Placental Types and Effective Perinatal Management of Vasa Previa: Lessons from 55 Cases in a Single Institution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
3.2. Clinical Management and Operative Outcomes
3.3. Neonatal Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catanzarite, V.; Maida, C.; Thomas, W.; Mendoza, A.; Stanco, L.; Piacquadio, K.M. Prenatal sonographic diagnosis of vasa previa: Ultrasound findings and obstetric outcome in ten cases. Ultrasound Obstet. Gynecol. 2001, 18, 109–115. [Google Scholar] [CrossRef]
- Oyelese, K.O.; Turner, M.; Lees, C.; Campbell, S. Vasa previa: An avoidable obstetric tragedy. Obstet. Gynecol. Surv. 1999, 54, 138–145. [Google Scholar] [CrossRef]
- Oyelese, K.O.; Schwärzler, P.; Coates, S.; Sanusi, F.A.; Hamid, R.; Campbell, S. A strategy for reducing the mortality rate from vasa previa using transvaginal sonography with color Doppler. Ultrasound Obstet. Gynecol. 1998, 12, 434–438. [Google Scholar] [CrossRef]
- Lee, W.; Lee, V.L.; Kirk, J.S.; Sloan, C.T.; Smith, R.S.; Comstock, C.H. Vasa previa: Prenatal diagnosis, natural evolution, and clinical outcome. Obstet. Gynecol. 2000, 95, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Oyelese, Y.; Catanzarite, V.; Prefumo, F.; Lashley, S.; Schachter, M.; Tovbin, Y.; Goldstein, V.; Smulian, J.C. Vasa Previa: The Impact of Prenatal Diagnosis on Outcomes. Obstet. Gynecol. 2004, 103, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Derbala, Y.; Grochal, F.; Jeanty, P. Vasa previa. J. Prenat. Med. 2007, 1, 2–13. [Google Scholar] [PubMed]
- Catanzarite, V.; Cousins, L.; Daneshmand, S.; Schwendemann, W.; Casele, H.; Adamczak, J.; Tith, T.; Patel, A. Prenatally Diagnosed Vasa Previa: A Single-Institution Series of 96 Cases. Obstet. Gynecol. 2016, 128, 1153–1161. [Google Scholar] [CrossRef]
