Analysis of Saliva Lipids in Breast and Prostate Cancer by IR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Saliva Collection and Storage Technique
2.3. Saliva Analysis by IR Spectroscopy
2.4. Statistical Analysis
3. Results
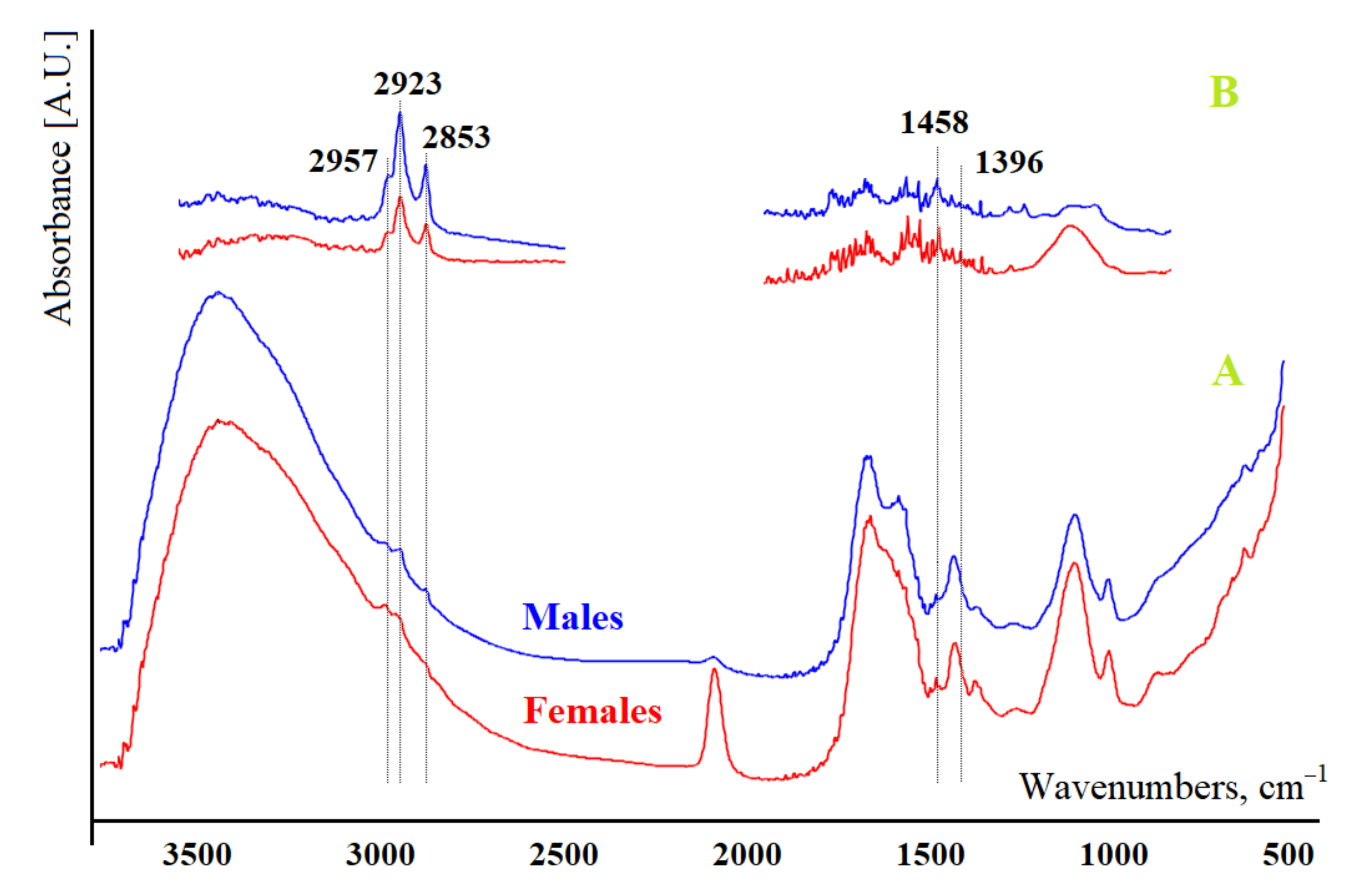
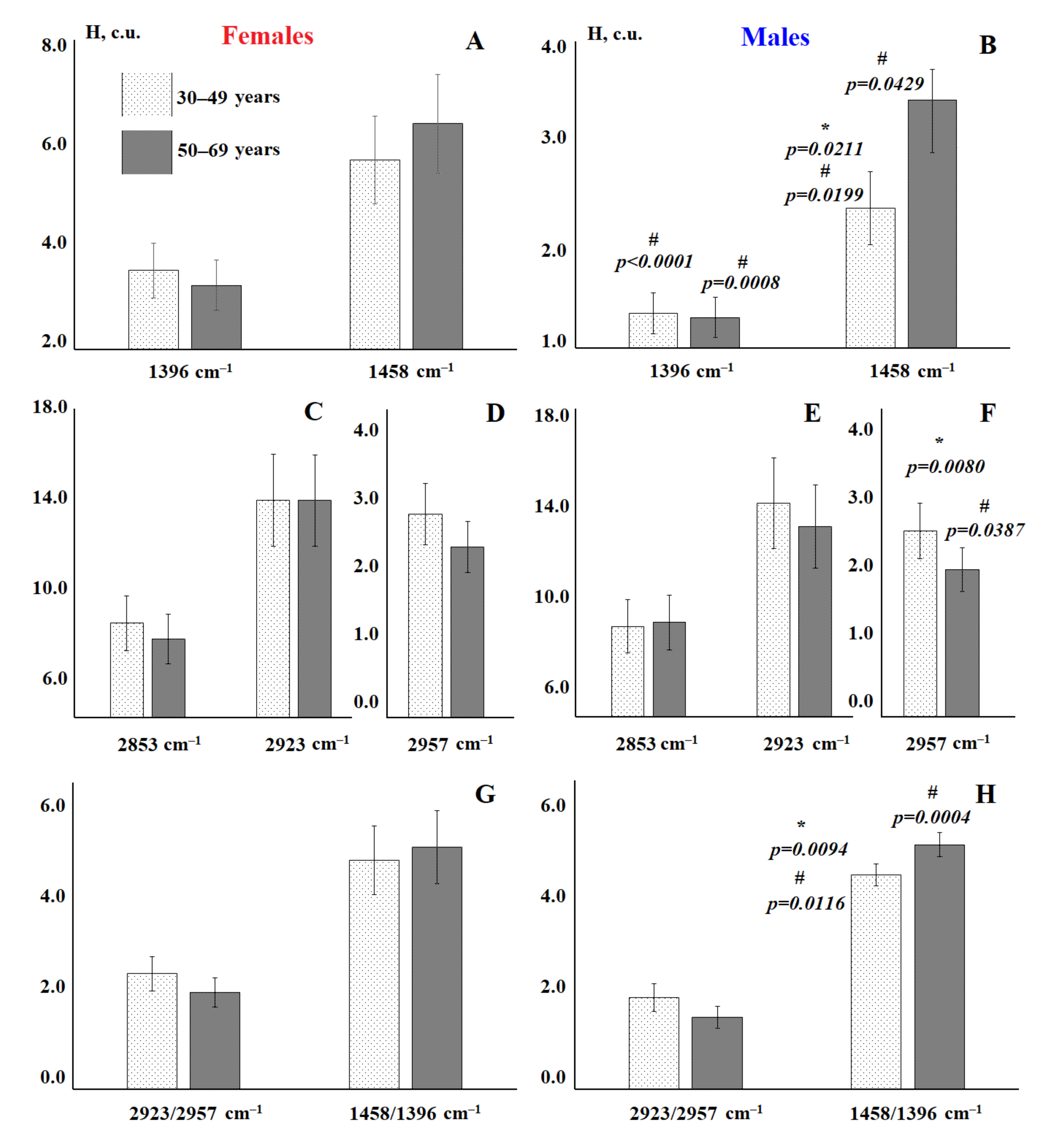
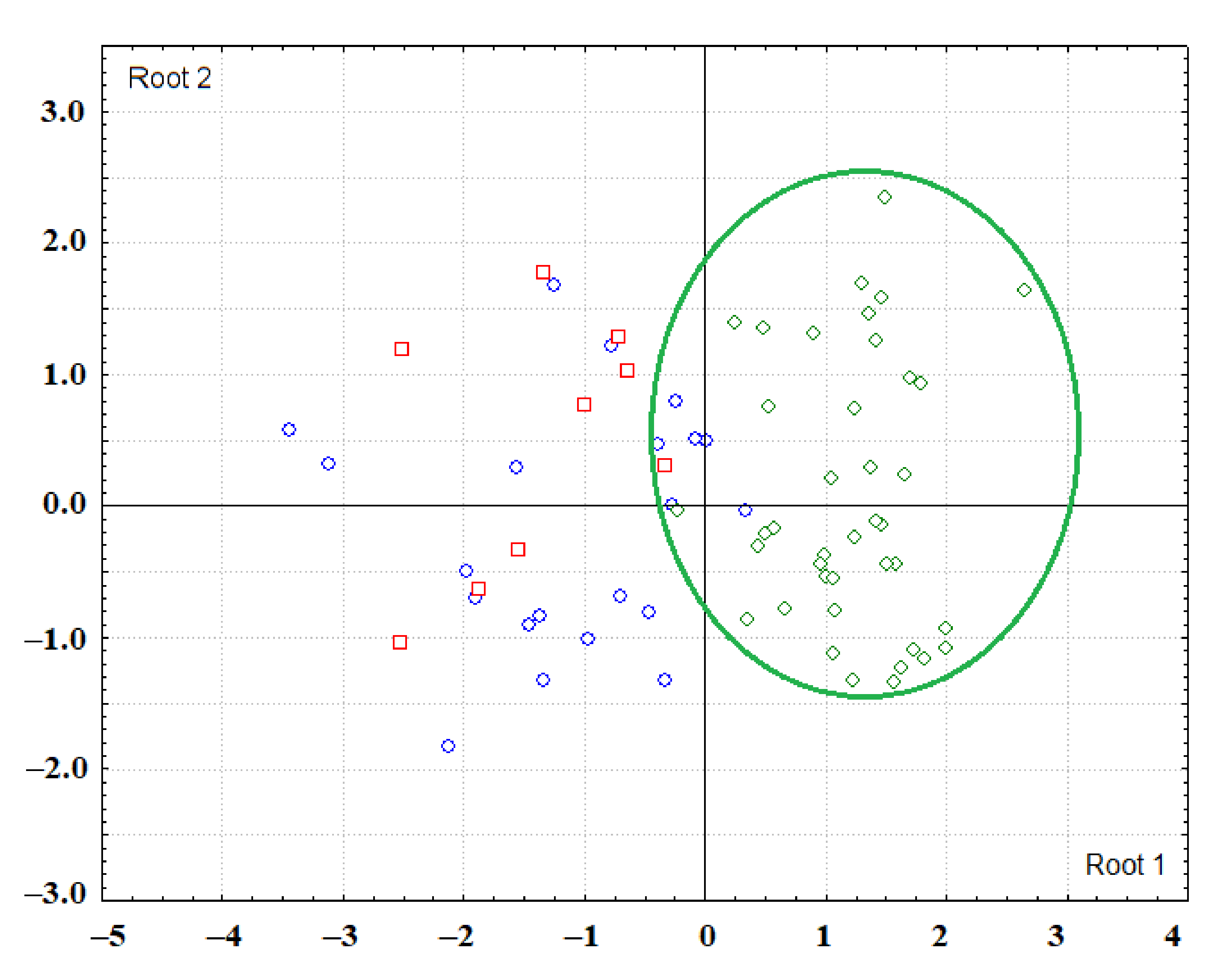
3.1. Analysis of the Salivary Lipid Profile of the Control Group
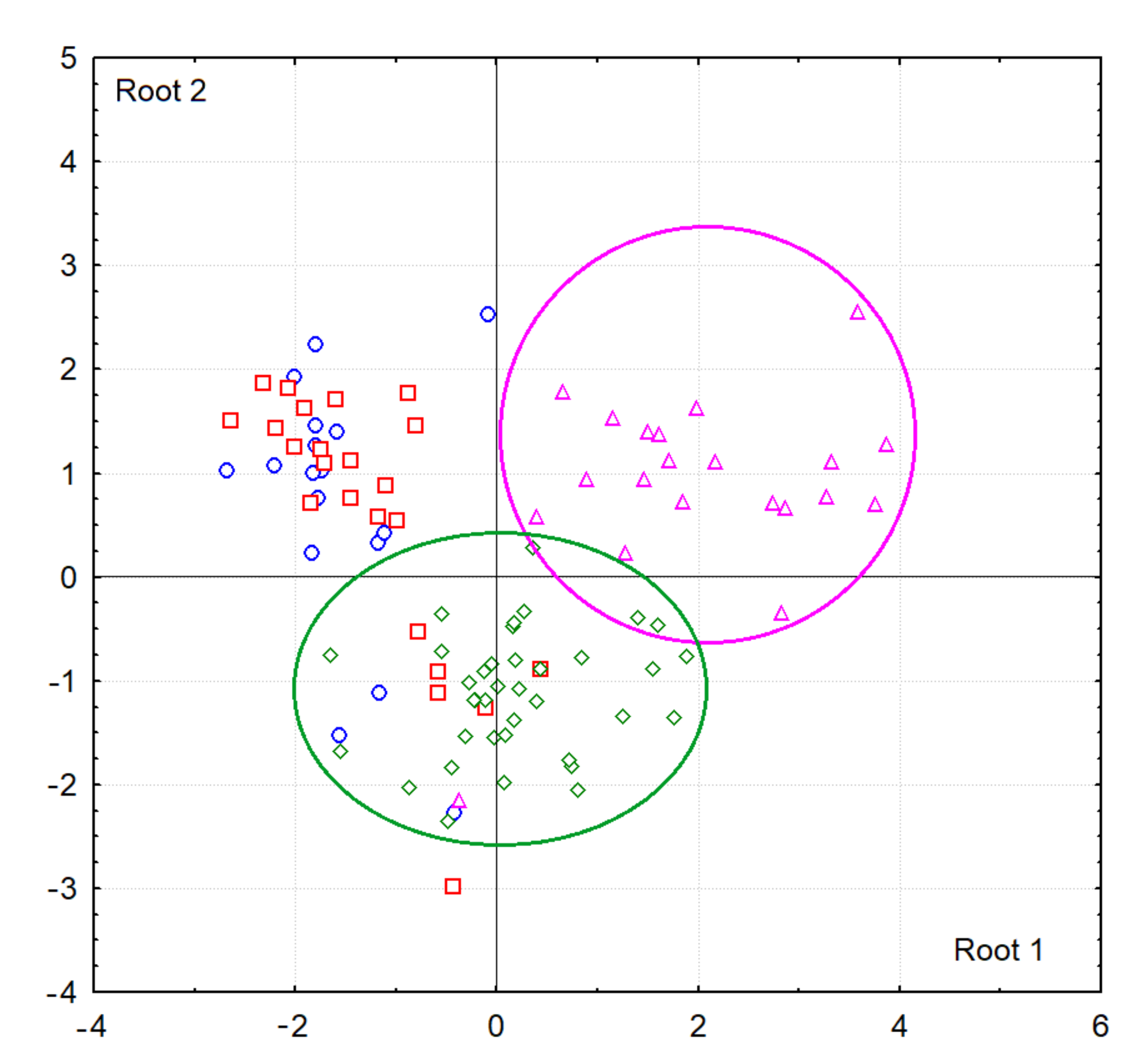
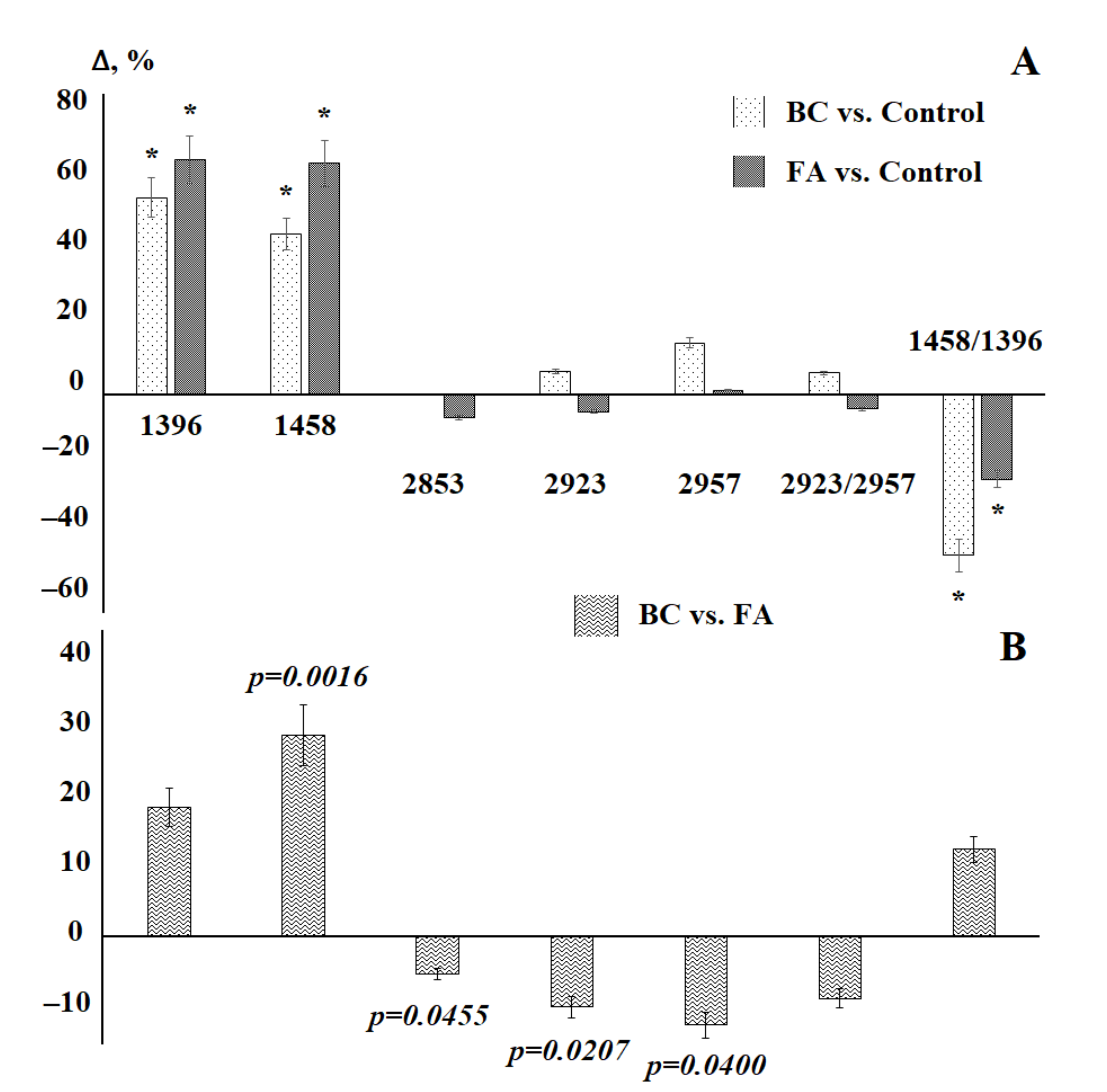
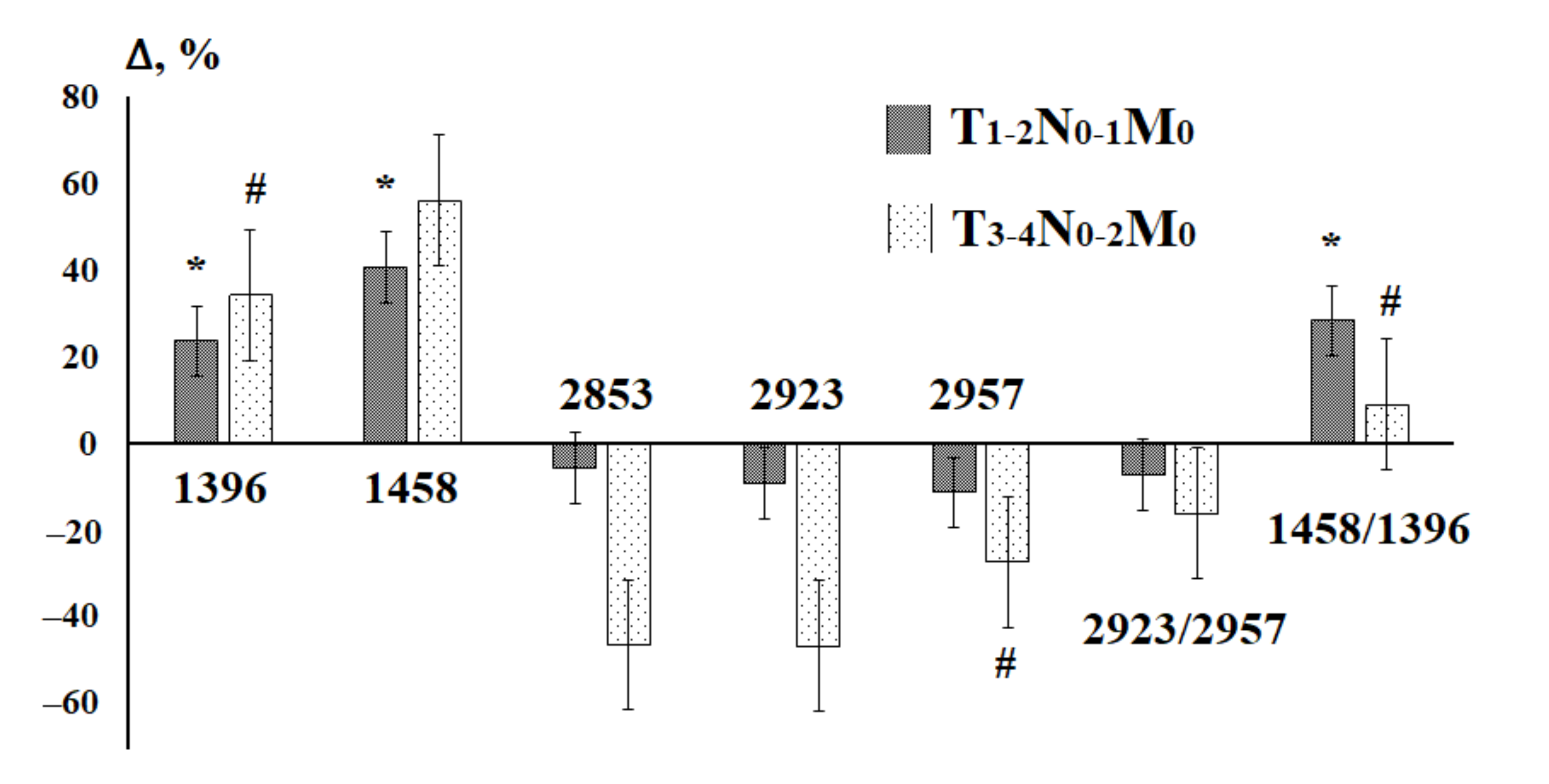
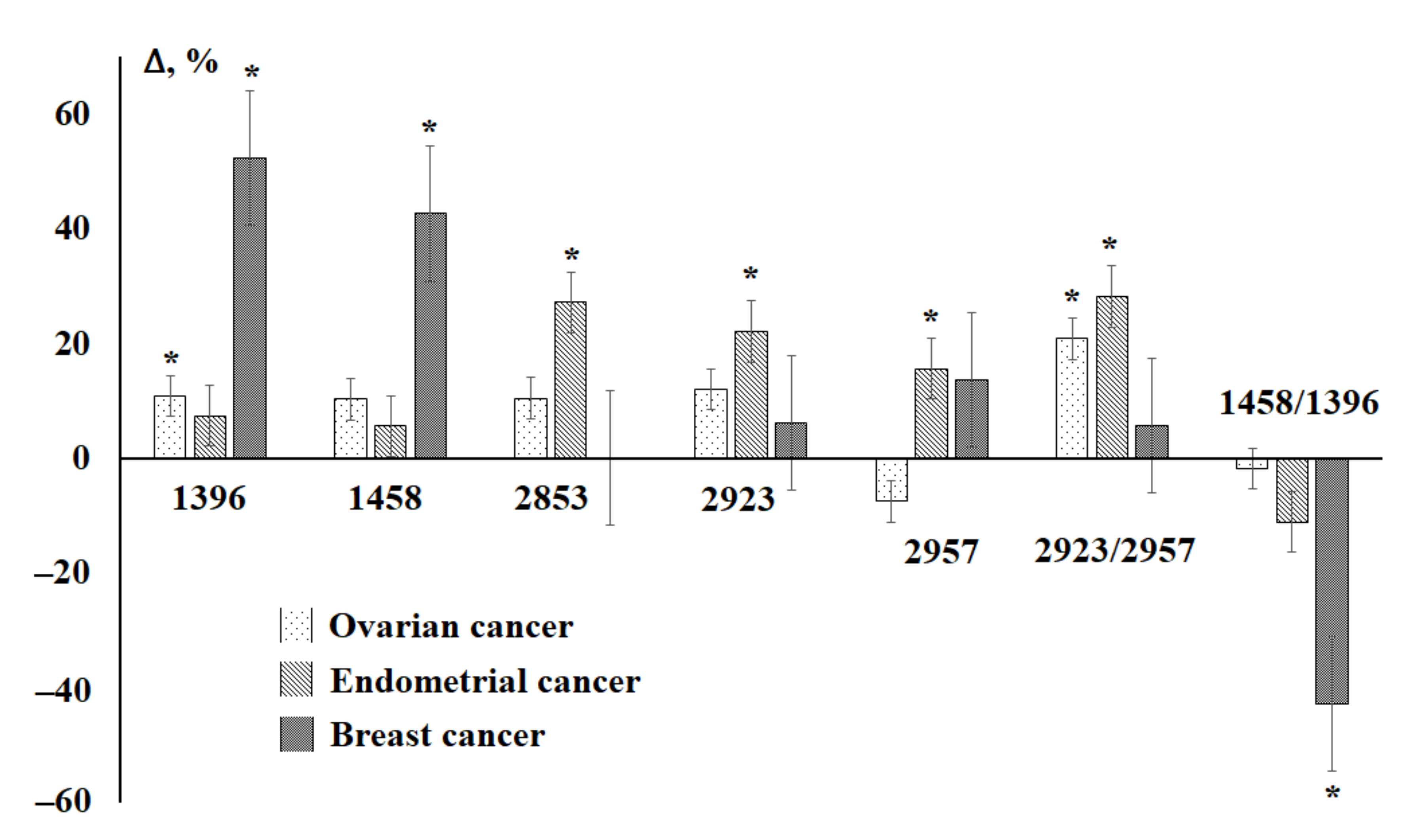
3.2. Saliva Lipid Profile in Breast Cancer
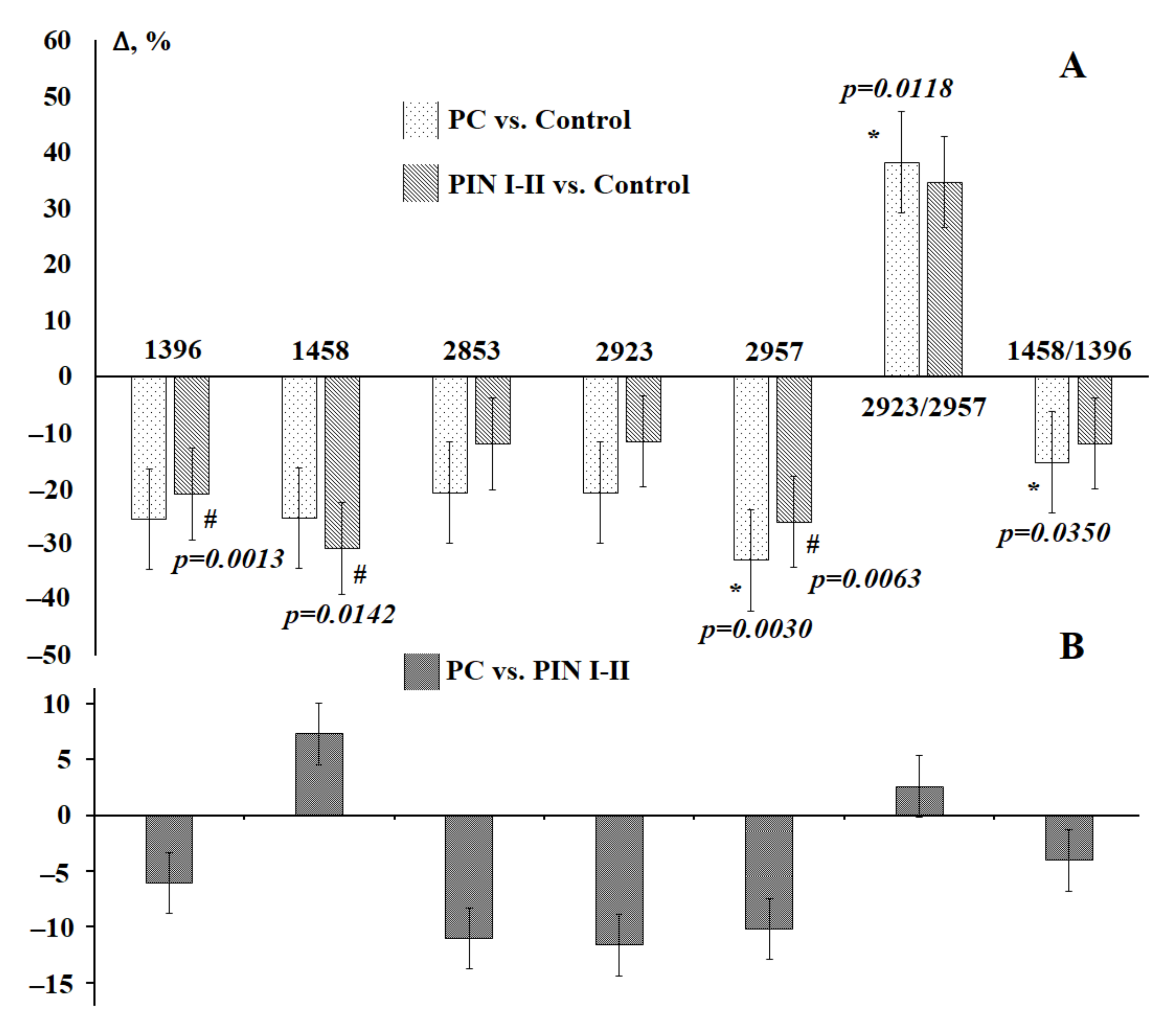
3.3. Saliva Lipid Profile in Prostate Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chojnowska, S.; Baran, T.; Wilińska, I.; Sienicka, P.; Cabaj-Wiater, I.; Knas, M. Human saliva as a diagnostic material. Adv. Med. Sci. 2018, 63, 185–191. [Google Scholar] [CrossRef]
- Roi, A.; Rusu, L.C.; Roi, C.I.; Luca, R.E.; Boia, S.; Munteanu, R.I. A New Approach for the Diagnosis of Systemic and Oral Diseases Based on Salivary Biomolecules. Hindawi Dis. Markers 2019, 8761860. [Google Scholar] [CrossRef] [Green Version]
- Kaczor-Urbanowicz, K.E.; Wei, F.; Rao, S.L.; Kim, J.; Shin, H.; Cheng, J.; Tu, M.; Wong, D.T.; Kim, Y. Clinical validity of saliva and novel technology for cancer detection. BBA-Rev. Cancer 2019, 1872, 49–59. [Google Scholar] [CrossRef]
- Gug, I.T.; Tertis, M.; Hosu, O.; Cristea, C. Salivary biomarkers detection: Analytical and immunological methods overview. TrAC Trends Anal. Chem. 2019, 113, 301–316. [Google Scholar] [CrossRef]
- Tierney, C.; Bazou, D.; Lê, G.; Dowling, P.; O’Gorman, P. Saliva-omics in plasma cell disorders- Proof of concept and potential as a non-invasive tool for monitoring disease burden. J. Proteom. 2021, 231, 104015. [Google Scholar] [CrossRef]
- Zubareva, G.M.; Mikin, V.M.; Bordina, G.Y.; Belyayeva, I.A.; Lopina, N.P.; Zubarev, S.M.; Kargapolov, A.V. Infrared spectrometry in the study of oral fluid for diagnostic purposes. Stomatologiya 2009, 5, 7–10. [Google Scholar]
- Caetano Júnior, P.C.; Ferreira-Strixino, J.; Raniero, L. Analysis of saliva by Fourier transform infrared spectroscopy for diagnosis of physiological stress in athletes. Res. Biomed. Eng. 2015, 31, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Bambery, K. The investigations of changes in mineral–organic and carbon–phosphate ratios in the mixed saliva by synchrotron infrared spectroscopy. Results Phys. 2016, 6, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Derruau, S.; Gobinet, C.; Mateu, A.; Untereiner, V.; Lorimier, S.; Piot, O. Shedding light on confounding factors likely to affect salivary infrared biosignatures. Anal. Bioanal. Chem. 2019, 411, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Smyth, H.E.; Gidley, M.J.; Cozzolino, D. Towards personalised saliva spectral fingerprints: Comparison of mid infrared spectra of dried and whole saliva samples. