Quantitative Multispectral Imaging Differentiates Melanoma from Seborrheic Keratosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Multispectral Imaging
2.3. Quantitative Intensity Descriptors
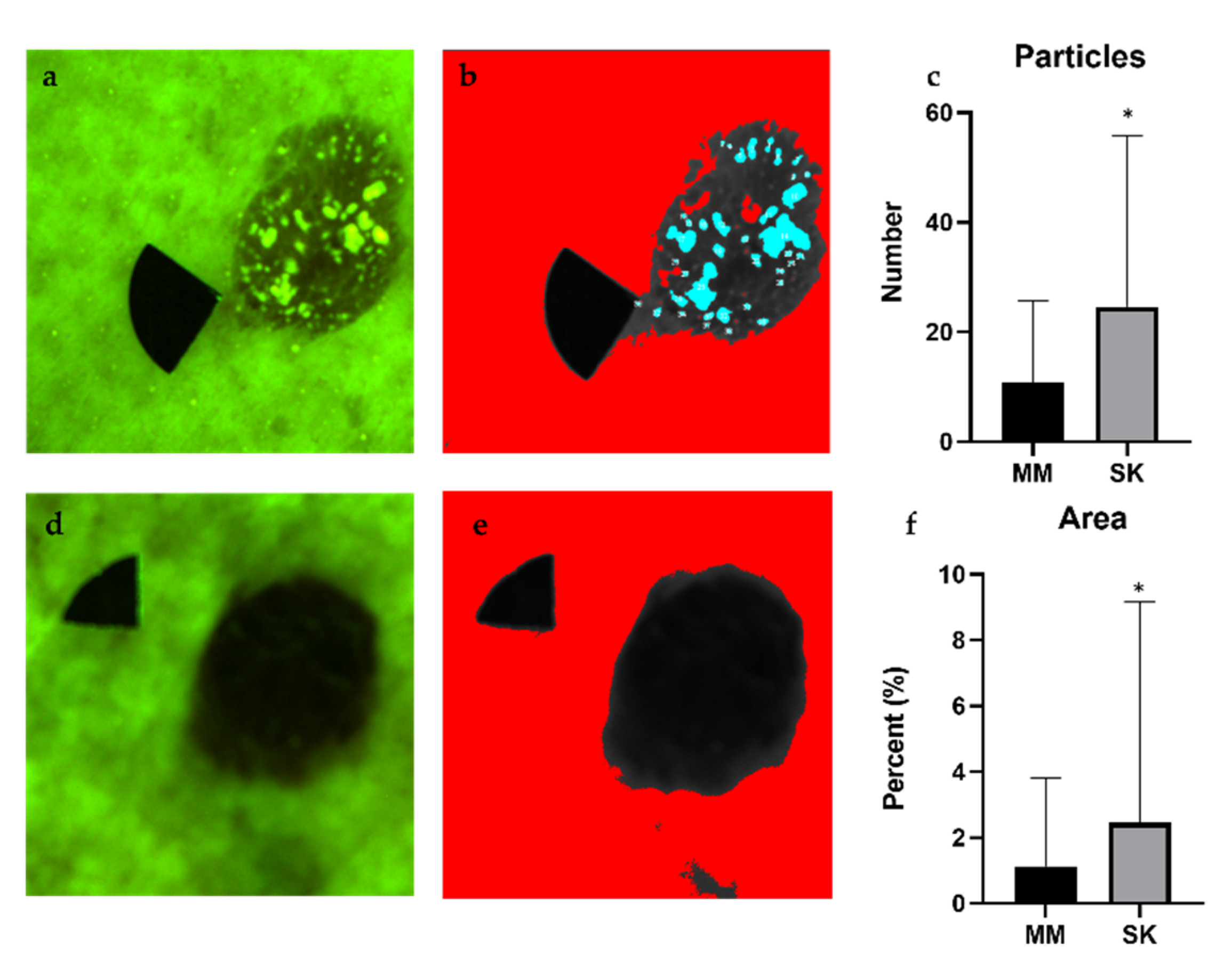
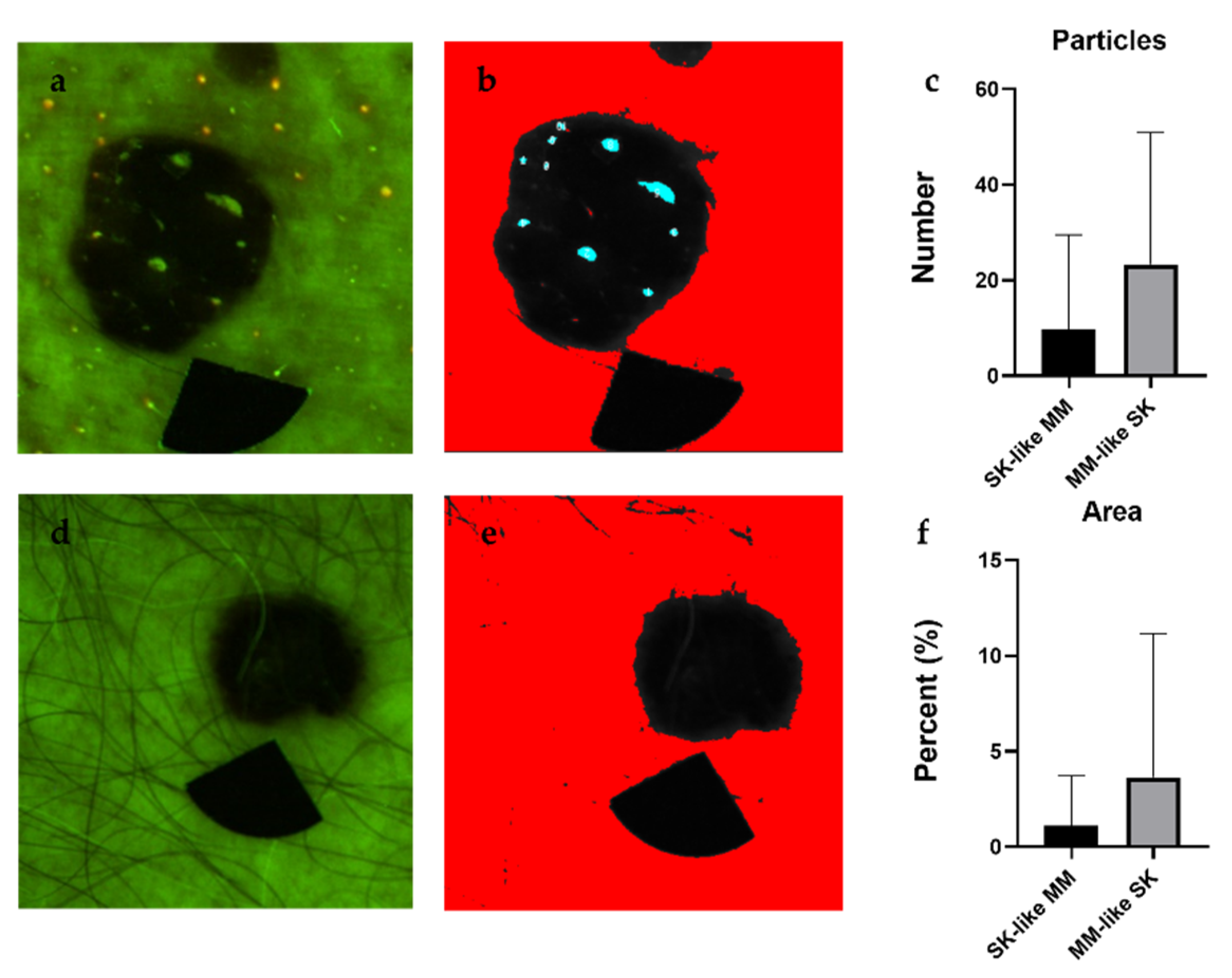
