Molecular Pathology of ALS: What We Currently Know and What Important Information Is Still Missing
Abstract
1. Introduction
2. Incidence and Prevalence of ALS
3. Etiology
4. Clinical Manifestation
- (1)
- ALS with cognitive impairment (ALSci);
- (2)
- ALS with behavioral impairment (ALSbi);
- (3)
- ALS with combined cognitive and behavioral impairment (ALS-cbi);
- (4)
- Fully developed behavioral variant of frontotemporal dementia (bvFTD) in combination with ALS (ALS-FTD);
- (5)
- Comorbid ALS and Alzheimer’s disease (AD) [55].
5. Differential Diagnosis
6. Types of ALS
6.1. Sporadic ALS
6.1.1. Background of SALS
6.1.2. Gross Findings in SALS
6.1.3. Neuropathological Findings in SALS
6.2. Familial ALS
6.2.1. Background of FALS
6.2.2. Neuropathological Findings in FALS
6.3. Amyotrophic Lateral Sclerosis-Frontotemporal Spectrum Disorder
6.3.1. Background of ALS-FTSD
6.3.2. Neuropathological Findings in ALS-FTSD
7. Molecular Biomarkers of ALS
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, C.E.; Rosenfeld, J. Motor neuron disease. Phys. Med. Rehabil. Clin. N. Am. 2001, 12, 335–352. [Google Scholar] [CrossRef]
- Ludolph, A.; Drory, V.; Hardiman, O.; Nakano, I.; Ravits, J.; Robberecht, W.; Shefner, J. WFN Research Group On ALS/MND. A revision of the El Escorial criteria—2015. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.A.; Statland, J.M.; Wolfe, G.I.; Barohn, R.J. Primary lateral sclerosis. Muscle Nerve 2007, 35, 291–302. [Google Scholar] [CrossRef]
- Liewluck, T.; Saperstein, D.S. Progressive Muscular Atrophy. Neurol. Clin. 2015, 33, 761–773. [Google Scholar] [CrossRef]
- Kim, W.K.; Liu, X.; Sandner, J.; Pasmantier, M.; Andrews, J.; Rowland, L.P.; Mitsumoto, H. Study of 962 patients indicates progressive muscular atrophy is a form of ALS. Neurology 2009, 73, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Rudnik-Schöneborn, S.; Zerres, K. Spinal muscular atrophies. In Emery and Rimoin’s Principles and Practice of Medical Genetics, 6th ed.; Rimoin, D.L., Pyeritz, R.E., Korf, B.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-012-383834-6. [Google Scholar]
- Batista, B.H.; Almeida, A.G.; Nunes, M.L.; Pitrez, P.M.; Ehlers, J.A. Paralisia bulbar progressiva juvenil doença de Fazio-Londe: Relato de caso Progressive bulbar palsy (Fazio-Londe disease): Case report. Arq. Neuropsiquiatr. 2002, 60, 830–834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- National Intitute of Neurological Disorders and Stroke. Motor Neuron Diseases Fact Sheet. Available online: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Motor-Neuron-Diseases-Fact-Sheet (accessed on 14 June 2021).
