Cerebral Autoregulation during Postural Change in Patients with Cervical Spinal Cord Injury—A Carotid Duplex Ultrasonography Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
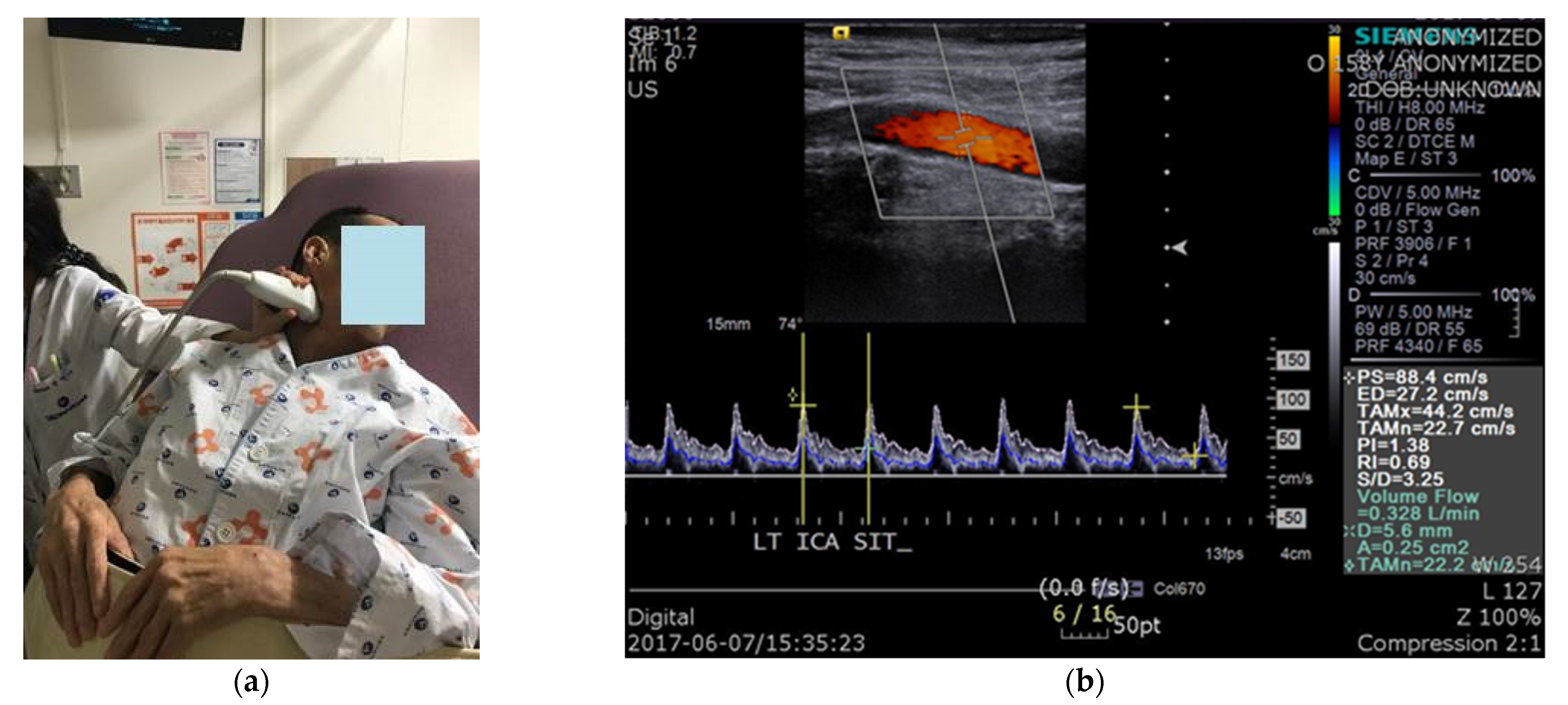
2.2. Protocol of Carotid Duplex Ultrasonography
2.3. Data Acquisition and Data Analysis
2.4. Statistical Analysis
3. Results
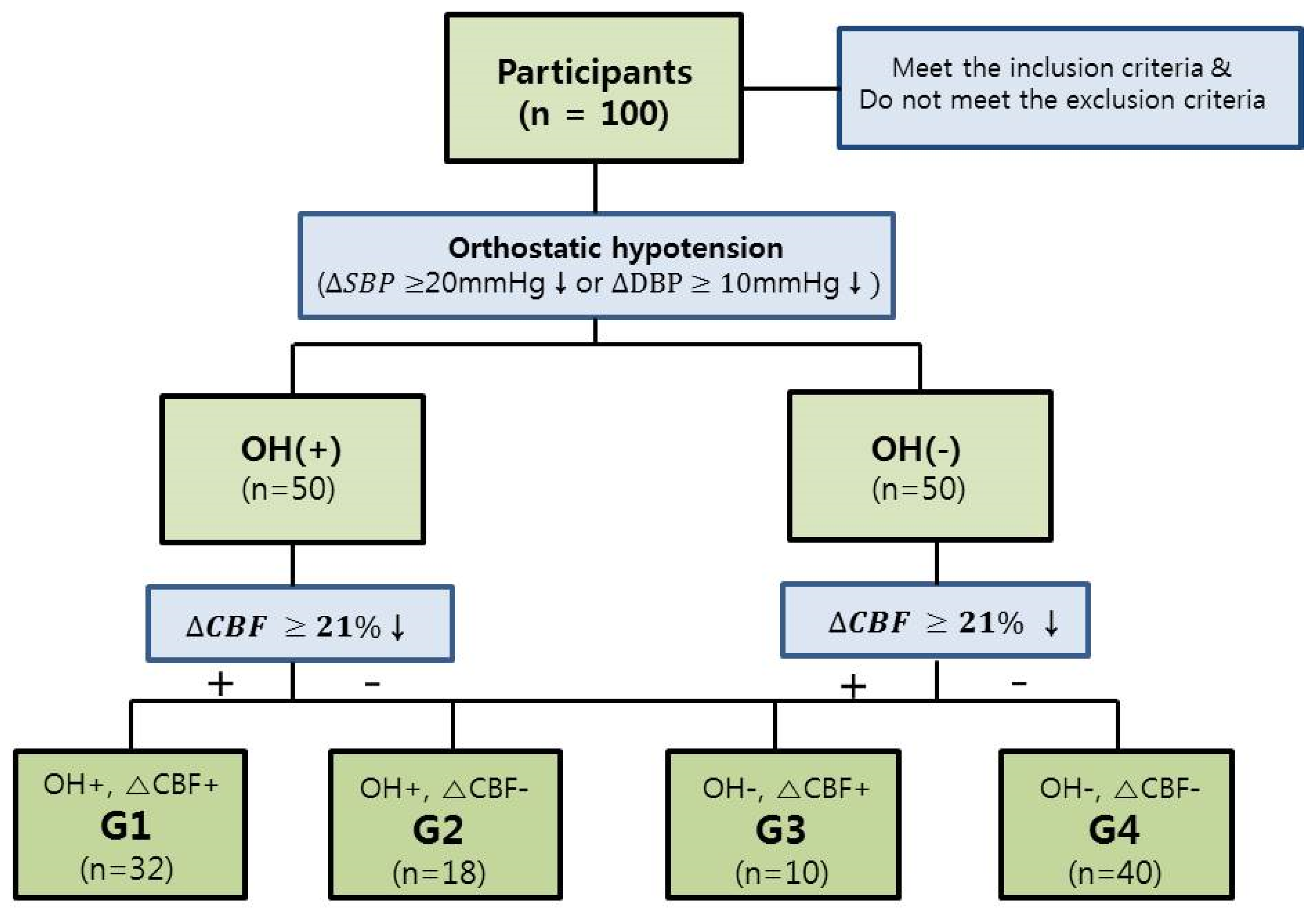
3.1. The Relationship between the Decrease in CBFV (L/min) after Tilt and Presence of Presyncopal Symptoms