2.4. Analyis of Particles with High Fluorescence
2.5. Calculating the SK Index
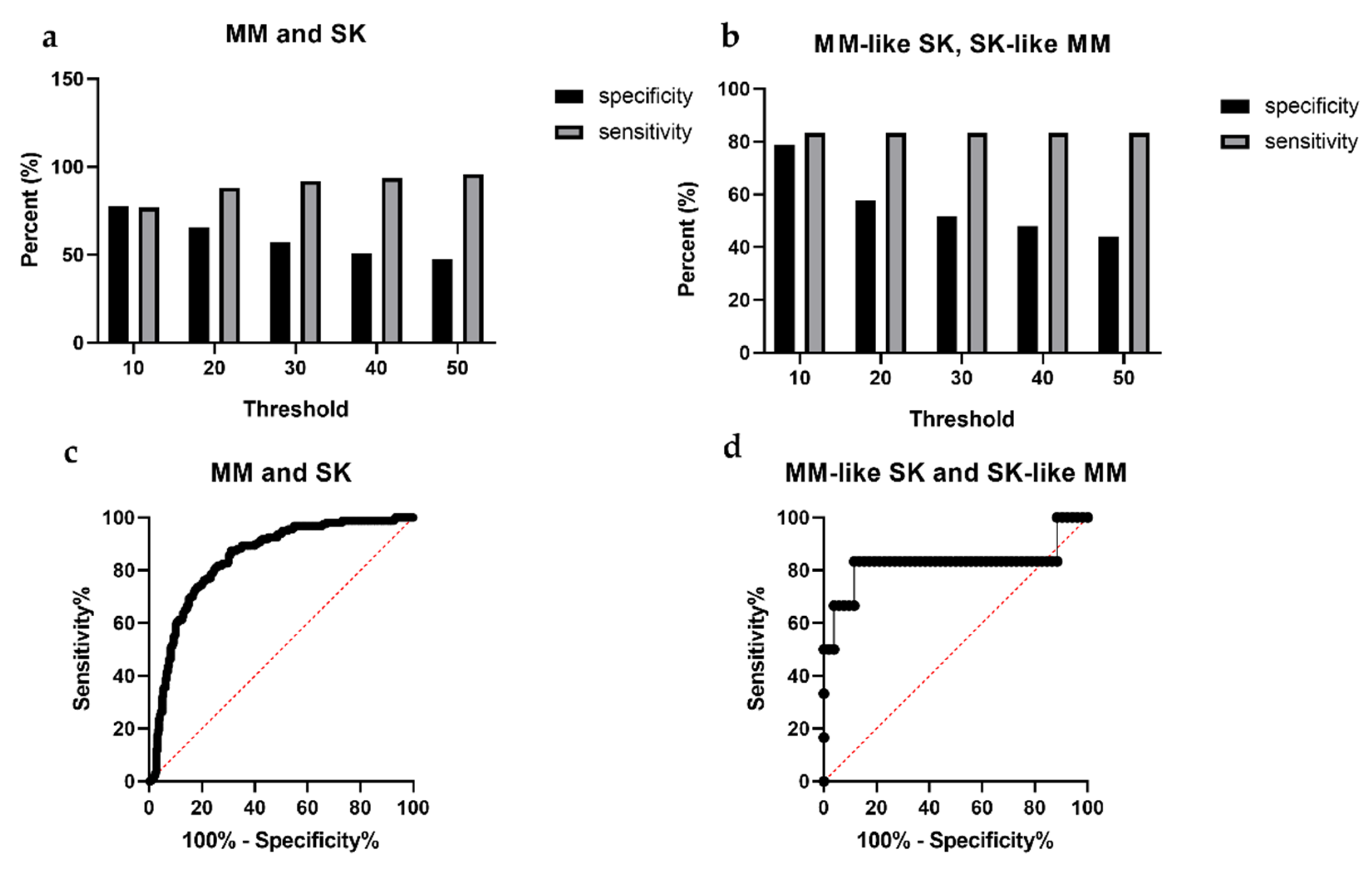
2.6. Statistical Analysis
2.7. Inclusion Criteria
2.8. Exclusion Criteria
3. Results
3.1. Intensity Values
3.2. Particle Analysis
3.3. SK Index
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 9 Regs Research Data, <Katrina/Rita Population Adjustment>-Linked to County Attributes-Total US, 1969–2017 Counties; Nov. 2018 Sub (1973–2016); National Cancer Institute: Bethesda, MD, USA, 2019.