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2021, 253, 119569. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, E.; Balercia, P.; Conti, C.; Ferraris, P.; Sabbatini, S.; Rubini, C.; Tosi, G. Insights on diagnosis of oral cavity pathologies by infrared spectroscopy: A review. J. Mol. Struct. 2013, 1051, 226–232. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Alva, T.D.M.; Martinho, H.D.S.; Almeida, J.D. Analysis of saliva composition in patients with burning mouth syndrome (BMS) by FTIR spectroscopy. Vib. Spectrosc. 2019, 100, 195–201. [Google Scholar] [CrossRef]
- Seredin, P.V.; Goloshchapov, D.L.; A Plotnikova, Y.; A Ippolitov, Y.; Vongsvivut, J. Diagnostic potential of the oral fluid for the observation people with multiple dental caries by means of FTIR. J. Phys. Conf. Ser. 2018, 1124, 031007. [Google Scholar] [CrossRef]
- Seredin, P.V.; Goloshchapov, D.L.; Ippolitov, Y.A.; Avraamova, O.G. Spectroscopic studies of the changes occurring in the molecular composition of oral fluid in people with multiple caries. Stomatologiya 2019, 98, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Saranya, S.K.; Maradi, A.; Chithresan, K.; Janakiram, S.H.; Krishna, P. Molecular signatures in infrared spectra of saliva in healthy, chronic and aggressive periodontitis. Vib. Spectrosc. 2020, 111, 103179. [Google Scholar] [CrossRef]
- Scott, D.A.; Renaud, D.E.; Krishnasamy, S.; Meriç, P.; Buduneli, N.; Çetinkalp, Ş.; Liu, K.-Z. Diabetes-related molecular signatures in infrared spectra of human saliva. Diabetol. Metab. Syndr. 2010, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Paluszkiewicz, C.; Pięta, E.; Woźniak, M.; Piergies, N.; Koniewska, A.; Ścierski, W.; Misiołek, M.; Kwiatek, W.M. Saliva as a first-line diagnostic tool: A spectral challenge for identification of cancer biomarkers. J. Mol. Liq. 2020, 307, 112961. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Aguiar, E.M.; Cardoso-Sousa, L.; Caixeta, D.C.; Guedes, C.C.; Siqueira, W.L.; Maia, Y.C.P.; Cardoso, S.V.; Sabino-Silva, R. Differential Molecular Signature of Human Saliva Using ATR-FTIR Spectroscopy for Chronic Kidney Disease Diagnosis. Braz. Dent. J. 2019, 30, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Ralbovsky, N.M.; Halámková, L.; Wall, K.; Anderson-Hanley, C.; Lednev, I.K. Screening for Alzheimer’s Disease Using Saliva: A New Approach Based on Machine Learning and Raman Hyperspectroscopy. J. Alzheimers Dis. 2019, 71, 1351–1359. [Google Scholar] [CrossRef]