3.2. Demographic Data of Groups and Comparison of Characteristics of Each Group
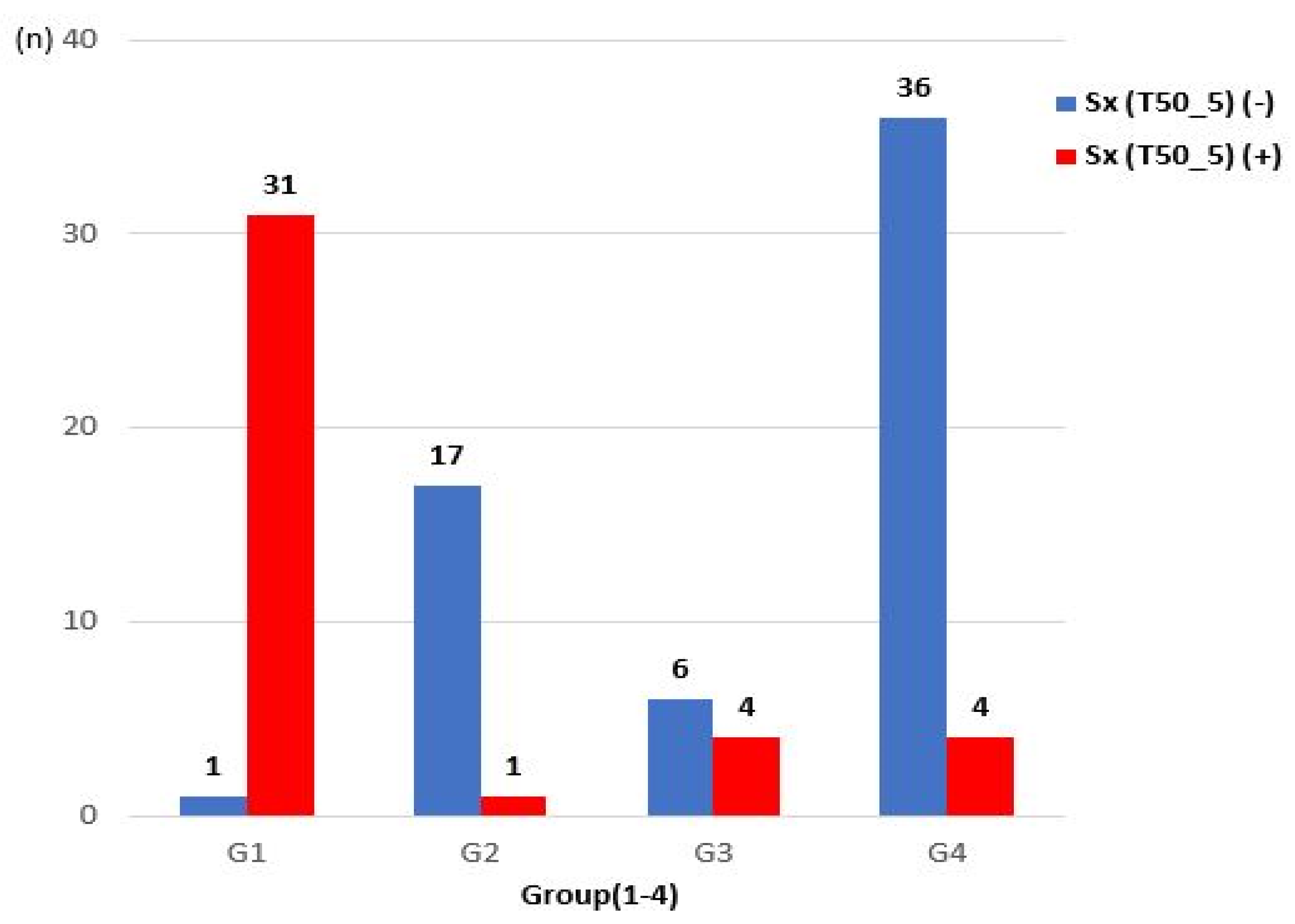
3.3. The Incidence of Presyncopal Symptoms in Each Group
4. Discussion
- Presyncopal symptoms occurred when the BFV of the ICA decreased by ≥21% after tilt in patients with a CSCI.
- In G1, i.e., patients who had OH and severe CBF decrease during tilt (because CA did not occur), the BMI was lower than that in G4 (patients who had neither OH nor CBF decrease); physical and functional scores such as MS (UE), MS (LE), MS (total), SS (LT), SS (PP), SS (total), and K-SCIM scores were low; and the proportions of AIS grades A, B, and C were high.
- In G2, i.e., the group of patients who had no decrease in CBF even though there was OH during tilt (because CA occurred), presyncopal symptoms rarely occurred (5.56%).