- Matthews, N.H.; Li, W.-Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Cutaneous Melanoma: Etiology and Therapy; Codon Publications: Brisbane, Australia, 2017; Chapter 1; p. 5. [Google Scholar]
- Schadendorf, D.; van Akkooi, A.C.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Albert, D.M.; Niffenegger, A.S.; Willson, J.K. Treatment of metastatic uveal melanoma: Review and recommendations. Surv. Ophthalmol. 1992, 36, 429–438. [Google Scholar] [CrossRef]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002, 3, 159–165. [Google Scholar] [CrossRef]
- Pehamberger, H.; Steiner, A.; Wolff, K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J. Am. Acad. Dermatol. 1987, 17, 571–583. [Google Scholar] [CrossRef]
- Carrera, C.; Marchetti, M.A.; Dusza, S.W.; Argenziano, G.; Braun, R.P.; Halpern, A.C.; Jaimes, N.; Kittler, H.J.; Malvehy, J.; Menzies, S.W.; et al. Validity and Reliability of Dermoscopic Criteria Used to Differentiate Nevi From Melanoma: A Web-Based International Dermoscopy Society Study. JAMA Derm. 2016, 152, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Que, S.K.T. Research techniques made simple: Noninvasive imaging technologies for the delineation of basal cell carcinomas. J. Investig. Dermatol. 2016, 136, e33–e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clebak, K. Dermatoscopy and Skin Cancer: A Handbook for Hunters of Skin Cancer and Melanoma. Fam. Med. 2020, 52, 148–149. [Google Scholar] [CrossRef] [Green Version]
- Marghoob, A.; Braun, R. An Atlas of Dermoscopy, 2nd ed.; Informa Healthcare: London, UK, 2013; p. 58. [Google Scholar]
- Weedon, D. Weedon’s Skin Pathology E-Book: Expert Consult-Online and Print, 3rd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010; Chapter 31; p. 673. [Google Scholar]
- Takenouchi, T. Key points in dermoscopic diagnosis of basal cell carcinoma and seborrheic keratosis in Japanese. J. Dermatol. 2011, 38, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, A. Dermoscopy–pathology relationship in seborrheic keratosis. J. Dermatol. 2017, 44, 518–524. [Google Scholar] [CrossRef]
- Braun, R.P.; Rabinovitz, H.S.; Krischer, J.; Kreusch, J.; Oliviero, M.; Naldi, L.; Kopf, A.W.; Saurat, J.H. Dermoscopy of pigmented seborrheic keratosis: A morphological study. Arch. Dermatol. 2002, 138, 1556–1560. [Google Scholar] [CrossRef] [Green Version]
- Wollina, U. Recent advances in managing and understanding seborrheic keratosis. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Izikson, L.; Sober, A.J.; Mihm, M.C.; Zembowicz, A. Prevalence of melanoma clinically resembling seborrheic keratosis: Analysis of 9204 cases. Arch. Dermatol. 2002, 138, 1562–1566. [Google Scholar] [CrossRef]
- Rubegni, P.; Feci, L.; Nami, N.; Burroni, M.; Taddeucci, P.; Miracco, C.; Munezero Butorano, M.A.; Fimiani, M.; Cevenini, G. Computer-assisted melanoma diagnosis: A new integrated system. Melanoma Res. 2015, 25, 537–542. [Google Scholar] [CrossRef]
- Carrera, C.; Segura, S.; Aguilera, P.; Takigami, C.M.; Gomes, A.; Barreiro, A.; Scalvenzi, M.; Longo, C.; Cavicchini, S.; Thomas, L. Dermoscopy improves the diagnostic accuracy of melanomas clinically resembling seborrheic keratosis: Cross-sectional study of the ability to detect seborrheic keratosis-like melanomas by a group of dermatologists with varying degrees of experience. Dermatology 2017, 233, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Braga, J.C.T.; Scope, A.; Klaz, I.; Mecca, P.; Spencer, P.; Marghoob, A.A. Melanoma mimicking seborrheic keratosis: An error of perception precluding correct dermoscopic diagnosis. J. Am. Acad. Dermatol. 2008, 58, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Kuehnl-Petzoldt, C.; Berger, H.; Wiebelt, H. Verrucous-keratotic variations of malignant melanoma: A clinicopathological study. Am. J. Dermatopathol. 1982, 4, 403–410. [Google Scholar] [PubMed]