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef]
- Burr, P.; Reddivari, A.K.R. Spinal Muscle Atrophy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Waldrop, M.A.; Elsheikh, B.H. Spinal Muscular Atrophy in the Treatment Era. Neurol. Clin. 2020, 38, 505–518. [Google Scholar] [CrossRef]
- Breza, M.; Koutsis, G. Kennedy’s disease (spinal and bulbar muscular atrophy): A clinically oriented review of a rare disease. J. Neurol. 2019, 266, 565–573. [Google Scholar] [CrossRef]
- Querin, G.; Sorarù, G.; Pradat, P.-F. Kennedy disease (X-linked recessive bulbospinal neuronopathy): A comprehensive review from pathophysiology to therapy. Rev. Neurol. 2017, 173, 326–337. [Google Scholar] [CrossRef]
- Jubelt, B. Post-polio syndrome. Curr. Treat. Options Neurol. 2004, 6, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Jay, V. The legacy of Jean-Martin Charcot. Arch. Pathol. Lab. Med. 2000, 124, 10–11. [Google Scholar] [CrossRef]
- Goetz, C.G. Amyotrophic lateral sclerosis: Early contributions of Jean-Martin Charcot. Muscle Nerve 2000, 23, 336–343. [Google Scholar] [CrossRef]
- Editorial. Dementia and motor neuron disease. Lancet 1990, 335, 1250–1251. [Google Scholar] [CrossRef]
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Bonafede, R.; Mariotti, R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front. Cell. Neurosci. 2017, 11, 80. [Google Scholar] [CrossRef]
- Chiò, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Traynor, B.G.; Eurals Consortium. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Rusina, R.; Mmatěj, R.; Cséfalvay, Z.; Keller, J.; Franková, V.; Vyhnálek, M. Frontotemporální demence. Cesk Slov. Neurol. N. 2021, 84/117, 9–29. [Google Scholar] [CrossRef]
- Talbott, E.O.; Malek, A.M.; Lacomis, D. The epidemiology of amyotrophic lateral sclerosis. Handb. Clin. Neurol. 2016, 138, 225–238. [Google Scholar] [CrossRef]
- Chen, S.; Sayana, P.; Zhang, X.; Le, W. Genetics of amyotrophic lateral sclerosis: An update. Mol. Neurodegener. 2013, 8, 28. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myl-lykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Siddique, T.; Ajroud-Driss, S. Familial amyotrophic lateral sclerosis, a historical perspective. Acta Myol. 2011, 30, 117–120. [Google Scholar] [PubMed]
- Mehta, P.R.; Jones, A.R.; Opie-Martin, S.; Shatunov, A.; Iacoangeli, A.; Al Khleifat, A.; Smith, B.N.; Topp, S.; Morrison, K.E.; Shaw, P.J.; et al. Younger age of onset in familial amyotrophic lateral sclerosis is a result of pathogenic gene variants, rather than ascertainment bias. J. Neurol. Neurosurg. Psychiatr. 2019, 90, 268–271. [Google Scholar] [CrossRef]
- Wijesekera, L.L.; Leigh, P.N. Amyotrophic lateral sclerosis. Orphanet. J. Rare Dis. 2009, 4, 3. [Google Scholar] [CrossRef]
- McCombe, P.A.; Henderson, R.D. Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 2010, 7, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.R.; Vucic, S.; Kiernan, M.C. Isolated bulbar phenotype of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2011, 12, 283–289. [Google Scholar] [CrossRef]
- Chen, H.; Richard, M.; Sandler, D.P.; Umbach, D.M.; Kamel, F. Head injury and amyotrophic lateral sclerosis. Am. J. Epidemiol. 2007, 166, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.R.; Rodríguez-Izquierdo, I.; Jiménez, J.L.; Muñoz-Fernández, M.A. Analysis of ALS-related proteins during herpes simplex virus-2 latent infection. J. Neuroinflamm. 2020, 17, 371. [Google Scholar] [CrossRef]
- Berger, M.M.; Kopp, N.; Vital, C.; Redl, B.; Aymard, M.; Lina, B. Detection and cellular localization of enterovirus RNA sequences in spinal cord of patients with ALS. Neurology 2000, 54, 20–25. [Google Scholar] [CrossRef]
- Oluwole, S.O.; Yao, Y.; Conradi, S.; Kristensson, K.; Karlsson, H. Elevated levels of transcripts encoding a human retroviral envelope protein (syncytin) in muscles from patients with motor neuron disease. Amyotroph. Lateral Scler. 2007, 8, 67–72. [Google Scholar] [CrossRef]
- Sundaram, R.S.; Gowtham, L.; Nayak, B.S. The role of excitatory neurotransmitter glutamate in brain physiology and pathology. Asian J Pharm. Clin. Res. 2012, 5, 1–7. [Google Scholar]
- Matej, R.; Botond, G.; Laszlo, L.; Kopitar-Jerala, N.; Rusina, R.; Budka, H.; Kovacs, G.G. Increased neuronal Rab5 immunoreactive endosomes do not colocalize with TDP-43 in motor neuron disease. Exp. Neurol. 2010, 225, 133–139. [Google Scholar] [CrossRef]
- Turner, M.R.; Goldacre, R.; Ramagopalan, S.; Talbot, K.; Goldacre, M.J. Autoimmune disease preceding amyotrophic lateral sclerosis: An epidemiologic study. Neurology 2013, 81, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Štětkářová, I.; Matěj, R.; Ehler, E. Nové poznatky v dia gnostice a léčbě amyotrofické laterální sklerózy. Cesk Slov Neurol. N. 2018, 81, 546–554. [Google Scholar] [CrossRef]