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krassioukov, A.V.; Karlsson, A.K.; Wecht, J.M.; Wuermser, L.A.; Mathias, C.J.; Marino, R.J.; Joint Committee of American Spinal Injury Association; International Spinal Cord Society. Assessment of Autonomic Dysfunction Following Spinal Cord Injury: Rationale for Additions to International Standards for Neurological Assessment. J. Rehabil. Res. Dev. 2007, 44, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, N.E.; Fyffe, D.; Morin, K.G.; Byrne, R.; Tulsky, D.S.; Victorson, D.; Lai, J.S.; Wecht, J.M. Impact of Blood Pressure Dysregulation on Health-Related Quality of Life in Persons with Spinal Cord Injury: Development of a Conceptual Model. Arch. Phys. Med. Rehabil. 2013, 94, 1721–1730. [Google Scholar] [CrossRef]
- Illman, A.; Stiller, K.; Williams, M. The prevalence of Orthostatic Hypotension during Physiotherapy Treatment in Patients with an Acute Spinal Cord Injury. Spinal Cord 2000, 38, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Claydon, V.E.; Steeves, J.D.; Krassioukov, A. Orthostatic Hypotension Following Spinal Cord Injury: Understanding Clinical Pathophysiology. Spinal Cord 2006, 44, 341–351. [Google Scholar] [CrossRef]
- Phillips, A.A.; Krassioukov, A.V.; Ainslie, P.N.; Warburton, D.E. Baroreflex function after Spinal Cord Injury. J. Neurotrauma 2012, 29, 2431–2445. [Google Scholar] [CrossRef]
- Gonzalez, F.; Chang, J.Y.; Banovac, K.; Messina, D.; Martinez-Arizala, A.; Kelley, R.E. Autoregulation of Cerebral Blood Flow in Patients with Orthostatic Hypotension after Spinal Cord Injury. Paraplegia 1991, 29, 1–7. [Google Scholar] [CrossRef]
- Brass, L.; Prohovnik, I.; Pavlakis, S.; DeVivo, D.; Piomelli, S.; Mohr, J. Middle Cerebral Artery Blood Velocity and Cerebral Blood Flow in Sickle Cell Disease. Stroke 1991, 22, 27–30. [Google Scholar] [CrossRef]
- Brooks, D.; Redmond, S.; Mathias, C.; Bannister, R.; Symon, L. The Effect of Orthostatic Hypotension on Cerebral Blood Flow and Middle Cerebral Artery Velocity in Autonomic Failure, with Observations on the Action of Ephedrine. J. Neurol. Neurosurg. Psychiatry 1989, 52, 962–966. [Google Scholar] [CrossRef]
