abuse: Morphologic and toxicologic findings in two fatal cases of bodybuilders. Int. J. Leg. Med. 2007, 121, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Thomas, A.; Schiwy-Bochat, K.H.; Geyer, H.; Thevis, M.; Glenewinkel, F.; Rothschild, M.A.; Andresen-Streichert, H.; Juebner, M. Death after misuse of anabolic substances (clenbuterol, stanozolol and metandienone). Forensic Sci. Int. 2019, 303, 109925. [Google Scholar] [CrossRef] [PubMed]
- Lichtenfeld, J.; Deal, B.J.; Crawford, S. Sudden cardiac arrest following ventricular fibrillation attributed to anabolic steroid use in an adolescent. Cardiol. Young 2016, 26, 996–998. [Google Scholar] [CrossRef]
- Lusetti, M.; Licata, M.; Silingardi, E.; Reggiani Bonetti, L.; Palmiere, C. Pathological changes in anabolic androgenic steroid users. J. Forensic Leg. Med. 2015, 33, 101–104. [Google Scholar] [CrossRef]
- Montisci, M.; El Mazloum, R.; Cecchetto, G.; Terranova, C.; Ferrara, S.D.; Thiene, G.; Basso, C. Anabolic androgenic steroids abuse and cardiac death in athletes: Morphological and toxicological findings in four fatal cases. Forensic Sci. Int. 2012, 217, e13–e18. [Google Scholar] [CrossRef]
- Inoue, H.; Nishida, N.; Ikeda, N.; Tsuji, A.; Kudo, K.; Hanagama, M.; Nata, M. The sudden and unexpected death of a female-to-male transsexual patient. J. Forensic Leg. Med. 2007, 14, 382–386. [Google Scholar] [CrossRef]
- Far, H.R.; Ågren, G.; Thiblin, I. Cardiac hypertrophy in deceased users of anabolic androgenic steroids: An investigation of autopsy findings. Cardiovasc. Pathol. 2012, 21, 312–316. [Google Scholar] [CrossRef]
- Darke, S.; Torok, M.; Duflou, J. Sudden or unnatural deaths involving anabolic-androgenic steroids. J. Forensic Sci. 2014, 59, 1025–1028. [Google Scholar] [CrossRef]
- Thiblin, I.; Mobini-Far, H.; Frisk, M. Sudden unexpected death in a female fitness athlete, with a possible connection to the use of anabolic androgenic steroids (AAS) and ephedrine. Forensic Sci. Int. 2009, 184, 7–11. [Google Scholar] [CrossRef]
- Dufayet, L.; Gorgiard, C.; Vayssette, F.; Barbet, J.; Hoizey, G.; Ludes, B. Death of an apprentice bodybuilder following 2,4-dinitrophenol and clenbuterol intake. Int. J. Leg. Med. 2020, 134, 1003–1006. [Google Scholar] [CrossRef]
- Dickerman, R.D.; Schaller, F.; Prather, I.; McConathy, W.J. Sudden cardiac death in a 20-year-old bodybuilder using anabolic steroids. Cardiology 1995, 86, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Scholz, D.G.; Hagen, P.T.; Ilstrup, D.M.; Edwards, W.D. Age-Related Changes in Normal Human Hearts During the First 10 Decades of Life. Part II (Maturity): A Quantitative Anatomic Study of 765 Specimens from Subjects 20 to 99 Years Old. Mayo Clin. Proc. 1988, 63, 137–146. [Google Scholar] [CrossRef]
- Mandal, R.; Loeffler, A.G.; Salamat, S.; Fritsch, M.K. Organ Weight Changes Associated with Body Mass Index Determined from a Medical Autopsy Population. Am. J. Forensic Med. Pathol. 2012, 33, 382–389. [Google Scholar] [CrossRef]
- Neri, M.; Riezzo, I.; Pomara, C.; Schiavone, S.; Turillazzi, E. Oxidative-Nitrosative Stress and Myocardial Dysfunctions in Sepsis: Evidence from the Literature and Postmortem Observations. Mediat. Inflamm. 2016, 2016, 3423450. [Google Scholar] [CrossRef] [PubMed]
- Lood, Y.; Eklund, A.; Garle, M.; Ahlner, J. Anabolic androgenic steroids in police cases in Sweden 1999–2009. Forensic Sci. Int. 2012, 219, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P.; Gheddar, L.; Ameline, A.; Dumestre-Toulet, V.; Verschoore, M.; Comte, J.; Raul, J.S. Complete post-mortem investigations in a death involving clenbuterol after long-term abuse. J. Anal. Toxicol. 2019, 43, 660–665. [Google Scholar] [CrossRef]