- Caixeta, D.C.; Aguiar, E.M.G.; Cardoso-Sousa, L.; Coelho, L.M.D.; Oliveira, S.W.; Espindola, F.S.; Raniero, L.; Crosara, K.T.B.; Baker, M.J.; Siquiera, W.L.; et al. Salivary molecular spectroscopy: A sustainable, rapid and non-invasive monitoring tool for diabetes mellitus during insulin treatment. PLoS ONE 2020, 15, e0223461. [Google Scholar] [CrossRef]
- Bottoni, U.; Tiriolo, R.; Pullano, S.A.; Dastoli, S.; Amoruso, G.F.; Nisticò, S.P.; Fiorillo, A.S. Infrared Saliva Analysis of Psoriatic and Diabetic Patients: Similarities in Protein Components. IEEE Trans. Biomed. Eng. 2016, 63, 379–384. [Google Scholar] [CrossRef]
- Bel’Skaya, L.V.; Sarf, E.A.; Gundyrev, I.A. Study of the IR Spectra of the Saliva of Cancer Patients. J. Appl. Spectrosc. 2019, 85, 1076–1084. [Google Scholar] [CrossRef]
- Ferreira, I.C.C.; Aguiar, E.M.G.; Silva, A.T.F.; Santos, L.L.D.; Cardoso-Sousa, L.; Araújo, T.G.; Santos, D.W.; Goulart, L.R.; Sabino-Silva, R.; Maia, Y.C.P. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy Analysis of Saliva for Breast Cancer Diagnosis. J. Oncol. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Falamas, A.; Faur, C.; Ciupe, S.; Chirila, M.; Rotaru, H.; Hedesiu, M.; Pinzaru, S.C. Rapid and noninvasive diagnosis of oral and oropharyngeal cancer based on micro-Raman and FT-IR spectra of saliva. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119477. [Google Scholar] [CrossRef]
- Byrne, H.J.; Behl, I.; Calado, G.; Ibrahim, O.; Toner, M.; Galvin, S.; Healy, C.M.; Flint, S.; Lyng, F.M. Biomedical Applications of Vibrational Spectroscopy: Oral Cancer Diagnostics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119470. [Google Scholar] [CrossRef] [PubMed]
- Maan, M.; Peters, J.; Dutta, M.; Patterson, A.D. Lipid metabolism and lipophagy in cancer. Biochem. Biophys. Res. Commun. 2018, 504, 582–589. [Google Scholar] [CrossRef]
- Omabe, M.; Ezeani, M.; Omabe, K.N. Lipid metabolism and cancer progression: The missing target in metastatic cancer treatment. J. Appl. Biomed. 2015, 13, 47–59. [Google Scholar] [CrossRef]
- Aripovskiy, A.B.; Kolesnik, P.O.; Vezhdel, M.I.; Titov, V.N. Sample preparation method for gas chromatographic determination of fatty acids without preliminary lipid extraction. Klin. Lab. Diagn. 2012, 1, 3–6. [Google Scholar]
- Kanskaya, N.V.; Ivanov, V.V.; Stepovaya, Y.A.; Pozdnyakova, I.A.; Fedorova, N.A. Modification of the method for determining lipids. Sib. Meditsinskiy Zhurnal 2014, 29, 69–73. [Google Scholar]