- Janssen, C.; Schmalbach, S.; Boeselt, S.; Sarlette, A.; Dengler, R.; Petri, S. Differential Histone Deacetylase mRNA Expression Patterns in Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2010, 69, 573–581. [Google Scholar] [CrossRef]
- Armon, C. An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyo-trophic lateral sclerosis. Neuroepidemiology 2003, 22, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Geser, F.; Brandmeir, N.J.; Kwong, L.K. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyo-trophic lateral sclerosis. Arch. Neurol. 2008, 65, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Rothstein, J.D.; Kiernan, M.C. Advances in treating amyotrophic lateral sclerosis: Insights from pathophysiological studies. Trends Neurosci. 2014, 37, 433–442. [Google Scholar] [CrossRef]
- Wu, C.H.; Fal-lini, C.; Ticozzi, N.; Keagle, P.J.; Sapp, P.C.; Piotrowska, K.; Lowe, P.; Koppers, M.; McKenna-Yasek, D.; Baron, D.M.; et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature 2012, 488, 499–503. [Google Scholar] [CrossRef]
- Gorges, M.; Vercruysse, P.; Müller, H.P.; Huppertz, H.J.; Rosenbohm, A.; Nagel, G.; Weydt, P.; Petersén, Å.; Ludolph, A.C.; Kassubek, J.; et al. Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatr. 2017, 88, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Braak, H.; Del Tredici, K.; Lemon, R.; Ludolph, A.C.; Kiernan, M.C. Cortical influences drive amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatr. 2017, 88, 917–924. [Google Scholar] [CrossRef]
- Kawakami, I.; Arai, T.; Hasegawa, M. The basis of clinicopathological heterogeneity in TDP-43 proteinopathy. Acta Neuropathol. 2019, 138, 751–770. [Google Scholar] [CrossRef]
- Graus, F.; Delattre, J.Y.; Antoine, J.C.; Dalmau, J.; Giometto, B.; Grisold, W.; Honnorat, J.; Smitt, P.S.; Vedeler, C.; Verschuuren, J.J.; et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatr. 2004, 75, 1135–1140. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2013, 377, 162–172. [Google Scholar] [CrossRef]
- Preston, D.C.; Shapiro, B.E. Amyotrophic Lateral Sclerosis and its Variants. In Electromyography and Neuromuscular Disorders, 3rd ed.; Preston, D.C., Shapiro, B.E., Eds.; W.B. Saunders: London, UK, 2013; pp. 417–431. ISBN 9781455726721. [Google Scholar]
- Statland, J.M.; Barohn, R.J.; Dimachkie, M.M.; Floeter, M.K.; Mitsumoto, H. Primary Lateral Sclerosis. Neurol. Clin. 2015, 33, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M. Amyotrophic lateral sclerosis mimic syndromes. Iran J. Neurol. 2016, 15, 85–91. [Google Scholar] [PubMed]
- Al-Ghawi, E.; Al-Harbi, T.; Al-Sarawi, A.; Binfalah, M. Monomelic amyotrophy with proximal upper limb involvement: A case report. J. Med. Case Rep. 2016, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.H.; Abrahams, S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurol. 2013, 12, 368–380. [Google Scholar] [CrossRef]
- Rusina, R.; Vandenberghe, R.; Bruffaerts, R. Cognitive and Behavioral Manifestations in ALS: Beyond Motor System Involvement. Diagnostics 2021, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- McGee, S. Examination of the Motor System: Approach to Weakness. In Evidence-Based Physical Diagnosis, 4th ed.; McGee, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 551–568. ISBN 9780323392761. [Google Scholar] [CrossRef]
- Gibbons, C.J.; Thornton, E.W.; Young, C.A. The patient experience of fatigue in motor neurone disease. Front. Psychol. 2013, 4. [Google Scholar] [CrossRef]
- Benny, R.; Shetty, K. The split hand sign. Ann. Indian Acad. Neurol. 2012, 15, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Cui, L.; Liu, M.; Zhang, K.; Liu, S.; Ding, Q.; Hu, Y. Reassessment of Split-Leg Signs in Amyotrophic Lateral Sclerosis: Differential Involvement of the Extensor Digitorum Brevis and Abductor Hallucis Muscles. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, H.; Gaigalat, T.; Wiedemuth-Catrinescu, U.; Graf, M.; Uttner, I.; Muche, R.; Ludolph, A.C. Cognitive function in bulbar– and spinal–onset amyotrophic lateral sclerosis. A longitudinal study in 52 patients. J. Neurol. 2005, 252, 772–781. [Google Scholar] [CrossRef]
- Beeldman, E.; Raaphorst, J.; Klein Twennaar, M.; Govaarts, R.; Pijnenburg, Y.A.L.; de Haan, R.J.; de Visser, M.; Schmand, B.A. The cognitive profile of behavioural variant FTD and its similarities with ALS: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatr. 2018, 89, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.J.; Abrahams, S.; Goldstein, L.H.; Woolley, S.; Mclaughlin, P.; Snowden, J.; Mioshi, E.; Roberts-South, A.; Benatar, M.; Hortobágyi, T.; et al. Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 153–174. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the di-agnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Rafalowska, J.; Wasowicz, B. Syringomyelia simulating amyotrophic lateral sclerosis. Pol. Med. J. 1968, 7, 1214–1218. [Google Scholar]
- Kennedy Disease. Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=481. (accessed on 15 June 2021).