- Thomas, A.; Guddat, S.; Kohler, M.; Krug, O.; Schänzer, W.; Petrou, M.; Thevis, M. Comprehensive plasma-screening for known and unknown substances in doping controls. Rapid Commun. Mass Spectrom. 2010, 24, 1124–1132. [Google Scholar] [CrossRef]
- Görgens, C.; Guddat, S.; Thomas, A.; Wachsmuth, P.; Orlovius, A.K.; Sigmund, G.; Thevis, M.; Schänzer, W. Simplifying and expanding analytical capabilities for various classes of doping agents by means of direct urine injection high performance liquid chromatography high resolution/high accuracy mass spectrometry. J. Pharm. Biomed. Anal. 2016, 131, 482–496. [Google Scholar] [CrossRef]
- Kintz, P.; Gheddar, L.; Raul, J.S. Simultaneous testing for anabolic steroids in human hair specimens collected from various anatomic locations has several advantages when compared with the standard head hair analysis. Drug Test. Anal. 2021. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Xiao, Z.-X.; Ji, A.; Ye, J.; Sun, Q.; Caoet, Y. A systematic analysis of miRNA markers and classification algorithms for forensic body fluid identification. Brief. Bioinform. 2020. [Google Scholar] [CrossRef]
- Zubakov, D.; Boersma, A.W.M.; Choi, Y.; Van Kuijk, P.F.; Wiemer, E.A.C.; Kayser, M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int. J. Leg. Med. 2010, 124, 217–226. [Google Scholar] [CrossRef]
- Silva, S.S.; Lopes, C.; Teixeira, A.L.; de Sousa, M.J.C.; Medeiros, R. Forensic miRNA: Potential biomarker for body fluids? Forensic Sci. Int. Genet. 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Courts, C.; Madea, B. Micro-RNA—A potential for forensic science? Forensic Sci. Int. 2010, 203, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Z.H.; Olgin, J.E.; Vittinghoff, E.; Ursell, P.C.; Kim, A.S.; Sporer, K.; Yeh, C.; Colburn, B.; Clark, N.M.; Khan, R.; et al. Prospective Countywide Surveillance and Autopsy Characterization of Sudden Cardiac Death: POST SCD Study. Circulation 2018, 137, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Esposito, M.; Messina, G.; Di Mizio, G.; Di Nunno, N.; Salerno, M. Sudden Death in Adults: A Practical Flow Chart for Pathologist Guidance. Healthcare 2021, 9, 870. [Google Scholar] [CrossRef]
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef]
- Doolan, A.; Langlois, N.; Semsarian, C. Causes of sudden cardiac death in young Australians. Med. J. Aust. 2004, 180, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Agrawal, R.; Arntz, H.R. How sudden is sudden cardiac death? Circulation 2006, 114, 1146–1150. [Google Scholar] [CrossRef]
- Melchert, R.B.; Welder, A.A. Cardiovascular effects of androgenic-anabolic steroids. Med. Sci. Sports Exerc. 1995. [Google Scholar] [CrossRef]
- Pomara, C.; D’Errico, S.; Riezzo, I.; de Cillis, G.P.; Fineschi, V. Sudden cardiac death in a child affected by Prader-Willi syndrome. Int. J. Leg. Med. 2005, 119, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Maior, A.S.; Menezes, P.; Pedrosa, R.C.; Carvalho, D.P.; Soares, P.P.; Nascimento, J.H.M. Abnormal cardiac repolarization in anabolic androgenic steroid users carrying out submaximal exercise testing. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1129–1133. [Google Scholar] [CrossRef]
- Lusetti, M.; Licata, M.; Silingardi, E.; Bonsignore, A.; Palmiere, C. Appearance/Image- and Performance-Enhancing Drug Users: A Forensic Approach. Am. J. Forensic Med. Pathol. 2018, 39, 325–329. [Google Scholar] [CrossRef]