- Jeannotte, R.; Hamel, C.; Jabaji, S.; Whalen, J.K. Comparison of solvent mixtures for pressurized solvent extraction of soil fatty acid biomarkers. Talanta 2008, 77, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Cox, B.E.; Liu, W.; Porter, N.A.; Morrow, J.D.; Milne, G.L. Identification of intact oxidation products of glycerophospholipids in vitro and in vivo using negative ion electrospray iontrap mass spectrometry. J. Mass Spectrom. 2009, 44, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Lu, J.; Zhuo, Y.; Xiao, M.; Xue, X.; Zhong, S.; Shen, X.; Yin, C.; Li, L.; Chen, Q.; et al. Endogenous cholesterol ester hydroperoxides modulate cholesterol levels and inhibit cholesterol uptake in hepatocytes and macrophages. Redox Biol. 2019, 21, 101069. [Google Scholar] [CrossRef]
- Oleszko, A.; Olsztyńska-Janus, S.; Walski, T.; Grzeszczuk-Kuć, K.; Bujok, J.; Gałecka, K.; Czerski, A.; Witkiewicz, W.; Komorowska, M. Application of FTIR-ATR Spectroscopy to Determine the Extent of Lipid Peroxidation in Plasma during Haemodialysis. BioMed. Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Olsztyńska-Janus, S.; Pietruszka, A.; Kiełbowicz, Z.; Czarnecki, M. ATR-IR study of skin components: Lipids, proteins and water. Part I: Temperature effect. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 37–49. [Google Scholar] [CrossRef]
- Dzhatdoyeva, A.A.; Polimova, A.M.; Proskurina, Y.V.; Proskurin, M.A.; Vladimirov, Y.A. Determination of lipids and their oxidation products using IR spectroscopy. Zhurnal Anal. Khimii 2016, 76, 570–576. [Google Scholar]
- Orphanou, C.-M. The detection and discrimination of human body fluids using ATR FT-IR spectroscopy. Forensic Sci. Int. 2015, 252, e10–e16. [Google Scholar] [CrossRef]
- Bel’Skaya, L.V.; Sarf, E.A.; Solomatin, D.V. Determination of the quantitative content of lipids in biological material by IR spectroscopy. Klin. Lab. Diagn. 2019, 64, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Bel’Skaya, L.V.; Sarf, E.A.; Solomatin, D.V.; Kosenok, V.K. Analysis of the lipid profile of saliva in ovarian and endometrial cancer by IR fourier spectroscopy. Vib. Spectrosc. 2019, 104, 102944. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K. Age and gender characteristics of the biochemical composition of saliva: Correlations with the composition of blood plasma. J. Oral Biol. Craniofac. Res. 2020, 10, 59–65. [Google Scholar] [CrossRef]