- Swank, M.L.; Garite, T.J.; Maurel, K.; Das, A.; Perlow, J.H.; Combs, C.A.; Fishman, S.; Vanderhoeven, J.; Nageotte, M.; Bush, M.; et al. Vasa previa: Diagnosis and management. Am. J. Obstet. Gynecol. 2016, 215, e1–e223. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.A.; Javid, N.; Duncombe, G.; Li, Z.; Safi, N.; Cincotta, R.; Homer, C.S.E.; Halliday, L.; Oyelese, Y. Vasa Previa Diagnosis, Clinical Practice, and Outcomes in Australia. Obstet. Gynecol. 2017, 130, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Suekane, T.; Tachibana, D.; Pooh, R.K.; Misugi, T.; Koyama, M. Type-3 vasa previa: Normal umbilical cord insertion cannot exclude vasa previa in cases with abnormal placental location. Ultrasound Obstet. Gynecol. 2020, 55, 556–557. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tachibana, D.; Tahara, M.; Misugi, T.; Koyama, M. How to differentiate the fetal velamentous vein from maternal blood flow in cases with vasa previa. Prenat. Diagn. 2020, 40, 1610–1611. [Google Scholar] [CrossRef]
- Ward, C.R. Avoiding an incision through the anterior previa at cesarean delivery. Obstet. Gynecol. 2003, 102, 552–554. [Google Scholar]
- Tahara, M.; Tachibana, D.; Hamuro, A.; Misugi, T.; Nakano, A.; Koyama, M. The ward technique for anterior placenta previa. Arch. Gynecol. Obstet. 2020, 303, 1375–1376. [Google Scholar] [CrossRef]
- Oyelese, Y.; Smulian, J.C. Placenta previa, placenta accreta, and vasa previa. Obstet. Gynecol. 2006, 107, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Powel, J.; Aziz, M.; Shah, L.; Lashley, S.; Benito, C.; Oyelese, Y. Vasa Previa: Prenatal Diagnosis and Outcomes: Thirty-five Cases From a Single Maternal-Fetal Medicine Practice. J. Ultrasound Med. 2018, 37, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebarber, A.; Dolin, C.; Fox, N.S.; Klauser, C.K.; Saltzman, D.H.; Roman, A.S. Natural