- Sculthorpe, N.; Grace, F.; Jones, P.; Davies, B. Evidence of altered cardiac electrophysiology following prolonged androgenic anabolic steroid use. Cardiovasc. Toxicol. 2010, 10, 239–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agriesti, F.; Tataranni, T.; Pacelli, C.; Scrima, R.; Laurenzana, I.; Ruggieri, V.; Cela, O.; Mazzoccoli, C.; Salerno, M.; Sessa, F.; et al. Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Urhausen, A.; Hölpes, R.; Kindermann, W. One- and two-dimensional echocardiography in bodybuilders using anabolic steroids. Eur. J. Appl. Physiol. Occup Physiol. 1989, 58, 633–640. [Google Scholar] [CrossRef]
- Barone, R.; Pitruzzella, A.; Marino Gammazza, A.; Rappa, F.; Salerno, M.; Barone, F.; Sangiorgi, C.; D’Amico, D.; Locorotondo, N.; Di Gaudio, F.; et al. Nandrolone decanoate interferes with testosterone biosynthesis altering blood-testis barrier components. J. Cell Mol. Med. 2017, 21, 1636–1647. [Google Scholar] [CrossRef]
- Patanè, F.G.; Liberto, A.; Maria Maglitto, A.N.; Malandrino, P.; Esposito, M.; Amico, F.; Cocimano, G.; Rosi, G.L.; Condorelli, D.; Nunno, N.D.; et al. Nandrolone Decanoate: Use, Abuse and Side Effects. Medicina 2020, 56, 606. [Google Scholar] [CrossRef]
- Nieschlag, E.; Vorona, E. Doping with anabolic androgenic steroids (AAS): Adverse effects on non-reproductive organs and functions. Rev. Endocr. Metab. Disord. 2015, 16, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Coward, R.M.; Rajanahally, S.; Kovac, J.R.; Smith, R.P.; Pastuszak, A.W.; Lipshultz, L.I. Anabolic steroid induced hypogonadism in young men. J. Urol. 2013, 190, 2200–2205. [Google Scholar] [CrossRef]
- Wadthaisong, M.; Witayavanitkul, N.; Bupha-Intr, T. Chronic high-dose testosterone treatment: Impact on rat cardiac contractile biology. Physiol. Rep. 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Phillis, B.D.; Abeywardena, M.Y.; Adams, M.J.; Kennedy, J.A.; Irvine, R.J. Nandrolone potentiates arrhythmogenic effects of cardiac ischemia in the Rat. Toxicol. Sci. 2007, 99, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, D.; Riezzo, I.; Fiaschi, A.I.; Centini, F.; Giorgi, G.; D’Errico, S.; Fiore, C.; Karch, S.B.; Neri, M.; Pomara, C.; et al. Cardiac oxidative stress determination and myocardial morphology after a single ecstasy (MDMA) administration in a rat model. Int. J. Leg. Med. 2008, 122, 461–469. [Google Scholar] [CrossRef]
- Rocha, F.L.; Carmo, E.C.; Roque, F.R.; Hashimoto, N.Y.; Rossoni, L.V.; Frimm, C.; Anéas, I.; Negrão, C.E.; Krieger, J.E.; Oliveira, E.M. Anabolic steroids induce cardiac renin-angiotensin system and impair the beneficial effects of aerobic training in rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 3575–3583. [Google Scholar] [CrossRef]
- Shirpoor, A.; Heshmatian, B.; Tofighi, A.; Eliasabad, S.N.; Kheradmand, F.; Zerehpoosh, M. Nandrolone administration with or without strenuous exercise increases cardiac fatal genes overexpression, calcium/calmodulin-dependent protein kinaseiiδ, and monoamine oxidase activities and enhances blood pressure in adult wistar rats. Gene 2019, 697, 131–137. [Google Scholar] [CrossRef]
- Ledda, C.; Pomara, C.; Bracci, M.; Mangano, D.; Ricceri, V.; Musumeci, A.; Ferrante, M.; Musumeci, G.; Loreto, C.; Fenga, C.; et al. Natural carcinogenic fiber and pleural plaques assessment in a general population: A cross-sectional study. Environ. Res. 2016, 150, 23–29. [Google Scholar] [CrossRef]
- Turillazzi, E.; La Rocca, G.; Anzalone, R.; Corrao, S.; Neri, M.; Pomara, C.; Riezzo, I.; Karch, S.B.; Fineschi, V. Heterozygous nonsense SCN5A mutation W822X explains a simultaneous sudden infant death syndrome. Virchows Arch. 2008, 453, 209–216. [Google Scholar] [CrossRef]