- Rothoerl, R.; Schebesch, K.; Woertgen, C.; Brawanski, A. Internal Carotid Artery Volume Flow Correlates to Rcbf Measurements. Acta Neurochir. 2003, 145, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Soustiel, J.F.; Levy, E.; Zaaroor, M.; Bibi, R.; Lukaschuk, S.; Manor, D. A New Angle-Independent Doppler Ultrasonic Device for Assessment of Blood Flow Volume in the Extracranial Internal Carotid Artery. J. Ultrasound Med. 2002, 21, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Soustiel, J.; Glenn, T.; Vespa, P.; Rinsky, B.; Hanuscin, C.; Martin, N. Assessment of Cerebral Blood Flow by Means of Blood-Flow-Volume Measurement in the Internal Carotid Artery: Comparative Study with a 133xenon Clearance Technique. Stroke 2003, 34, 1876–1880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schebesch, K.-M.; Woertgen, C.; Schlaier, J.; Brawanski, A.; Rothoerl, R.D. Doppler Ultrasound Measurement of Blood Flow Volume in the Extracranial Internal Carotid Artery for Evaluation of Brain Perfusion after Aneurysmal Subarachnoid Hemorrhage. Neurol. Res. 2007, 29, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Claydon, V.E.; Krassioukov, A.V. Orthostatic Hypotension and Autonomic Pathways after Spinal Cord Injury. J. Neurotrauma 2006, 23, 1713–1725. [Google Scholar] [CrossRef]
- Johnson, R.; Park, D.; Frankel, H. Orthostatic Hypotension and the Renin-Angiotensin System in Paraplegia. Spinal Cord 1971, 9, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, A.; Reinhard, M.; Guschlbauer, B.; Braune, S. Challenging Cerebral Autoregulation in Patients with Preganglionic Autonomic Failure. Clin. Auton. Res. 2003, 13, 27–35. [Google Scholar] [CrossRef]
- Krassioukov, A. Autonomic Function following Cervical Spinal Cord Injury. Resp. Physiol. Neurobiol. 2009, 169, 157–164. [Google Scholar] [CrossRef]
- Osborn, J.W.; Taylor, R.F.; Schramm, L.P. Determinants of Arterial Pressure after Chronic Spinal Transection in Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1989, 256, R666–R673. [Google Scholar] [CrossRef] [PubMed]
- Šerić, V.; Blažić-Čop, N.; Demarin, V. Haemodynamic Changes in Patients with Whiplash Injury Measured by Transcranial Doppler Sonography (TCD). Coll. Antropol. 2000, 24, 197–204. [Google Scholar]
- Bleton, H.; Sejdić, E. A Cerebral Blood Flow Evaluation during Cognitive Tasks Following a Cervical Spinal Cord Injury: A Case Study Using Transcranial Doppler Recordings. Cogn. Neurodyn. 2015, 9, 615–626. [Google Scholar] [CrossRef]
- Phillips, A.A.; Ainslie, P.N.; Krassioukov, A.V.; Warburton, D.E. Regulation of Cerebral Blood Flow after Spinal Cord Injury. J. Neurotrauma 2013, 30, 1551–1563. [Google Scholar] [CrossRef]