- Kamino, H.; Tam, S.T.; Alvarez, L. Malignant melanoma with pseudocarcinomatous hyperplasia--an entity that can simulate squamous cell carcinoma. A light-microscopic and immunohistochemical study of four cases. Am. J. Dermatopathol. 1990, 12, 446–451. [Google Scholar] [CrossRef]
- Urbancek, S.; Fedorcova, P.; Tomkova, J.; Sutka, R. Misdiagnosis of Melanoma: A 7 Year Single-Center Analysis. Pigment. Disord. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Carrera, C.; Segura, S.; Palou, J.; Puig, S.; Segura, J.; Martí, R.M.; Malvehy, J. Seborrheic keratosislike melanoma with folliculotropism. Arch. Dermatol. 2007, 143, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Katz, B.; Rabinovitz, H.S. Introduction to dermoscopy. Dermatol. Clin. 2001, 19, 221–258. [Google Scholar] [CrossRef]
- Marghoob, A.A.; Usatine, R.; Jaimes, N. Dermoscopy for the family physician. Am. Fam. Physician 2013, 88, 441–450. [Google Scholar] [PubMed]
- Chappuis, P.; Duru, G.; Marchal, O.; Girier, P.; Dalle, S.; Thomas, L. Dermoscopy, a useful tool for general practitioners in melanoma screening: A nationwide survey. Br. J. Dermatol. 2016, 175, 744–750. [Google Scholar] [CrossRef] [Green Version]
- Gülseren, D.; Hofmann-Wellenhof, R. Evaluation of dermoscopic criteria for seborrheic keratosis on non-polarized versus polarized dermoscopy. Ski. Res. Technol. 2019, 25, 801–804. [Google Scholar] [CrossRef]
- DeJong, H.M.; Abbott, S.; Zelesco, M.; Kennedy, B.F.; Ziman, M.R.; Wood, F.M. The validity and reliability of using ultrasound elastography to measure cutaneous stiffness, a systematic review. Int. J. Burn. Trauma 2017, 7, 124. [Google Scholar]
- Hernandez-Ibanez, C.; Blazquez-Sanchez, N.; Aguilar-Bernier, M.; Fúnez-Liébana, R.; Rivas-Ruiz, F.; de Troya-Martin, M. Usefulness of high-frequency ultrasound in the classification of histologic subtypes of primary basal cell carcinoma. Actas Dermo-Sifiliográficas 2017, 108, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Calzavara-Pinton, P.; Longo, C.; Venturini, M.; Sala, R.; Pellacani, G. Reflectance confocal microscopy for in vivo skin imaging. Photochem. Photobiol. 2008, 84, 1421–1430. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Jee, S.-H.; Dong, C.-Y.; Lin, S.-J. Multiphoton microscopy in dermatological imaging. J. Dermatol. Sci. 2009, 56, 1–8. [Google Scholar] [CrossRef]
- Kiss, N.; Haluszka, D.; Lőrincz, K.; Gyöngyösi, N.; Bozsányi, S.; Bánvölgyi, A.; Szipőcs, R.; Wikonkál, N. Quantitative analysis on ex vivo nonlinear microscopy images of basal cell carcinoma samples in comparison to healthy skin. Pathol. Oncol. Res. 2019, 25, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Krolopp, Á.; Lőrincz, K.; Bánvölgyi, A.; Szipőcs, R.; Wikonkál, N. Stain-free histopathology of basal cell carcinoma by dual vibration resonance frequency CARS microscopy. Pathol. Oncol. Res. 2018, 24, 927–930. [Google Scholar] [CrossRef]
- Lihachev, A.; Derjabo, A.; Ferulova, I.; Lange, M.; Lihacova, I.; Spigulis, J. Autofluorescence imaging of basal cell carcinoma by smartphone RGB camera. J. Biomed. Opt. 2015, 20, 120502. [Google Scholar] [CrossRef]