- Rowland, L.P. Diagnosis of amyotrophic lateral sclerosis. J. Neurol. Sci. 1998, 160 (Suppl. 1), S6–S24. [Google Scholar] [CrossRef]
- Mélé, N.; Berzero, G.; Maisonobe, T.; Salachas, F.; Nicolas, G.; Weiss, N.; Beaudonnet, G.; Ducray, F.; Psimaras, D.; Lenglet, T. Motor neuron disease of paraneoplastic origin: A rare but treatable condition. J. Neurol. 2018, 265, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Hudgson, P. Polymyositis and Dermatomyositis in Adults. Clin. Rheum. Dis. 1984, 10, 85–93. [Google Scholar] [CrossRef]
- Dabby, R.; Lange, D.J.; Trojaborg, W.; Hays, A.P.; Lovelace, R.E.; Brannagan, T.H.; Rowland, L.P. Inclusion body myositis mimicking motor neuron disease. Arch. Neurol. 2001, 58, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Wicks, P.; Abrahams, S.; Papps, B.; Al-Chalabi, A.; Shaw, C.E.; Leigh, P.N.; Goldstein, L.H. SOD1 and cognitive dysfunction in familial amyotrophic lateral sclerosis. J. Neurol. 2009, 256, 234–241. [Google Scholar] [CrossRef]
- Hayashi, Y.; Homma, K.; Ichijo, H. SOD1 in neurotoxicity and its controversial roles in SOD1 mutation-negative ALS. Adv. Biol. Regul. 2016, 60, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ajroud-Driss, S.; Siddique, T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim. Biophys. Acta 2015, 1852, 679–684. [Google Scholar] [CrossRef]
- Liscic, R.M.; Grinberg, L.T.; Zidar, J.; Gitcho, M.A.; Cairns, N.J. ALS and FTLD: Two faces of TDP-43 proteinopathy. Eur. J. Neurol. 2008, 15, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Mc Cann, E.P.; Henden, L.; Fifita, J.A.; Zhang, K.Y.; Grima, N.; Bauer, D.C.; Chan Moi Fat, S.; Twine, N.A.; Pamphlett, R.; Kiernan, M.C.; et al. Evidence for polygenic and oligogenic basis of Australian sporadic amyotrophic lateral sclerosis. J. Med. Genet. 2020, 14, 06866. [Google Scholar]
- Ellison, D.; Love, S.; Chimelli, L. Neuropathology—A Reference Text of CNS Pathology, 3rd ed.; Mosby: London, UK, 2013. [Google Scholar]
- Roeben, B.; Wilke, C.; Bender, B.; Ziemann, U.; Synofzik, M. The motor band sign in ALS: Presentations and frequencies in a consecutive series of ALS patients. J. Neurol. Sci. 2019, 406, 116440. [Google Scholar] [CrossRef]
- Kiernan, J.A.; Hudson, A.J. Frontal lobe atrophy in motor neuron diseases. Brain 1994, 117, 747–757. [Google Scholar] [CrossRef]
- Bede, P.; Chipika, R.H.; Finegan, E.; Li Hi Shing, S.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Hutchinson, S.; Donaghy, C.; McLaughlin, R.L.; et al. Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: A longitudinal neuroimaging study. NeuroImage Clin. 2019, 24, 102054. [Google Scholar] [CrossRef]
- Hirano, A. Neuropathology of ALS: An overview. Neurology 1996, 47 (Suppl. 2), 63–66. [Google Scholar] [CrossRef]
- Pun, S.; Santos, A.F.; Saxena, S.; Xu, L.; Caroni, P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 2006, 9, 408–419. [Google Scholar] [CrossRef]
- Hegedus, J.; Putman, C.T.; Gordon, T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyo-trophic lateral sclerosis. Neurobiol. Dis. 2007, 28, 154–164. [Google Scholar] [CrossRef]
- Shanmukha, S.; Narayanappa, G.; Nalini, A.; Alladi, P.A.; Raju, T.R. Sporadic amyotrophic lateral sclerosis (SALS)—skeletal muscle response to cerebrospinal fluid from SALS patients in a rat model. Dis. Model. Mech. 2018, 11, dmm031997. [Google Scholar] [CrossRef]