- Willie, C.; Colino, F.; Bailey, D.; Tzeng, Y.; Binsted, G.; Jones, L.; Haykowsky, M.; Bellapart, J.; Ogoh, S.; Smith, K. Utility of Transcranial Doppler Ultrasound for the Integrative Assessment of Cerebrovascular Function. J. Neurosci. Methods 2011, 196, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Sahota, I.S.; Ravensbergen, H.J.; McGrath, M.S.; Claydon, V.E. Cerebrovascular Responses to Orthostatic Stress after Spinal Cord Injury. J. Neurotrauma 2012, 29, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.C.; Cotter, J.D.; Fan, J.-L.; Lucas, R.A.; Thomas, K.N.; Ainslie, P.N. Cerebrovascular Reactivity and Dynamic Autoregulation in Tetraplegia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1035–R1042. [Google Scholar] [CrossRef] [PubMed]
- Aries, M.J.; Elting, J.W.; De Keyser, J.; Kremer, B.P.; Vroomen, P.C. Cerebral Autoregulation in Stroke: A Review of Transcranial Doppler Studies. Stroke 2010, 41, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A.; Krassioukov, A.V.; Ainslie, P.N.; Warburton, D.E. Perturbed and Spontaneous Regional Cerebral Blood Flow Responses to Changes in Blood Pressure after High-level Spinal Cord Injury: The effect of midodrine. J. Appl. Physiol. 2014, 116, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, E.; Townson, A.; Dvorak, M.; Kwon, B.; Steeves, J.; Krassioukov, A. Orthostatic Hypotension in the First Month following Acute Spinal Cord Injury. Spinal Cord 2008, 46, 65–69. [Google Scholar] [CrossRef]
- Azevedo, E.; Castro, P.; Santos, R.; Freitas, J.; Coelho, T.; Rosengarten, B.; Panerai, R. Autonomic Dysfunction Affects Cerebral Neurovascular Coupling. Clin. Auton. Res. 2011, 21, 395–403. [Google Scholar] [CrossRef]
- Sato, K.; Fisher, J.P.; Seifert, T.; Overgaard, M.; Secher, N.H.; Ogoh, S. Blood Flow in Internal Carotid and Vertebral Arteries during Orthostatic Stress. Exp. Physiol. 2012, 97, 1272–1280. [Google Scholar] [CrossRef]
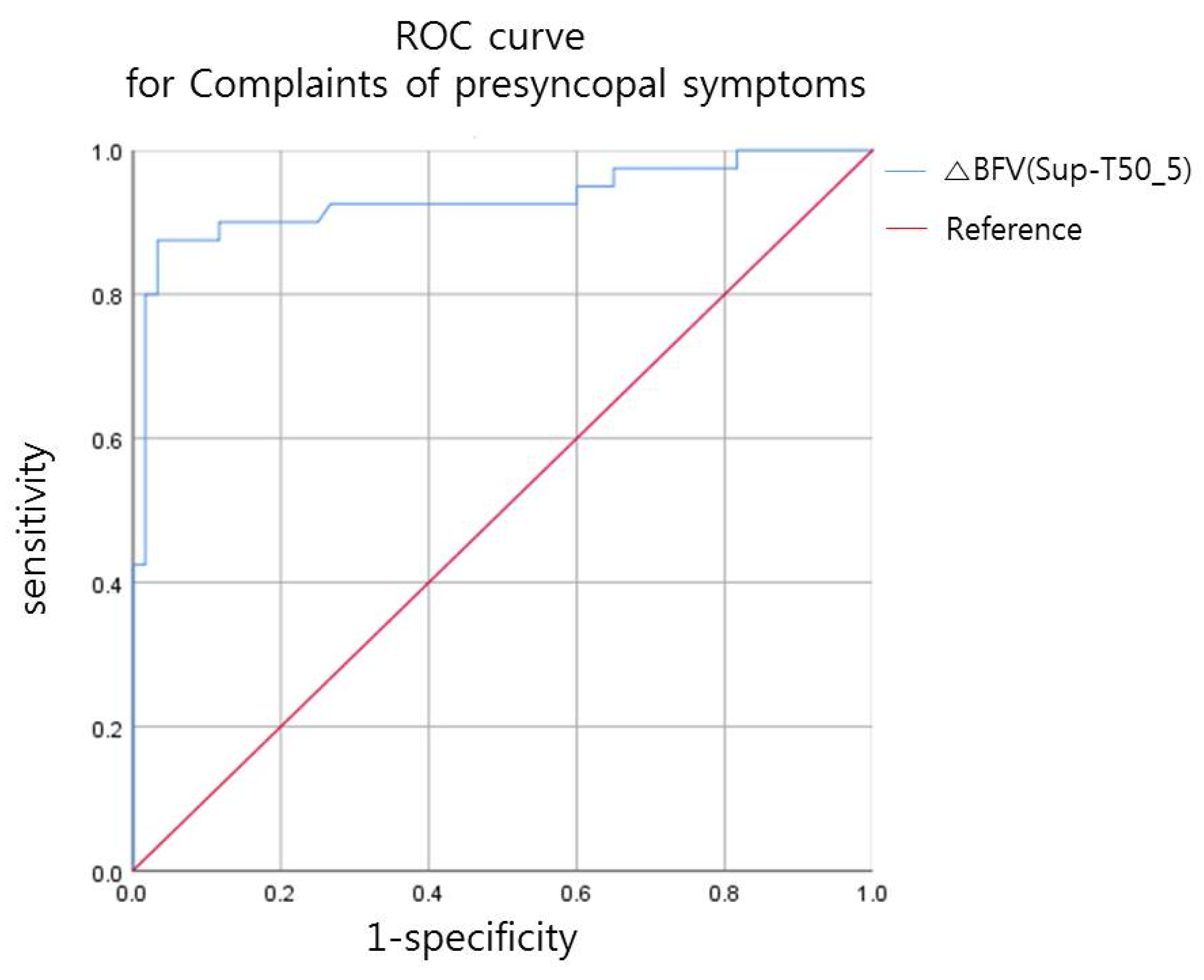
| AUC | 95% CI | Cut-Off Value (%) | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| ΔBFV (Sup-T50_5) | 0.93 | (0.870~0.991) | 21.23 | 0.875 | 0.967 |
| Characteristic, Mean ± SD | G1 (n = 32) | G2 (n = 18) | G3 (n = 10) | G4 (n = 40) | Total (n = 100) |
|---|---|---|---|---|---|
| Age (years) | 58.5 ± 16.97 | 60.61 ± 14.74 | 63.00 ± 18.94 | 57.22 ± 13.50 | 58.82 ± 15.34 |
| Height (cm) | 169.41 ± 7.98 | 163.63 ± 9.31 | 164.18 ± 13.50 | 167.20 ± 8.41 | 166.96 ± 9.16 |
| BMI (kg/m2) * | 20.92 ± 3.44 | 23.38 ± 4.54 | 23.078 ± 2.60 | 24.01 ± 3.49 | 22.82 ± 3.80 |
| DOI | 185.17 ± 432.04 | 145.95 ± 188.82 | 118.89 ± 142.34 | 158.29 ± 222.59 | 160.74 ± 293.85 |
| G1 (n = 32) | G2 (n = 18) | G3 (n = 10) | G4 (n = 40) | Total (n = 100) | ||
|---|---|---|---|---|---|---|
| Characteristic | ||||||