History of Vasa Previa Across Gestation Using a Screening Protocol. J. Ultrasound Med. 2014, 33, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Arakaki, T.; Ichizuka, K.; Sekizawa, A. Management of vasa previa during pregnancy. J. Perinat. Med. 2015, 43, 783–784. [Google Scholar] [CrossRef] [PubMed]
- Bartal, M.F.; Sibai, B.M.; Ilan, H.; Katz, S.; Eisen, I.S.; Kassif, E.; Yoeli, R.; Yinon, Y.; Mazaki-Tovi, S. Prenatal Diagnosis of Vasa Previa: Outpatient versus Inpatient Management. Am. J. Perinatol. 2019, 36, 422–427. [Google Scholar] [CrossRef]
- Society of Maternal-Fetal (SMFM) Publications Committee; Sinkey, R.G.; Odibo, A.O.; Dashe, J.S. #37: Diagnosis and management of vasa previa. Am. J. Obstet. Gynecol. 2015, 213, 615–619. [Google Scholar]
- Gagnon, R.J. No.231-Guidelines for the Management of Vasa Previa. Obstet. Gynaecol. Can. 2017, 39, e415–e421. [Google Scholar] [CrossRef]
- Jauniaux, E.; Alfirevic, Z.; Bhide, A.G.; Burton, G.J.; Collins, S.L.; Silver, R.; Royal College of Obstetricians and Gynaecologists. Vasa Praevia: Diagnosis and Management: Green-top Guideline No. 27b. BJOG 2019, 126, e49–e61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erfani, H.; Haeri, S.; Shainker, S.A.; Saad, A.F.; Ruano, R.; Dunn, T.N.; Rezaei, A.; Aalipour, S.; Nassr, A.A.; Shamshirsaz, A.A.; et al. Vasa previa: A multicenter retrospective cohort study. Am. J. Obstet. Gynecol. 2019, 221, 644.e1–644.e5. [Google Scholar] [CrossRef]
- Klahr, R.; Fox, N.S.; Zafman, K.; Hill, M.B.; Connolly, C.T.; Rebarber, A. Frequency of spontaneous resolution of vasa previa with ad-vancing gestational age. Am. J. Obstet. Gynecol. 2019, 221, e1–e646. [Google Scholar] [CrossRef]