- Kuzmina, I.; Diebele, I.; Spigulis, J.; Valeine, L.; Berzina, A.; Abelite, A. Contact and contactless diffuse reflectance spectroscopy: Potential for recovery monitoring of vascular lesions after intense pulsed light treatment. J. Biomed. Opt. 2011, 16, 040505. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.; Segura, S.; Aguilera, P.; Scalvenzi, M.; Longo, C.; Barreiro, A.; Broganelli, P.; Cavicchini, S.; Llambrich, A.; Zaballos, P. Dermoscopic clues for diagnosing melanomas that resemble seborrheic keratosis. JAMA Dermatol. 2017, 153, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Farnetani, F.; Pedroni, G.; Lippolis, N.; Giovani, M.; Ciardo, S.; Chester, J.; Kaleci, S.; Pezzini, C.; Cantisani, C.; Dattola, A. Facial Seborrheic Keratosis with Unusual Dermoscopic Patterns can be differentiated from other skin malignancies by In Vivo Reflectance Confocal Microscopy. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Kuzmina, I.; Diebele, I.; Asare, L.; Kempele, A.; Abelite, A.; Jakovels, D.; Spigulis, J. Multispectral imaging of pigmented and vascular cutaneous malformations: The influence of laser treatment. In Proceedings of the Laser Applications in Life Sciences 2010, Oulu, Finland, 24 November 2010; p. 73760J. [Google Scholar]
- Kuzmina, I.; Diebele, I.; Jakovels, D.; Spigulis, J.; Valeine, L.; Kapostinsh, J.; Berzina, A. Towards noncontact skin melanoma selection by multispectral imaging analysis. J. Biomed. Opt. 2011, 16, 060502. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.E.; Palmer MD, L.C.; Shuler, M.; Franklin, D. Smartphone Mobile Application to Enhance Diagnosis of Skin Cancer: A Guide for the Rural Practitioner. West Va. Med. J. 2015, 111, 22–29. [Google Scholar]
- Kuzmina, I.; Lacis, M.; Spigulis, J.; Berzina, A.; Valeine, L. Study of smartphone suitability for mapping of skin chromophores. J. Biomed. Opt. 2015, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamošiūnas, M.; Plorina, E.V.; Lange, M.; Derjabo, A.; Kuzmina, I.; Bļizņuks, D.; Spigulis, J. Autofluorescence imaging for recurrence detection in skin cancer postoperative scars. J. Biophotonics 2020, 13, e201900162. [Google Scholar] [CrossRef]
- Lange, M.; Bozsányi, S.; Plorina, E.V.; Lihachev, A.; Derjabo, A. Spectral imaging as a tool for the evaluation of skin cancer post-operative scars. In Proceedings of the Biophotonics—Riga 2020, Riga, Lativia, 28 October 2020; p. 1158506. [Google Scholar]
- Lihachev, A.; Lihacova, I.; Plorina, E.V.; Lange, M.; Derjabo, A.; Spigulis, J. Differentiation of seborrheic keratosis from basal cell carcinoma, nevi and melanoma by RGB autofluorescence imaging. Biomed. Opt. Express 2018, 9, 1852–1858. [Google Scholar] [CrossRef] [Green Version]
- Farkas, K.; Bozsányi, S.; Plázár, D.; Bánvölgyi, A.; Fésűs, L.; Anker, P.; Zakariás, S.; Lihacova, I.; Lihachev, A.; Lange, M. Autofluorescence Imaging of the Skin Is an Objective Non-Invasive Technique for Diagnosing Pseudoxanthoma Elasticum. Diagnostics 2021, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Anker, P.; Fésűs, L.; Kiss, N.; Noll, J.; Becker, K.; Kuroli, E.; Mayer, B.; Bozsányi, S.; Lőrincz, K.; Lihacova, I. Visualization of Keratin with Diffuse Reflectance and Autofluorescence Imaging and Nonlinear Optical Microscopy in a Rare Keratinopathic Ichthyosis. Sensors 2021, 21, 1105. [Google Scholar] [CrossRef] [PubMed]
- Borisova, E.G.; Angelova, L.P.; Pavlova, E.P. Endogenous and exogenous fluorescence skin cancer diagnostics for clinical applications. IEEE J. Sel. Top. Quantum Electron. 2013, 20, 211–222. [Google Scholar] [CrossRef]
- Zonios, G.; Dimou, A.; Bassukas, I.; Galaris, D.; Tsolakidis, A.; Kaxiras, E. Melanin absorption spectroscopy: New method for noninvasive skin investigation and melanoma detection. J. Biomed. Opt. 2008, 13, 014017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliznuks, D.; Jakovels, D.; Saknite, I.; Spigulis, J. Mobile platform for online processing of multimodal skin optical images: Using online Matlab server for processing remission, fluorescence and laser speckle images, obtained by using novel handheld device. In Proceedings of the 2015 International Conference on BioPhotonics (BioPhotonics), Florence, Italy, 20–22 May 2015; pp. 1–4. [Google Scholar]