- Telerman-Toppet, N.; Coers, C. Motor innervation and fiber type pattern in amyotrophic lateral sclerosis and in Charcot-Marie-Tooth disease. Muscle Nerve 1978, 1, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Weller, R.O. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2011. [Google Scholar]
- Okamoto, K.; Hirai, S.; Shoji, M.; Senoh, Y.; Yamazaki, T. Axonal swellings in the corticospinal tracts in amyotrophic lateral sclerosis. Acta Neuropathol. 1990, 80, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Mizuno, Y.; Fujita, Y. Bunina bodies in amyotrophic lateral sclerosis. Neuropathology 2008, 28, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Knock, J.; Hewitt, C.; Highley, J.R.; Brockington, A.; Milano, A.; Man, S.; Martindale, J.; Hartley, J.; Walsh, T.; Gelsthorpe, C.; et al. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain 2012, 135, 751–764. [Google Scholar] [CrossRef]
- Rea, S.L.; Foster, A.D.; Rea, S.L. The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis. Neural Regen. Res. 2020, 15, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, H.; Nakamura, H.; Wakayama, I.; Yen, S.H.; Hirano, A. Skein-like inclusions in the anterior horn cells in motor neuron disease. J. Neurol. Sci. 1991, 105, 14–21. [Google Scholar] [CrossRef]
- Strong, M.J. The evidence for altered RNA metabolism in amyotrophic lateral sclerosis (ALS). J. Neurol. Sci. 2010, 288, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.J.; Donison, N.S.; Volkening, K. Alterations in Tau Metabolism in ALS and ALS-FTSD. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, I.R.; Frick, P.; Neumann, M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2013, 127, 347–357. [Google Scholar] [CrossRef]
- Cohen, T.J.; Lee, V.M.; Trojanowski, J.Q. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol. Med. 2011, 17, 659–667. [Google Scholar] [CrossRef]
- Deng, H.X.; Bigio, E.H.; Zhai, H.; Fecto, F.; Ajroud, K.; Shi, Y.; Yan, J.; Mishra, M.; Ajroud-Driss, S.; Heller, S.; et al. Differential Involvement of Optineurin in Amyotrophic Lateral Sclerosis with or Without SOD1 Mutations. Arch. Neurol. 2011, 68, 1057–1061. [Google Scholar] [CrossRef]
- Saez-Atienzar, S.; Bandres-Ciga, S.; Langston, R.G.; Kim, J.J.; Choi, S.W.; Reynolds, R.H.; Abramzon, Y.; Dewan, R.; Ahmed, S.; Landers, J.E.; et al. Genetic analysis of amyotrophic lateral sclerosis identifies contributing pathways and cell types. Sci. Adv. 2021, 7, eabd9036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef]
- Brettschneider, J.; del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Moisse, K.; Mepham, J.; Volkening, K.; Welch, I.; Hill, T.; Strong, M.J. Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL−/− mice: Support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009, 1296, 176–186. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Polymenidou, M.; Hutt, K.R.; Vu, A.Q.; Baughn, M.; Huelga, S.C.; Clutario, K.M.; Ling, S.-C.; Liang, T.Y.; Mazur, C.; et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat. Neurosci. 2012, 15, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Orban, P.; Devon, R.S.; Hayden, M.R.; Leavitt, B.R. Juvenile amyotrophic lateral sclerosis. Hum. Hypothal. Neuropsychiatr. Disord. 2007, 82, 301–312. [Google Scholar] [CrossRef]