- Turillazzi, E.; Baroldi, G.; Silver, M.D.; Parolini, M.; Pomara, C.; Fineschi, V. A systematic study of a myocardial lesion: Colliquative myocytolysis. Int. J. Cardiol. 2005, 104, 152–157. [Google Scholar] [CrossRef]
- Kanayama, G.; Hudson, J.I.; Pope, H.G. Anabolic-Androgenic Steroid Use and Body Image in Men: A Growing Concern for Clinicians. Psychother. Psychosom. 2020, 89, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Pope, H., Jr.; Kanayama, G. Treatment of Anabolic-Androgenic Steroid–Related Disorders. In The American Psychiatric Publishing Textbook of Substance Abuse Treatment; American Psychiatric Publishing: Washington, DC, USA, 2014. [Google Scholar]
- Bertozzi, G.; Sessa, F.; Albano, G.D.; Sani, G.; Maglietta, F.; Roshan, M.H.K.; Volti, G.L.; Bernardini, R.; Avola, R.; Pomara, C.; et al. The Role of Anabolic Androgenic Steroids in Disruption of the Physiological Function in Discrete Areas of the Central Nervous System. Mol. Neurobiol. 2018, 55, 5548–5556. [Google Scholar] [CrossRef]
- Salerno, M.; Cascio, O.; Bertozzi, G.; Sessa, F.; Messina, A.; Monda, V.; Cipolloni, L.; Biondi, A.; Daniele, A.; Pomara, C. Anabolic androgenic steroids and carcinogenicity focusing on Leydig cell: A literature review. Oncotarget 2018, 9, 19415–19426. [Google Scholar] [CrossRef]
- El-Reshaid, W.; El-Reshaid, K.; Al-Bader, S.; Ramadan, A.J. Complementary bodybuilding: A potential risk for permanent kidney disease. Saudi J. Kidney Dis. Transplant. 2018, 29, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil. Steril. 2003, 79, 1659–1661. [Google Scholar] [CrossRef]
- Albano, G.D.; Amico, F.; Cocimano, G.; Liberto, A.; Maglietta, F.; Esposito, M.; Rosi, G.L.; Di Nunno, N.; Salerno, M.; Montana, A. Adverse Effects of Anabolic-Androgenic Steroids: A Literature Review. Healthcare 2021, 9, 97. [Google Scholar] [CrossRef]
- Fineschi, V.; Baroldi, G.; Monciotti, F.; Reattelli, L.P.; Turillazzi, E. Anabolic steroid abuse and cardiac sudden death: A pathologic study. Arch. Pathol. Lab. Med. 2001, 125, 253–255. [Google Scholar] [CrossRef]
- Sessa, F.; Maglietta, F.; Bertozzi, G.; Salerno, M.; Di Mizio, G.; Messina, G.; Montana, A.; Ricci, P.; Pomara, C. Human brain injury and mirnas: An experimental study. Int. J. Mol. Sci. 2019, 20, 1546. [Google Scholar] [CrossRef]



| Reference | Study Design | Number of Cases | Autopsy/Histopathological Findings | Immunohistochemical Findings | AASs/Toxicological Analysis Samples | Toxicological Method | Cause of Death |
|---|---|---|---|---|---|---|---|
| Lehmann S. [37] | Case report | 1 young adult | Hypertrophy of the heart with enlargement of the ventricular walls as well as a small vascular lumen (3 mm diameter) of the right coronary artery | Immunohistochemistry staining was conducted with antibodies against primary antibodies against troponin T that showed inflammation, fibrosis or necrosis |
| HPLC | Pathological changes of the heart (left ventricular hypertrophy) and atherosclerosis of the coronaries |
| Bertozzi G. [6] | Case report | 1 young adult | Left thigh necrotizing myofasciitis | Immunohistochemistry with anti-myoglobin antibodies showed myofibrillar rexis on heart samples |
| GC–MS | AASs adversely influenced the immune response, affecting leucocyte growth or activity, and antibody and cytokine production |