- Spigulis, J. Multispectral, fluorescent and photoplethysmographic imaging for remote skin assessment. Sensors 2017, 17, 1165. [Google Scholar] [CrossRef] [Green Version]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Sondermann, W.; Zimmer, L.; Schadendorf, D.; Roesch, A.; Klode, J.; Dissemond, J. Initial misdiagnosis of melanoma located on the foot is associated with poorer prognosis. Medicine 2016, 95, e4332. [Google Scholar] [CrossRef]
- Petrie, T.; Samatham, R.; Witkowski, A.M.; Esteva, A.; Leachman, S.A. Melanoma Early Detection: Big Data, Bigger Picture. J. Invest. Derm. 2019, 139, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Kandel, M.; Allayous, C.; Dalle, S.; Mortier, L.; Dalac, S.; Dutriaux, C.; Leccia, M.; Guillot, B.; Saiag, P.; Lacour, J. Update of survival and cost of metastatic melanoma with new drugs: Estimations from the MelBase cohort. Eur. J. Cancer 2018, 105, 33–40. [Google Scholar] [CrossRef]
- Mun, J.H.; Kim, G.W.; Jwa, S.W.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B. Dermoscopy of subungual haemorrhage: Its usefulness in differential diagnosis from nail-unit melanoma. Br. J. Dermatol. 2013, 168, 1224–1229. [Google Scholar] [CrossRef]
- Fargnoli, M.C.; Kostaki, D.; Piccioni, A.; Micantonio, T.; Peris, K. Dermoscopy in the diagnosis and management of non-melanoma skin cancers. Eur. J. Dermatol. 2012, 22, 456–463. [Google Scholar] [CrossRef]
- Zalaudek, I.; Ferrara, G.; Leinweber, B.; Mercogliano, A.; D’Ambrosio, A.; Argenziano, G. Pitfalls in the clinical and dermoscopic diagnosis of pigmented actinic keratosis. J. Am. Acad. Dermatol. 2005, 53, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Elbaum, M.; Kopf, A.W.; Rabinovitz, H.S.; Langley, R.G.; Kamino, H.; Mihm Jr, M.C.; Sober, A.J.; Peck, G.L.; Bogdan, A.; Gutkowicz-Krusin, D. Automatic differentiation of melanoma from melanocytic nevi with multispectral digital dermoscopy: A feasibility study. J. Am. Acad. Dermatol. 2001, 44, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Moscarella, E.; Piana, S.; Lallas, A.; Carrera, C.; Pellacani, G.; Zalaudek, I.; Argenziano, G. Not all lesions with a verrucous surface are seborrheic keratoses. J. Am. Acad. Dermatol. 2014, 70, e121–e123. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, R.; Ballerini, M.; Bartoli, C.; Pignoli, E.; Sichirollo, A.E.; Tomatis, S.; Zurrida, S.; Cascinelli, N. Telespectrophotometry of human skin diseases by means of a ccd camera. In Proceedings of the Europto Biomedical Optics ’93, Budapest, Hungary, 29 August–3 September 1993; pp. 168–173. [Google Scholar]
- Setiadi, I.C.; Nasution, A.M.; Chandra, T.G. A new LED-based multispectral imaging system for blood and melanin content estimation: The validation. In Proceedings of the AIP Conference, Padang, Indonesia, 22–24 July 2019; p. 050017. [Google Scholar]
- Moncrieff, M.; Cotton, S.; Hall, P.; Schiffner, R.; Lepski, U.; Claridge, E. SIAscopy assists in the diagnosis of melanoma by utilizing computer vision techniques to visualise the internal structure of the skin. Med. Image Underst Anal. 2001, 53–56. [Google Scholar]
- Tomatis, S.; Carrara, M.; Bono, A.; Bartoli, C.; Lualdi, M.; Tragni, G.; Colombo, A.; Marchesini, R. Automated melanoma detection with a novel multispectral imaging system: Results of a prospective study. Phys. Med. Biol. 2005, 50, 1675. [Google Scholar] [CrossRef] [PubMed]
- March, J.; Hand, M.; Truong, A.; Grossman, D. Practical application of new technologies for melanoma diagnosis: Part II. Molecular approaches. J. Am. Acad. Dermatol. 2015, 72, 943–958. [Google Scholar] [CrossRef]