- Ben Hamida, M.; Hentati, F.; Ben Hamida, C. Hereditary motor system diseases (chronic juvenile amyotrophic lateral sclerosis). Conditions combining a bilateral pyramidal syndrome with limb and bulbar amyotrophy. Brain 1990, 113, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.X.; Chen, W.; Hong, S.T.; Boycott, K.M.; Gorrie, G.H.; Siddique, N.; Yang, Y.; Fecto, F.; Shi, Y.; Zhai, H.; et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 2011, 477, 211–215. [Google Scholar] [CrossRef]
- Blokhuis, A.M.; Groen, E.J.; Koppers, M.; van den Berg, L.H.; Pasterkamp, R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013, 125, 777–794. [Google Scholar] [CrossRef]
- Shin, W.H.; Park, J.H.; Chung, K.C. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson’s disease. BMB Rep. 2020, 53, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Kaplan, V.; Livneh, I.; Avni, N.; Fabre, B.; Ziv, T.; Kwon, Y.T.; Ciechanover, A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc. Natl. Acad. Sci. USA 2016, 113, E7490–E7499. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Johansen, T. p62/SQSTM1: A Missing Link between Protein Aggregates and the Autophagy Machinery. Autophagy 2006, 2, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.L.; Zhang, X.; Chen, W.W.; Huang, W.J. Genetics insight into the amyotrophic lateral sclerosis/frontotemporal dementia spectrum. J. Med. Genet. 2017, 54, 145–154. [Google Scholar] [CrossRef]
- Lopate, G.; Baloh, R.H.; Al-Lozi, M.T.; Miller, T.M.; Fernandes Filho, J.A.; Ni, O.; Leston, A.; Florence, J.; Schierbecker, J.; Allred, P. Familial ALS with extreme phenotypic variability due to the I113T SOD1 mutation. Amyotroph. Lateral Scler. 2010, 11, 232–236. [Google Scholar] [CrossRef]
- Kato, S.; Hayashi, H.; Nakashima, K.; Nanba, E.; Kato, M.; Hirano, A.; Nakano, I.; Asayama, K.; Ohama, E. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am. J. Pathol. 1997, 151, 611–620. [Google Scholar] [PubMed]
- Rusina, R.; Ridzon, P.; Kulišťák, P.; Keller, O.; Bartos, A.; Buncova, M.; Fialová, L.; Koukolík, F.; Matej, R. Relationship between ALS and the degree of cognitive impairment, markers of neurodegeneration and predictors for poor outcome. A prospective study. Eur. J. Neurol. 2009, 17, 23–30. [Google Scholar] [CrossRef]
- Gregory, J.M.; Fagegaltier, D.; Phatnani, H.; Harms, M.H. Genetics of Amyotrophic Lateral Sclerosis. Curr. Genet. Med. Rep. 2020, 8, 121–131. [Google Scholar] [CrossRef]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; Vasta, R.; Brunetti, M.; Barberis, M.; Corrado, L.; D’Alfonso, S.; Bersano, E.; et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 2019, 93, e984–e994. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.M.; Nicholas, J.; Grossman, M.; McMillan, C.T.; Irwin, D.J.; Massimo, L.; van Deerlin, V.M.; Warren, J.D.; Fox, N.C.; Rossor, M.N.; et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: An international retrospective cohort study. Lancet Neurol. 2020, 19, 145–156. [Google Scholar] [CrossRef]
- Millecamps, S.; Boillée, S.; Le Ber, I.; Seilhean, D.; Teyssou, E.; Giraudeau, M.; Moigneu, C.; Vandenberghe, N.; Danel-Brunaud, V.; Corcia, P.; et al. Phenotype difference between ALS patients with expanded repeats inC9ORF72and patients with mutations in other ALS-related genes. J. Med. Genet. 2012, 49, 258–263. [Google Scholar] [CrossRef]