- Westcott, J.M.; Simpson, M.S.; Chasen, S.; Vieira, L.; Stone, J.; Doulaveris, G.; Dar, P.; Bernstein, P.S.; Atallah, F.; Dolin, C.D.; et al. Prenatally diagnosed vasa previa: Association with adverse obstetrical and neonatal outcomes. Am. J. Obstet. Gynecol. MFM 2020, 2, 100206. [Google Scholar] [CrossRef] [PubMed]
- Maymon, R.; Melcer, Y.; Tovbin, J.; Pekar-Zlotin, M.; Smorgick, N.; Jauniaux, E. The Rate of Cervical Length Shortening in the Management of Vasa Previa. J. Ultrasound Med. 2018, 37, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Quintero, R.A.; Kontopoulos, E.V.; Bornick, P.W.; Allen, M.H. In utero laser treatment of type II vasa previa. J. Matern. Neonatal Med. 2007, 20, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, P.; Shamshirsaz, A.A.; Cass, D.L.; Espinoza, J.; Lee, W.; Salmanian, B.; Ruano, R.; Belfort, M.A. Fetoscopic laser ablation of vasa previa in pregnancy complicated by giant fetal cervical lymphatic malformation. Ultrasound Obstet. Gynecol. 2015, 46, 507–508. [Google Scholar] [CrossRef] [Green Version]
- Johnston, R.; Shrivastava, V.; Chmait, R. Term Vaginal Delivery following Fetoscopic Laser Photocoagulation of Type II Vasa Previa. Fetal Diagn. Ther. 2014, 35, 62–64. [Google Scholar] [CrossRef]
- Chmait, R.H.; Chavira, E.; Kontopoulos, E.V.; Quintero, R.A. Third trimester fetoscopic laser ablation of type II vasa previa. J. Matern. Neonatal Med. 2010, 23, 459–462. [Google Scholar] [CrossRef]
- Chmait, R.H.; Catanzarite, V.; Chon, A.H.; Korst, L.; Llanes, A.; Ouzounian, J.G. Fetoscopic Laser Ablation Therapy for Type II Vasa Previa. Fetal Diagn. Ther. 2020, 47, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Mönckeberg, M.; Valdés, R.; Kusanovic, J.P.; Schepeler, M.; Nien, J.K.; Pertossi, E.; Silva, P.; Silva, K.; Venegas, P.; Guajardo, U.; et al. Patients with acute cervical insufficiency