- Bel’Skaya, L.V.; Sarf, E.A.; Solomatin, D.V. Age and gender characteristics of IR spectra of normal human saliva. Appl. Spectrosc. 2020, 74, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Bel’Skaya, L.V.; Kosenok, V.K.; Sarf, E.A. Chronophysiological features of the normal mineral composition of human saliva. Arch. Oral Biol. 2017, 82, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.R.; Souza, R.O.; Dias, L.B.; Ribas, T.B.; De Oliveira, L.C.F.; Sumida, D.H.; Dornelles, R.C.M.; Nakamune, A.C.D.M.S.; Chaves-Neto, A.H. The effects of storage time and temperature on the stability of salivary phosphatases, transaminases and dehydrogenase. Arch. Oral Biol. 2018, 85, 160–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radišauskas, R.; Kuzmickienė, I.; Milinavičienė, E.; Everatt, R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina 2016, 52, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Nohturfft, A.; Zhang, S.C. Coordination of Lipid Metabolism in Membrane Biogenesis. Annu. Rev. Cell Dev. Biol. 2009, 25, 539–566. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.; Torres, C.; Ramírez, M.E.; Epuñán, M.J.; Valladares, L.E.; Sierralta, W.D. Proliferation of human mammary cancer cells exposed to 27-hydroxycholesterol. Exp. Ther. Med. 2010, 1, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.C.; Holmberg, L.; Rohrmann, S.; Loda, M.; Van Hemelrijck, M. Serum Lipid Profiles and Cancer Risk in the Context of Obesity: Four Meta-Analyses. J. Cancer Epidemiol. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannikoppa, P.; Dhayanand, J.; Madhukumar, R.; Padmanabhan, A.; Bafna, U.; Vijayakumar, M.; Devi, K.U.; Pramod, K.; Thomas, T.; Jayshree, R.; et al. Fatty acid intake and erythrocyte fatty acid profile in women with breast, ovarian and cervical cancers. Clin. Nutr. ESPEN 2017, 19, 59–63. [Google Scholar] [CrossRef]
- Peck, B.; Schug, Z.T.; Zhang, Q.; Dankworth, B.; Jones, D.T.; Smethurst, E.; Patel, R.; Mason, S.; Jiang, M.; Saunders, R.; et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016, 4, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Baumann, J.; Sevinsky, C.; Conklin, D.S. Lipid biology of breast cancer. Biochim. Biophys. Acta 2013, 1831, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Gadomska, H.; Grzechocin’ska, B.; Janecki, J.; Nowicka, G.; Powolny, M.; Marianowski, L. Serum lipids concentration in women with benign and malignant ovarian tumours. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 87–90. [Google Scholar] [CrossRef]
- Munir, R.; Usman, H.; Hasnain, S.; Smans, K.; Kalbacher, H.; Zaidi, N. Atypical plasma lipid profile in cancer patients: Cause or consequence? Biochimie 2014, 102, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.M.; de Cedrón, M.G.; Rubio, J.M.; Martínez, S.F.; Martínez, R.S.; Casado, E.; de Molina, A.R.; Sereno, M. Lipid metabolism and lung cancer. Crit. Rev. Oncol. Hematol. 2017, 112, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Laura, G.; Hing, L.Y.; Imran, A. Lipid Pathway Deregulation in Advanced Prostate Cancer. Pharmacol. Res. 2018, 131, 177–184. [Google Scholar]