- Khan, B.K.; Yokoyama, J.S.; Takada, L.T.; Sha, S.J.; Rutherford, N.J.; Fong, J.C.; Karydas, A.M.; Wu, T.; Ketelle, R.S.; Baker, M.C.; et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated withC9ORF72hexanucleotide expansion. J. Neurol. Neurosurg. Psychiatr. 2012, 83, 358–364. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Neumann, M.; Baborie, A.; Sampathu, D.M.; Du, P.D.; Jaros, E.; Perry, R.H.; Trojanowski, J.Q.; Mann, D.M.A.; Lee, V.M.Y. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011, 122, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Davidson, Y.; Kelley, T.; MacKenzie, I.R.A.; Pickering-Brown, S.; Du Plessis, D.; Neary, D.; Snowden, J.S.; Mann, D.M.A. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007, 113, 521–533. [Google Scholar] [CrossRef]
- MacKenzie, I.R.; Bigio, E.H.; Ince, P.G.; Geser, F.; Neumann, M.; Cairns, N.J.; Kwong, L.K.; Forman, M.S.; Ravits, J.; Stewart, H.; et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis withSOD1 mutations. Ann. Neurol. 2007, 61, 427–434. [Google Scholar] [CrossRef]
- Al-Sarraj, S.; King, A.; Troakes, C.; Smith, B.; Maekawa, S.; Bodi, I.; Rogelj, B.; Al-Chalabi, A.; Hortobágyi, T.; Shaw, C. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011, 122, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Geser, F.; Lee, V.M.-Y.; Trojanowski, J.Q. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: A spectrum of TDP-43 proteinopathies. Neuropathology 2010, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Fichou, Y.; Al-Hilaly, Y.K.; Devred, F.; Smet-Nocca, C.; Tsvetkov, P.O.; Verelst, J.; Winderickx, J.; Geukens, N.; Vanmechelen, E.; Perrotin, A.; et al. The elusive tau molecular structures: Can we translate the recent breakthroughs into new targets for intervention? Acta Neuropathol. Commun. 2019, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Stejskalova, Z.; Rohan, Z.; Rusina, R.; Tesar, A.; Kukal, J.; Kovacs, G.G.; Bartos, A.; Matej, R. Pyramidal system involvement in progressive supranuclear palsy—A clinicopathological correlation. BMC Neurol. 2019, 19, 42. [Google Scholar] [CrossRef]
- Ahmed, Z.; Doherty, K.M.; Silveira-Moriyama, L.S.; Bandopadhyay, R.; Lashley, T.; Mamais, A.; Hondhamuni, G.; Wray, S.; Newcombe, J.; O’Sullivan, S.S.; et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: An emerging group of 4-repeat tauopathies. Acta Neuropathol. 2011, 122, 415–428. [Google Scholar] [CrossRef]
- Lantos, P.L.; Quinn, N. Multiple system atrophy. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 1st ed.; Dickson, D.W., Ed.; ISN Neuropath Press: Basel, Switzerland, 2003; pp. 203–214. [Google Scholar]
- Bowser, R.; Hamilton, R.L. Alzheimer disease pathology in amyotrophic lateral sclerosis. Acta Neuropathol. 2004, 107, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.L.; Crockford, D.R.; Sizemova, A.; McCann, H.; Shepherd, C.E.; McGeachie, A.B.; Affleck, A.J.; Carew-Jones, F.; Bartley, L.; Kwok, J.B.; et al. Coexisting Lewy body disease and clinical parkinsonism in frontotemporal lobar degeneration. Neurology 2019, 92, e2472–e2482. [Google Scholar] [CrossRef] [PubMed]
- Brait, K.; Fahn, S.; Schwarz, G.A. Sporadic and familial parkinsonism and motor neuron disease. Neurology 1973, 23, 990. [Google Scholar] [CrossRef]
- Manno, C.; Lipari, A.; Bono, V.; Taiello, A.C.; La Bella, V. Sporadic Parkinson disease and Amyotrophic Lateral Sclerosis complex (Brait–Fahn–Schwartz Disease). J. Neurol. Sci. 2013, 326, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Singh, S. Progranulin and TDP-43: Mechanistic Links and Future Directions. J. Mol. Neurosci. 2011, 45, 561–573. [Google Scholar] [CrossRef]