- Ganga, R.S.; Gundre, D.; Bansal, S.; Shirsat, P.M.; Prasad, P.; Desai, R.S. Evaluation of the diagnostic efficacy and spectrum of autofluorescence of benign, dysplastic and malignant lesions of the oral cavity using VELscope. Oral Oncol. 2017, 75, 67–74. [Google Scholar] [CrossRef]
- Bliznakova, I.; Borisova, E.; Avramov, L. Laser-and light-induced autofluorescence spectroscopy of human skin in dependence on excitation wavelengths. Acta Phys. Pol. Ser. A 2007, 112, 1131. [Google Scholar] [CrossRef]
- Pal, R.; Edward, K.; Ma, L.; Qiu, S.; Vargas, G. Spectroscopic characterization of oral epithelial dysplasia and squamous cell carcinoma using multiphoton autofluorescence micro-spectroscopy. Lasers Surg. Med. 2017, 49, 866–873. [Google Scholar] [CrossRef]
- Takahama Jr, A.; Kurachi, C.; Cosci, A.; Faustino, I.S.P.; Camisasca, D.R.; Fontes, K.B.d.C.F.; Pires, F.R.; Azevedo, R.S. Usefulness of tissue autofluorescence imaging in actinic cheilitis diagnosis. J. Biomed. Opt. 2013, 18, 076023. [Google Scholar] [CrossRef]
- Pratavieira, S.; Andrade, C.; Salvio, A.; Bagnato, V.; Kurachi, C. Optical imaging as auxiliary tool in skin cancer diagnosis. Ski. Cancers Risk Factors Prev. Ther. 2011, 159–173. [Google Scholar]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Zeng, H.; Hamzavi, I.; Alajlan, A.; Tan, E.; McLean, D.I.; Lui, H. Cutaneous melanin exhibiting fluorescence emission under near-infrared light excitation. J. Biomed. Opt. 2006, 11, 034010. [Google Scholar] [CrossRef] [Green Version]
- di Ruffano, L.F.; Takwoingi, Y.; Dinnes, J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Matin, R.N.; Godfrey, K.; O’Sullivan, C.; Gulati, A. Computer-assisted diagnosis techniques (dermoscopy and spectroscopy-based) for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, H.; Kalia, S.; Lui, H. Incorporating patient demographics into Raman spectroscopy algorithm improves in vivo skin cancer diagnostic specificity. Transl. Biophotonics 2019, 1, e201900016. [Google Scholar] [CrossRef]
- Bratchenko, I.A.; Bratchenko, L.A.; Moryatov, A.A.; Khristoforova, Y.A.; Artemyev, D.N.; Myakinin, O.O.; Orlov, A.E.; Kozlov, S.V.; Zakharov, V.P. In vivo diagnosis of skin cancer with a portable Raman spectroscopic device. Exp. Dermatol. 2021, 30, 652–663. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozsányi, S.; Farkas, K.; Bánvölgyi, A.; Lőrincz, K.; Fésűs, L.; Anker, P.; Zakariás, S.; Jobbágy, A.; Lihacova, I.; Lihachev, A.; et al. Quantitative Multispectral Imaging Differentiates Melanoma from Seborrheic Keratosis. Diagnostics 2021, 11, 1315. https://doi.org/10.3390/diagnostics11081315
Bozsányi S, Farkas K, Bánvölgyi A, Lőrincz K, Fésűs L, Anker P, Zakariás S, Jobbágy A, Lihacova I, Lihachev A, et al. Quantitative Multispectral Imaging Differentiates Melanoma from Seborrheic Keratosis. Diagnostics. 2021; 11(8):1315. https://doi.org/10.3390/diagnostics11081315
Chicago/Turabian StyleBozsányi, Szabolcs, Klára Farkas, András Bánvölgyi, Kende Lőrincz, Luca Fésűs, Pálma Anker, Sára Zakariás, Antal Jobbágy, Ilze Lihacova, Alexey Lihachev, and et al. 2021. "Quantitative Multispectral Imaging Differentiates Melanoma from Seborrheic Keratosis" Diagnostics 11, no. 8: 1315. https://doi.org/10.3390/diagnostics11081315
APA StyleBozsányi, S., Farkas, K., Bánvölgyi, A., Lőrincz, K., Fésűs, L., Anker, P., Zakariás, S., Jobbágy, A., Lihacova, I., Lihachev, A., Lange, M., Bliznuks, D., Medvecz, M., Kiss, N., & Wikonkál, N. M. (2021). Quantitative Multispectral Imaging Differentiates Melanoma from Seborrheic Keratosis. Diagnostics, 11(8), 1315. https://doi.org/10.3390/diagnostics11081315