without intra-amniotic infection/inflammation treated with cerclage have a good prognosis. J. Perinat. Med. 2019, 47, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Shen, J.; Hua, K. Cerclage for women with twin pregnancies: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2019, 220, 543–557.e1. [Google Scholar] [CrossRef] [PubMed]
- Chatzakis, C.; Efthymiou, A.; Sotiriadis, A.; Makrydimas, G. Emergency cerclage in singleton pregnancies with painless cervical dilatation: A meta-analysis. Acta Obstet. Gynecol. Scand. 2020, 99, 1444–1457. [Google Scholar] [CrossRef]
- Englert, Y.; Imbert, M.; Van Rosendael, E.; Belaisch, J.; Segal, L.; Feichtinger, W.; Wilkin, P.; Frydman, R.; Leroy, F. Morphological anomalies in the placentae of IVF pregnancies: Preliminary report of a multicentric study. Hum. Reprod. 1987, 2, 155–157. [Google Scholar] [CrossRef]
- Jauniaux, E.; Englert, Y.; Vanesse, M.; Hiden, M.; Wilkin, P. Pathologic features of placentas from singleton pregnancies obtained by in vitro fertilization and embryo transfer. Obstet. Gynecol. 1990, 76, 61–64. [Google Scholar] [PubMed]
- Torpin, R. Classification of Human Pregnancy based on Depth of Intrauterine Implantation of the Ovum*. Am. J. Obstet. Gynecol. 1953, 66, 791–800. [Google Scholar] [CrossRef]
- Chiofalo, B.; Laganà, A.S.; Vaiarelli, A.; La Rosa, V.L.; Rossetti, D.; Palmara, V.; Valenti, G.; Rapisarda, A.M.C.; Granese, R.; Sapia, F.; et al. Do miRNAs Play a Role in Fetal Growth Restriction? A Fresh Look to a Busy Corner. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Laganà, A.S.; Vitale, S.G.; Sapia, F.; Valenti, G.; Corrado, F.; Padula, F.; Rapisarda, A.M.C.; D’Anna, R. miRNA expression for early di-agnosis of preeclampsia onset: Hope or hype? J. Matern. Fetal Neonatal Med. 2018, 31, 817–821. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Marotta, C.; Pisani, L.; Veronese, N.; Pisani, V.; Lippolis, V.; Pellizer, G.; Pizzol, D.; Tognon, F.; Bavaro, D.F.; et al. Maternal caesarean section infection (MACSI) in Sierra Leone: A case-control study. Epidemiol. Infect. 2020, 27, 148e40. [Google Scholar] [CrossRef] [Green Version]
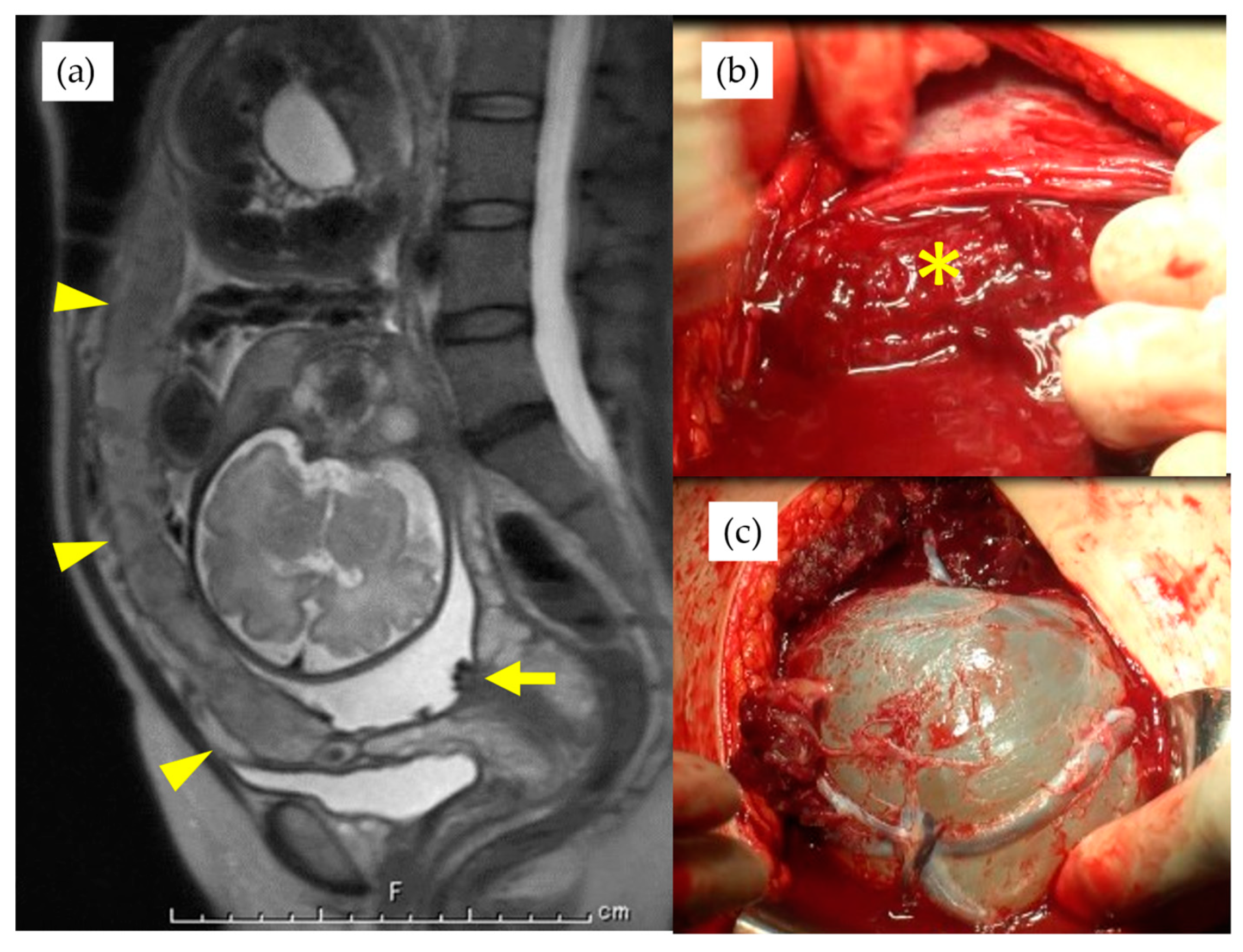
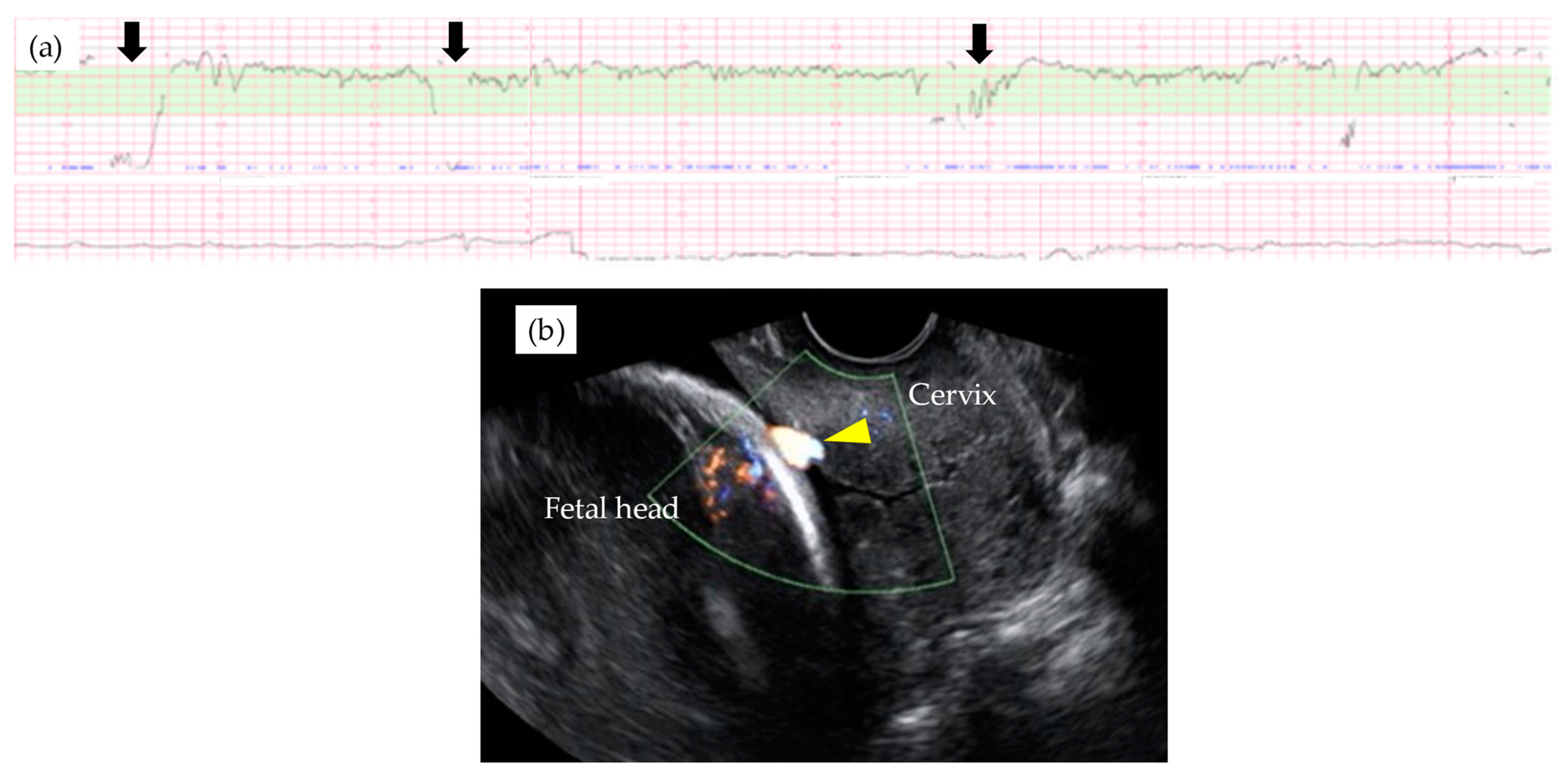
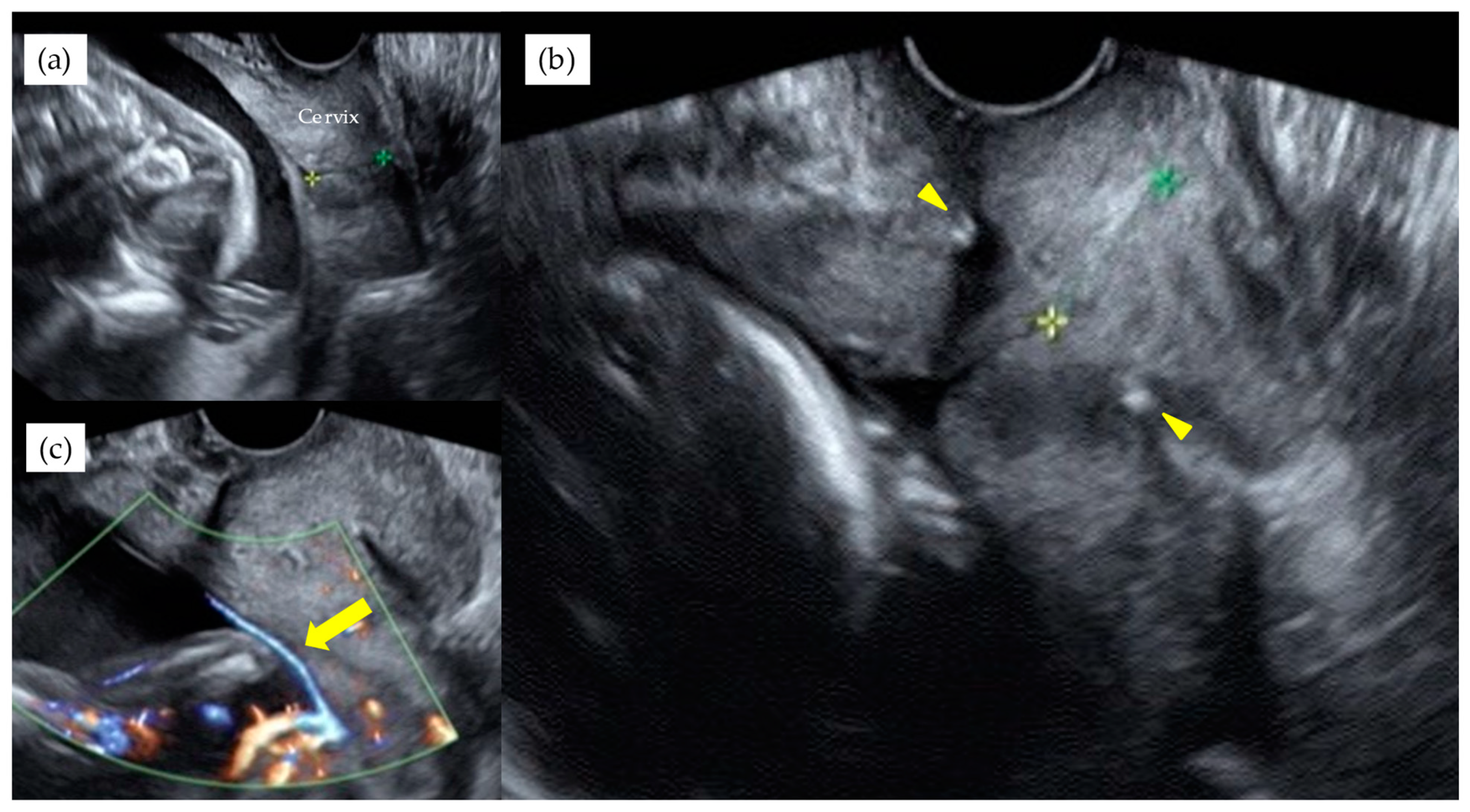
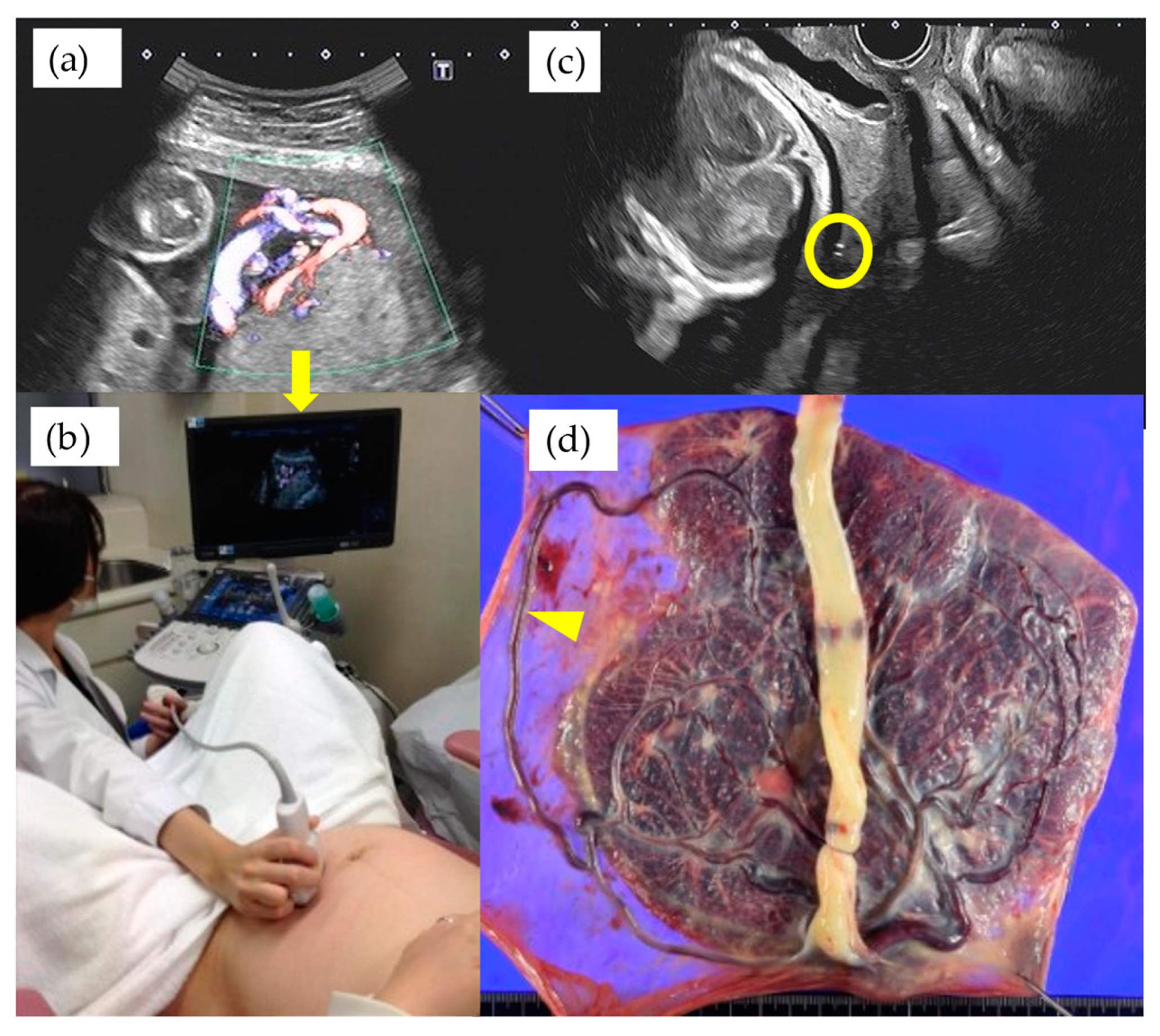
| Total n = 55 | Type 1 n = 35 | Non-Type 1 n = 20 | p-Value | |
|---|---|---|---|---|
| Age | 36 (21–49) | 38 (21–43) | 34 (29–49) | 0.759 |
| Gravida | 1 (1–6) | 1 (1–6) | 2 (1–4) | 0.308 |
| Parity | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0.192 |
| ART, n (%) | 26 (47.3%) | 21 (60.0%) | 5 (25.0%) | 0.024 |
| Twin pregnancy, n (%) | 3 (5.5%) | 3 (8.6%) | 0 (0%) | 0.293 |
| Referred after VP diagnosis, n (%) | 32 (58.2%) | 25 (71.4%) | 7 (35.0%) | 0.019 |
| Total n = 55 | Type 1 n = 35 | Non-Type 1 n = 20 | p-Value | |
|---|---|---|---|---|
| Diagnosis of GW | 25.1 (18.0–39.0) | 21.4 (18.0–39.0) | 28.6 (18.3–35.0) | 0.027 |
| Diagnosis ≦ 20th GW, n (%) | n = 16 (29.1%) | n = 14 (40.0%) | n = 2 (10.0%) | 0.029 |
| Diagnosis ≦ 24th GW, n (%) | n = 26 (47.3%) | n = 20 (57.1%) | n = 6 (30.0%) | 0.097 |
| Diagnosis ≦ 28th GW, n (%) | n = 34 (61.8%) | n = 25 (71.4%) | n = 9 (45.0%) | 0.099 |
| Diagnosis ≦ 32th GW, n (%) | n = 45 (81.8%) | n = 30 (85.7%) | n = 15 (75.0%) | 0.530 |
| Non-sagittal plane, n (%) | n = 37 (67.3%) | n = 24 (68.6%) | n = 13 (65.0%) | 1.000 |
| Valsalva maneuver, n (%) | n = 11 (20.0%) | n = 4 (11.4%) | n = 7 (35.0%) | 0.076 |
| Low-lying placenta at diagnosis, n (%) | n = 34 (61.8%) | n = 18 (51.4%) | n = 16 (80.0%) | 0.070 |