- Majumder, V.; Gregory, J.M.; Barria, M.A.; Green, A.; Pal, S. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: A systematic review and meta-analysis. BMC Neurol. 2018, 18, 90. [Google Scholar] [CrossRef]
- Junttila, A.; Kuvaja, M.; Hartikainen, P.; Siloaho, M.; Helisalmi, S.; Moilanen, V.; Kiviharju, A.; Jansson, L.; Tienari, P.J.; Remes, A.M.; et al. Cerebrospinal Fluid TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis Patients with and without the C9ORF72 Hexanucleotide Expansion. Dement. Geriatr. Cogn. Disord. Extra 2016, 6, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ji, Y.; Wang, W.; Zhang, L.; Chen, Z.; Yu, M.; Shen, Y.; Ding, F.; Gu, X.; Sun, H. Amyotrophic Lateral Sclerosis: Molecular Mechanisms, Biomarkers, and Therapeutic Strategies. Antioxidants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Elden, A.C.; Kim, H.-J.; Hart, M.P.; Chen-Plotkin, A.S.; Johnson, B.S.; Fang, X.; Armakola, M.; Geser, F.; Greene, R.; Lu, M.M.; et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 2010, 466, 1069–1075. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Ke, Y.; Hao, J.; Lu, L.; Lu, N.; Chen, X. Serum uric acid levels in patients with amyotrophic lateral sclerosis: A meta-analysis. Sci. Rep. 2018, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Feneberg, E.; Halbgebauer, S.; Witzel, S.; Verde, F.; Oeckl, P.; van Damme, P.; Gaur, N.; Gray, E.; Grosskreutz, J.; et al. Chitotriosidase as biomarker for early stage amyotrophic lateral sclerosis: A multicenter study. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 276–286. [Google Scholar] [CrossRef]
- Verde, F.; Steinacker, P.; Weishaupt, J.H.; Kassubek, J.; Oeckl, P.; Halbgebauer, S.; von Tumani, H.; Arnim, C.A.F.; Dorst, J.; Feneberg, E.; et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatr. 2019, 90, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Colletti, T.; Lo Sasso, B.; Vidali, M.; Spataro, R.; Gambino, C.M.; Giglio, R.V.; Piccoli, T.; Bivona, G.; La Bella, V.; et al. Tau protein as a diagnostic and prognostic biomarker in amyotrophic lateral sclerosis. Eur. J. Neurol. 2021, 28, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
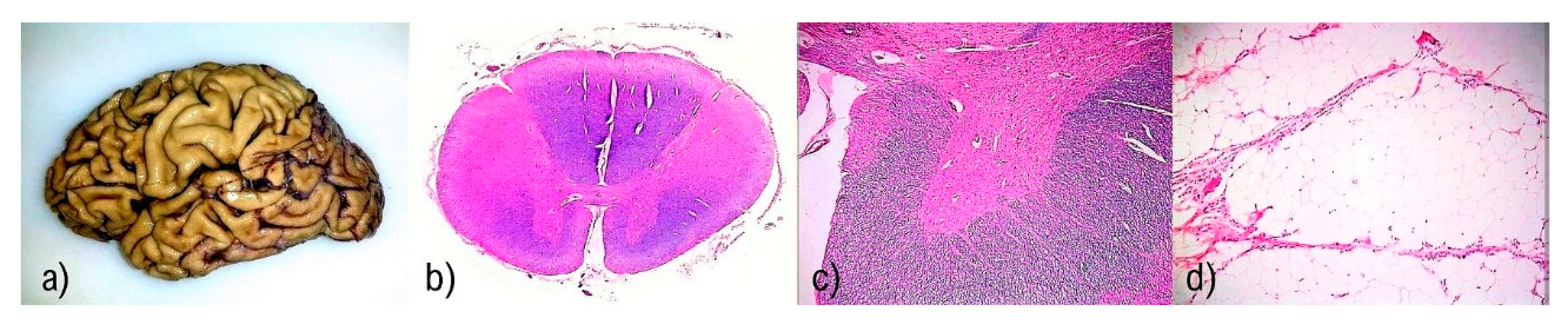

| Disease-Associated Genes | Inclusions |
|---|---|
|
|
| Disease-Associated Genes | Inclusions |
|---|---|
|
|
| Disease-Associated Genes | Inclusions |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankovska, N.; Matej, R. Molecular Pathology of ALS: What We Currently Know and What Important Information Is Still Missing. Diagnostics 2021, 11, 1365. https://doi.org/10.3390/diagnostics11081365
Jankovska N, Matej R. Molecular Pathology of ALS: What We Currently Know and What Important Information Is Still Missing. Diagnostics. 2021; 11(8):1365. https://doi.org/10.3390/diagnostics11081365
Chicago/Turabian StyleJankovska, Nikol, and Radoslav Matej. 2021. "Molecular Pathology of ALS: What We Currently Know and What Important Information Is Still Missing" Diagnostics 11, no. 8: 1365. https://doi.org/10.3390/diagnostics11081365
APA StyleJankovska, N., & Matej, R. (2021). Molecular Pathology of ALS: What We Currently Know and What Important Information Is Still Missing. Diagnostics, 11(8), 1365. https://doi.org/10.3390/diagnostics11081365