| AIS grade (n) * | AIS A | 12 | 0 | 0 | 1 | 13 |
| AIS B | 3 | 0 | 1 | 0 | 4 | |
| AIS C | 11 | 3 | 1 | 4 | 19 | |
| AIS D | 6 | 15 | 8 | 35 | 64 | |
| Sex (n) | Male | 26 | 13 | 8 | 30 | 77 |
| Female | 6 | 5 | 2 | 10 | 23 | |
| NLI (n) | C2 | 1 | 0 | 0 | 1 | 2 |
| C3 | 4 | 3 | 1 | 2 | 10 | |
| C4 | 12 | 7 | 5 | 16 | 40 | |
| C5 | 14 | 8 | 4 | 18 | 44 | |
| C6 | 0 | 0 | 0 | 2 | 2 | |
| C8 | 1 | 0 | 0 | 1 | 2 | |
| DM (n) | (−) | 31 | 12 | 7 | 38 | 88 |
| (+) | 1 | 6 | 3 | 2 | 12 | |
| HTN (n) | (−) | 23 | 13 | 7 | 31 | 74 |
| (+) | 9 | 5 | 3 | 9 | 26 |
| Functional Score (Mean ± SD) | G1 (n = 32) | G2 (n = 18) | G3 (n = 10) | G4 (n = 40) | Total | p-Value (ANOVA) |
|---|---|---|---|---|---|---|
| MS (UE) | 22.25 ± 11.92 | 34.11 ± 5.95 | 34.2 ± 7.56 | 36.33 ± 6.60 | 31.21 ± 10.59 | 0.000 |
| MS (LE) | 12.03 ± 15.23 | 33.06 ± 12.71 | 33.70 ± 18.28 | 36.93 ± 10.07 | 27.94 ± 17.12 | 0.000 |
| MS (total) | 34.28 ± 23.11 | 67.17 ± 15.89 | 67.90 ± 22.66 | 73.25 ± 14.92 | 59.15 ± 25.39 | 0.000 |
| SS (LT) | 56.28 ± 22.49 | 65.56 ± 13.98 | 65.30 ± 19.03 | 70.78 ± 20.92 | 64.65 ± 20.87 | 0.032 |
| SS (PP) | 52.66 ± 25.03 | 64.00 ± 13.23 | 60.80 ± 9.39 | 73.23 ± 23.11 | 63.74 ± 22.83 | 0.002 |
| SS (total) | 108.94 ± 46.01 | 129.56 ± 26.41 | 126.10 ± 25.80 | 144.00 ± 43.00 | 128.39 ± 42.35 | 0.005 |
| K-SCIM | 23.44 ± 21.76 | 62.44 ± 25.80 | 57.70 ± 25.74 | 64.78 ± 25.82 | 50.42 ± 30.59 | 0.00000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kee, J.-H.; Han, J.-H.; Moon, C.-W.; Cho, K.H. Cerebral Autoregulation during Postural Change in Patients with Cervical Spinal Cord Injury—A Carotid Duplex Ultrasonography Study. Diagnostics 2021, 11, 1321. https://doi.org/10.3390/diagnostics11081321
Kee J-H, Han J-H, Moon C-W, Cho KH. Cerebral Autoregulation during Postural Change in Patients with Cervical Spinal Cord Injury—A Carotid Duplex Ultrasonography Study. Diagnostics. 2021; 11(8):1321. https://doi.org/10.3390/diagnostics11081321
Chicago/Turabian StyleKee, Joo-Hyun, Jun-Hyeong Han, Chang-Won Moon, and Kang Hee Cho. 2021. "Cerebral Autoregulation during Postural Change in Patients with Cervical Spinal Cord Injury—A Carotid Duplex Ultrasonography Study" Diagnostics 11, no. 8: 1321. https://doi.org/10.3390/diagnostics11081321
APA StyleKee, J.-H., Han, J.-H., Moon, C.-W., & Cho, K. H. (2021). Cerebral Autoregulation during Postural Change in Patients with Cervical Spinal Cord Injury—A Carotid Duplex Ultrasonography Study. Diagnostics, 11(8), 1321. https://doi.org/10.3390/diagnostics11081321