| Low-lying placenta at CS, n (%) | n = 29 (52.7%) | n = 14 (40.0%) | n = 15 (75.0%) | 0.026 |
| Total n = 55 | Type 1 n = 35 | Non-Type 1 n = 20 | p-Value | |
|---|---|---|---|---|
| GW of admission | 31.4 (24.3–39.3) | 30.8 (24.3–39.3) | 31.8 (24.3–35.8) | 0.457 |
| Usage of tocolytic agent, n (%) | 31 (56.4%) | 20 (57.1%) | 11 (55.0%) | 1.000 |
| Duration of tocolysis (days) | 5 (0–69) | 5 (0–68) | 4 (0–69) | 0.762 |
| First treatment with tocolytics (GW) | 30.9 (24.3–34.6) | 30.8 (24.3–34.6) | 32.1 (24.3–33.1) | 0.580 |
| Steroid administration, n/N (%) | 15/55 (27.3%) | 10/35 (28.6%) | 5/20 (25.0%) | 1.000 |
| Steroid administration for the cases delivered before 34th GW, n/N (%) | 9/13 (69.2%) | 8/9 (88.9%) | 1/4 (25.0%) | 0.052 |
| Abnormal CTG, n (%) | 1 (1.8%) | 1 (2.9%) | 0 (0%) | 1.000 |
| Cervical cerclage, n (%) | 2 (3.6%) | 2 (5.7%) | 0 (0%) | 0.529 |
| Resolution, n (%) | 12 (21.8%) | 9 (25.7%) | 3 (15%) | 0.503 |
| GW of delivery (GW) | 35.1 (30.3–39.3) | 35.1 (31.0–39.3) | 35.1 (30.3–36.7) | 0.937 |
| Emergent CS, n (%) | 26 (47.3) | 17 (48.6%) | 9 (45.0%) | 1.000 |
| Operation time (minutes) | 56.0 (30–95) | 59.0 (30–95) | 52.5 (35–95) | 0.426 |
| Estimated blood loss (mL) | 1290 (400–3400) | 1330 (400–3400) | 1190 (560–3225) | 0.618 |
| Transfusion, n (%) | 21 (38.2%) | 11 (31.4%) | 10 (50.0%) | 0.282 |
| Ward method, n (%) | 17 (30.9%) | 5 (14.3%) | 12 (60.0%) | <0.001 |
| Total n = 58 | Type 1 n = 38 | Non-Type 1 n = 20 | p-Value | |
|---|---|---|---|---|
| Birth weight (gram) | 2221 (1472–2940) | 2173 (1472–2775) | 2277 (1541–2940) | 0.314 |
| Standard deviations of birth weight | −0.02 (−1.30–1.34) | −0.05 (−1.30–1.34) | 0.25 (−1.14–1.16) | 0.239 |
| Female/Male | 27/31 | 20/18 | 7/13 | 0.316 |
| Apgar score 1 min | 8 (1–9) | 8 (1–9) | 8 (4–8) | 0.178 |
| Apgar score 5 min | 9 (6–10) | 9 (6–10) | 9 (7–9) | 0.850 |