- Perrotti, F.; Rosa, C.; Cicalini, I.; Sacchetta, P.; Del Boccio, P.; Genovesi, D.; Pieragostino, D. Advances in Lipidomics for Cancer Biomarkers Discovery. Int. J. Mol. Sci. 2016, 17, 1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, A.; Maurhofer, O.; Beyoglu, D.; Lanz, C.; Krausz, K.W.; Pabst, T.; Gonzalez, F.J.; Dufour, J.-F.; Idle, J. Aberrant Lipid Metabolism in Hepatocellular Carcinoma Revealed by Plasma Metabolomics and Lipid Profiling. Cancer Res. 2011, 71, 6590–6600. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, H.; Dai, M.; Ai, J.; Li, Y.; Mahon, B.; Dai, S.; Deng, Y. Plasma lipidomics profiling identified lipid biomarkers in distinguishing early-stage breast cancer from benign lesions. Oncotarget 2016, 7, 36622–36631. [Google Scholar] [CrossRef]
- Meleh, M.; Požlep, B.; Mlakar, A.; Meden-Vrtovec, H.; Zupančič-Kralj, L. Determination of serum lysophosphatidic acid as a potential biomarker for ovarian cancer. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 858, 287–291. [Google Scholar] [CrossRef]
- Lee, G.B.; Lee, J.C.; Moon, M.H. Plasma lipid profile comparison of five different cancers by nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2019, 1063, 117–126. [Google Scholar] [CrossRef]
- Li, Z.; Kang, Y. Lipid Metabolism Fuels Cancer’s Spread. Cell Metab. 2017, 25, 228–230. [Google Scholar] [CrossRef] [Green Version]
- Petibois, C.; Déléris, G. Chemical mapping of tumor progression by FT-IR imaging: Towards molecular histopathology. Trends Biotechnol. 2006, 24, 455–462. [Google Scholar] [CrossRef]
- Sheng, D.; Xu, F.; Yu, Q.; Fang, T.; Xia, J.; Li, S.; Wang, X. A study of structural differences between liver cancer cells and normal liver cells using FTIR spectroscopy. J. Mol. Struct. 2015, 1099, 18–23. [Google Scholar] [CrossRef]
- Le Bivic, A.; Sari, H.; Reynier, M.; Lebec, S.; Bardin, F. Differences in lipid characteristics of autologous human melanoma cell lines with distinct biological properties. J. Natl. Cancer Inst. 1987, 79, 1181–1188. [Google Scholar] [PubMed]
- Reynier, M.; Sari, H.; D’Anglebermes, M.; A Kye, E.; Pasero, L. Differences in lipid characteristics of undifferentiated and enterocytic-differentiated HT29 human colonic cells. Cancer Res. 1991, 51, 1270–1277. [Google Scholar] [PubMed]
- Galeotti, T.; Borrello, S.; Minotti, G.; Masotti, L. Membrane alterations in cancer cells: The role of oxy radicals. Ann. N. Y. Acad. Sci. 1986, 488, 468–480. [Google Scholar] [CrossRef]