| Lichtenfeld J. [38] | Case report | 1 young adult | Left ventricular myocardium findings: foci of myofibrillar disarray, proliferation of fibroblasts consistent with early fibrosis, and enlarged myofibers with heterogeneity of nuclear size including box car nuclei | Not available |
| Not available | Cardiac arrest attributed to hypertrophic cardiomyopathy from anabolic steroid use, with documented ventricular fibrillation as the initiating arrhythmia |
| Fineschi V. [36] | Case series | 2 young adults | Hepatic injury, including cholestasis, peliosis hepatis, hyperplasia, ventricular fibrillation | Not available |
| GC–MS | Overstimulation of the sympathetic system followed by a transient functional and structural destabilization of the sympathetic axon terminals |
| Fanton L. [35] | Original article (retrospective study) | 6 of 2250 young adults | Coronary thrombosis associated with left ventricle hypertrophy, toxic, adrenergic myocarditis, dilated cardiomyopathy associated with a recent coronary thrombosis | Immunohistochemical staining was conducted with hematoxylin–phloxin–saffron (HPS) antibodies, which showed interstitial inflammatory cells, interstitial reticular fibrosis, concentric stenosing and intimal sclerosis of the heart |
| GC–MS | Various cardiac lesions: misshapen cell nuclei, myolysis, fibrosis and interstitial lesions |
| Lusetti M. [39] | Original article (retrospective study) | 6 of 98 young adults | Pathological changes consisted of various degrees of interstitial and perivascular fibrosis as well as fibroadipose metaplasia and perineural fibrosis within the myocardium of the left ventricle | Immunohistochemistry, antibodies against fibronectin and c5b9 showed a myocardium fibrosis |
| GC–MS | Left ventricular hypertrophy and myocardial fibrosis can create a predisposition to sudden cardiac death |
| Montisci M. [40] | Case report | 4 young adults | Concentric cardiac hypertrophy with focal fibrosis (one case), dilated cardiomyopathy with patchy myocyte death (two cases) and eosinophilic myocarditis (one case). The most typical cardiac abnormality in AAS abusers is left ventricular hypertrophy, associated with fibrosis and myocytolysis | Immunohistochemical analysis through primary antibody against Troponin T showed myocytolysis in the sub-endocardial trabeculae, hypertrophic myocytes with dysmetric and dysmorphic nuclei |
| GC–MS | Three cases of sudden cardiac death (SCD) and one of death due to congestive heart failure of a previously healthy athlete |
| Inoue H. [41] | Case report | 1 young adult | Concentric cardiac hypertrophy was macroscopically observed. In the left and right coronary arteries, atherosclerosis was generally observed within the endothelium | Immunohistochemical analysis through primary antibody against Troponin T showed myocytolysis in the sub-endocardial trabeculae, hyper-trophic myocytes with dysmetric and dysmorphic nuclei |
| Not available | Ischemic heart disease due to coronary stenosis |
| Far M. [42] | Original article (retrospective study) | 87 of 260 adults (1989 to 2009) | A significantly greater cardiac mass among deceased users of AASs compared to individuals with no suspected use of AASs. An elevated risk of developing concentric LVH among AAS users | Not available |
| GC–MS | Cardiac hypertrophy with a direct cardiotropic effect |
| Darke S. [43] | Original article (retrospective study) | 24 adults (1995–2012) | In 23 of 24 cases, substances other than steroids were detected, most commonly psychostimulants (66.7%); in nearly half, testicular atrophy was noted, as was testicular fibrosis and arrested spermatogenesis; left ventricular hypertrophy was noted in 30.4%, and moderate to severe narrowing of the coronary arteries in 26.1% | Not available |