| pH of umbilical artery | 7.299 (7.172–7.403) | 7.308 (7.172–7.403) | 7.279 (7.204–7.397) | 0.086 |
| Hemoglobin at birth (g/dL) | 13.6 (10.9–18.4) | 13.6 (10.9–17.4) | 13.9 (11.6–18.4) | 0.507 |
| Respiratory support, n (%) | 39 (70.9%) | 26 (74.3%) | 13 (65.0%) | 0.674 |
| Respiratory support (days) | 3 (0–42) | 3 (0–30) | 3 (0–42) | 0.913 |
| Congenital disorders, n (%) | 4 (6.9%) | 2 (5.3%) | 2 (10%) | 0.428 |
| Admission days | 16 (6–73) | 14 (6–59) | 16 (6–73) | 0.616 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, D.; Misugi, T.; Pooh, R.K.; Kitada, K.; Kurihara, Y.; Tahara, M.; Hamuro, A.; Nakano, A.; Koyama, M. Placental Types and Effective Perinatal Management of Vasa Previa: Lessons from 55 Cases in a Single Institution. Diagnostics 2021, 11, 1369. https://doi.org/10.3390/diagnostics11081369
Tachibana D, Misugi T, Pooh RK, Kitada K, Kurihara Y, Tahara M, Hamuro A, Nakano A, Koyama M. Placental Types and Effective Perinatal Management of Vasa Previa: Lessons from 55 Cases in a Single Institution. Diagnostics. 2021; 11(8):1369. https://doi.org/10.3390/diagnostics11081369
Chicago/Turabian StyleTachibana, Daisuke, Takuya Misugi, Ritsuko K. Pooh, Kohei Kitada, Yasushi Kurihara, Mie Tahara, Akihiro Hamuro, Akemi Nakano, and Masayasu Koyama. 2021. "Placental Types and Effective Perinatal Management of Vasa Previa: Lessons from 55 Cases in a Single Institution" Diagnostics 11, no. 8: 1369. https://doi.org/10.3390/diagnostics11081369
APA StyleTachibana, D., Misugi, T., Pooh, R. K., Kitada, K., Kurihara, Y., Tahara, M., Hamuro, A., Nakano, A., & Koyama, M. (2021). Placental Types and Effective Perinatal Management of Vasa Previa: Lessons from 55 Cases in a Single Institution. Diagnostics, 11(8), 1369. https://doi.org/10.3390/diagnostics11081369