- Cejas, P.; Casado, E.; Belda–Iniesta, C.; De Castro, J.; Espinosa, E.; Redondo, A.; Sereno, M.; García-Cabezas, M.A.; Vara, J.A.; Domínguez-Cáceres, A.; et al. Implications of Oxidative Stress and Cell Membrane Lipid Peroxidation in Human Cancer (Spain). Cancer Causes Control. 2004, 15, 707–719. [Google Scholar] [CrossRef] [PubMed]

| Group | Subgroup | Number of Patients (%) |
|---|---|---|
| Breast cancer | 30 | |
| Stage | T1-2N0-1M0 | 20 (66.7%) |
| T3-4N0-2M0 | 10 (33.3%) | |
| Histological type | Ductal carcinoma | 18 (60.0%) |
| Lobular carcinoma | 12 (40.0%) | |
| The degree of anaplasia | G1 | 8 (26.7%) |
| G2 | 12 (40.0%) | |
| G3 | 10 (33.3%) | |
| Molecular subtypes | Luminal A | 3 (10.0%) |
| Luminal B | 20 (66.7%) | |
| HER2+ | 7 (23.3%) | |
| Fibroadenomas | 47 | |
| Group | Subgroup | Number of Patients (%) |
|---|---|---|
| Prostate cancer | 21 | |
| Stage | T2N0-1M0 | 16 (76.2%) |
| T3N0-1M0 | 5 (23.8%) | |
| Gleason grade | G 2–6 | 9 (42.9%) |
| G 7–10 | 12 (57.1%) | |
| Prostatic intraepithelial neoplasia | 21 | |
| PIN | PIN I | 10 (47.6%) |
| PIN II | 11 (52.4%) | |
| Indicator | Males, n = 58 | Females, n = 42 | p-Value | |
|---|---|---|---|---|
| Age, years | 46.4 [37.5; 53.2] | 50.5 [41.5; 54.8] | 0.1726 | |
| 1396 cm−1 | H | 1.33 [1.15; 2.44] | 3.24 [1.78; 4.03] | 0.0000 * |
| S | 6.94 [5.92; 12.99] | 18.30 [9.62; 24.80] | 0.0000 * | |
| 1458 cm−1 | H | 3.21 [2.22; 4.53] | 6.04 [2.95; 7.12] | 0.0020 * |
| S | 13.7 [10.0; 22.3] | 33.6 [12.3; 38.1] | 0.0037 * | |
| 2853 cm−1 | H | 8.14 [6.27; 10.49] | 7.85 [6.22; 11.65] | 0.7171 |
| S | 140.0 [106.0; 182.0] | 142.8 [106.4; 225.5] | 0.3200 | |
| 2923 cm−1 | H | 13.20 [10.60; 16.02] | 13.86 [10.22; 19.10] | 0.4119 |
| S | 326.9 [252.1; 395.7] | 341.4 [251.0; 492.2] | 0.2882 | |
| 2957 cm−1 | H | 2.27 [1.81; 2.57] | 2.60 [1.89; 3.00] | 0.0514 |
| S | 23.4 [18.0; 29.0] | 27.2 [22.4; 48.8] | 0.0026 * | |
| 2923/2957 | H | 5.70 [4.94; 7.18] | 5.50 [4.37; 7.27] | 0.2896 |
| S | 13.5 [10.1; 20.8] | 9.5 [7.2; 15.1] | 0.0419 * | |
| 1458/1396 | H | 2.09 [1.74; 2.56] | 1.72 [1.55; 1.98] | 0.0001 * |
| S | 1.79 [1.49; 2.20] | 1.50 [1.24; 1.76] | 0.0005 * | |
| Indicator | Breast Cancer, n = 30 | Fibroadenomas, n = 47 | Kruskal–Wallis Criterion; p-Value | |
|---|---|---|---|---|
| Age, years | 56.5 [48.0; 61.5] | 49.3 [40.5; 56.0] | 3.028; 0.1296 | |
| 1396 cm−1 | H | 1.55 [0.87; 1.96] | 1.22 [0.63; 1.56] | 41.04; 0.0000 * |
| S | 9.02 [6.13; 10.2] | 5.71 [2.89; 6.89] | 49.24; 0.0000 * | |
| 1458 cm−1 | H | 3.47 [2.6; 3.83] | 2.33 [1.93; 3.62] | 25.88; 0.0000 * |
| S | 18.1 [12; 20.6] | 9.77 [8.16; 22] | 17.98; 0.0001 * | |
| 2853 cm−1 | H | 7.85 [3.97; 9.06] | 8.34 [6.97; 10.9] | 4.428; 0.1093 |
| S | 140.0 [68.2; 157.0] | 151.0 [121; 206.0] | 4.843; 0.0888 | |
| 2923 cm−1 | H | 13 [7.76; 14.7] | 14.5 [12; 18.4] | 5.445; 0.0657 |