| HPLC | Particularly notable extensive cardiovascular disease |
| Thiblin I. [44] | Case report | 1 young adult | Both the foci of replacement fibrosis and the perivascular inflammatory changes were rather moderate, and probably not severe enough to cause arrhythmia by themselves, both fibrosis and myocardial inflammation are known risk factors for arrhythmia | Not available |
| GC–MS | Sudden cardiac arrhythmia possibly related to a combination of AASs and ephedrine |
| Hernández-Guerra, A. I. [1] | Case report and literature review | 1 young adult | Cardiomegaly (420 g) with a ventricular thickness that was within the upper normal ranges (left ventricular free wall 15 mm, ventricular septum 15 mm, right ventricular free wall 5 mm); acute myocardial infarction at the anterior third of the septum and the left ventricle (LV) anterior wall, subacute myocardial infarction at apical septum and apical posterior LV wall | Immunohistochemical analysis with primary antibodies against troponin T showed small intramyocardial vessels disease with media hypertrophy |
| HS–GC–FID | Myocardial infarction with severe coronary atherosclerosis and acute occlusive thrombosis affecting left main trunk and left anterior descending coronary artery (LAD) (single vessel disease) |
| Dufayet L. [45] | Case report | 1 young adult | Yellow discoloration of the skin, nonspecific signs of asphyxia (cyanosis, pulmonary edema and congestion); Heart: occasional foci of vascular congestion in the connective tissue surrounding coronary arteries; Lungs: edematous and congestive, with some areas of alveolar hemorrhage; Mild congestion was alsoobserved in the centrilobular region of the liver as well as in both kidneys. | Immunohistochemical analysis through primary antibody against Troponin T revealed occasional foci of vascular congestion in the connective tissue surrounding coronary arteries |
| GC– MSHPLC | Toxicological analysis showed high levels of clenbuterol and DNP, confirming an intoxication |
| Dickerman R. D. [46] | Case report | 1 young adult | The heart weighed 250 g with signs of concentric hypertrophy of the left ventricle, atherosclerosis of the vessels | Not available |
| Not available | Cardiac hypertrophy with a direct cardiotropic effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, M.; Licciardello, G.; Privitera, F.; Iannuzzi, S.; Liberto, A.; Sessa, F.; Salerno, M. Forensic Post-Mortem Investigation in AAS Abusers: Investigative Diagnostic Protocol. A Systematic Review. Diagnostics 2021, 11, 1307. https://doi.org/10.3390/diagnostics11081307
Esposito M, Licciardello G, Privitera F, Iannuzzi S, Liberto A, Sessa F, Salerno M. Forensic Post-Mortem Investigation in AAS Abusers: Investigative Diagnostic Protocol. A Systematic Review. Diagnostics. 2021; 11(8):1307. https://doi.org/10.3390/diagnostics11081307
Chicago/Turabian StyleEsposito, Massimiliano, Gabriele Licciardello, Federico Privitera, Salvatore Iannuzzi, Aldo Liberto, Francesco Sessa, and Monica Salerno. 2021. "Forensic Post-Mortem Investigation in AAS Abusers: Investigative Diagnostic Protocol. A Systematic Review" Diagnostics 11, no. 8: 1307. https://doi.org/10.3390/diagnostics11081307
APA StyleEsposito, M., Licciardello, G., Privitera, F., Iannuzzi, S., Liberto, A., Sessa, F., & Salerno, M. (2021). Forensic Post-Mortem Investigation in AAS Abusers: Investigative Diagnostic Protocol. A Systematic Review. Diagnostics, 11(8), 1307. https://doi.org/10.3390/diagnostics11081307