| S | 319.5 [180; 371] | 371.0 [303.0; 497.0] | 6.388; 0.0410 * | |
| 2957 cm−1 | H | 2.24 [1.48; 2.5] | 2.57 [2.11; 2.99] | 5.078; 0.0790 |
| S | 20.0 [14.2; 25.1] | 23.5 [16.1; 29.05] | 14.27; 0.0008 * | |
| 2923/2957 | H | 5.18 [4.54; 6.02] | 5.71 [4.46; 6.54] | 1.029; 0.5977 |
| S | 15.17 [8.55; 18.87] | 16.37 [11.57; 20.83] | 8.422; 0.0148 * | |
| 1458/1396 | H | 2.45 [1.95; 3.38] | 2.11 [1.73; 3.16] | 23.48; 0.0000 * |
| S | 2.19 [1.82; 2.77] | 2.21 [1.65; 3.68] | 29.21; 0.0000 * | |
| Indicator | Control (50–69 Years), n = 21 | Prostate Cancer, n = 21 | PINI-II, n = 21 | Kruskal–Wallis Criterion; p-Value | |
|---|---|---|---|---|---|
| Age, years | 66.1 [62.9; 68.3] | 65.0 [61.8; 69.0] | 66.0 [62.0; 69.3] | 2.043; 0.2889 | |
| 1396 cm−1 | H | 1.30 [1.05; 2.46] | 0.99 [0.90; 2.17] | 1.05 [0.80; 1.18] | 4.071; 0.1306 |
| S | 6.75 [5.88; 13.43] | 5.32 [4.97; 13.73] | 5.34 [4.42; 6.00] | 5.734; 0.0569 | |
| 1458 cm−1 | H | 3.42 [3.04; 5.01] | 2.40 [1.07; 5.04] | 2.22 [1.28; 2.66] | 9.745; 0.0077 * |
| S | 14.47 [12.63; 25.10] | 10.69 [4.24; 26.13] | 9.59 [5.17; 14.08] | 8.521; 0.0141 * | |
| 2853 cm−1 | H | 8.29 [6.08; 8.74] | 6.44 [5.08; 8.68] | 7.15 [5.80; 8.99] | 1.535; 0.4641 |
| S | 124.2 [88.4; 145.5] | 109.9 [91.7; 150.3] | 122.4 [96.7; 159.2] | 0.6748; 0.7136 | |
| 2923 cm−1 | H | 12.64 [9.64; 13.64] | 10.44 [9.46; 13.99] | 11.66 [9.47; 14.28] | 0.5786; 0.7488 |
| S | 315.2 [234.4; 346.4] | 279.0 [236.2; 343.7] | 287.9 [225.1; 347.8] | 0.4080; 0.8155 | |
| 2957 cm−1 | H | 1.91 [1.59; 2.28] | 1.52 [1.11; 1.84] | 1.68 [1.28; 1.95] | 3.443; 0.1788 |
| S | 25.52 [18.30; 30.75] | 18.75 [11.46; 27.45] | 20.37 [13.60; 32.15] | 1.726; 0.4219 | |
| 2923/2957 | H | 6.34 [5.27; 7.76] | 7.88 [6.09; 10.43] | 7.67 [4.15; 9.87] | 1.529; 0.4657 |
| S | 12.87 [7.94; 15.85] | 15.79 [10.54; 26.11] | 15.26 [8.47; 22.72] | 1.505; 0.4713 | |
| 1458/1396 | H | 2.28 [1.95; 3.07] | 1.77 [1.08; 2.21] | 1.84 [1.36; 3.09] | 6.853; 0.0325 * |
| S | 2.04 [1.73; 2.32] | 1.63 [0.83; 2.06] | 1.60 [1.11; 2.20] | 6.644; 0.0361 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K. Analysis of Saliva Lipids in Breast and Prostate Cancer by IR Spectroscopy. Diagnostics 2021, 11, 1325. https://doi.org/10.3390/diagnostics11081325
Bel’skaya LV, Sarf EA, Kosenok VK. Analysis of Saliva Lipids in Breast and Prostate Cancer by IR Spectroscopy. Diagnostics. 2021; 11(8):1325. https://doi.org/10.3390/diagnostics11081325
Chicago/Turabian StyleBel’skaya, Lyudmila V., Elena A. Sarf, and Victor K. Kosenok. 2021. "Analysis of Saliva Lipids in Breast and Prostate Cancer by IR Spectroscopy" Diagnostics 11, no. 8: 1325. https://doi.org/10.3390/diagnostics11081325







