46,XX DSD: Developmental, Clinical and Genetic Aspects
Abstract
:1. Introduction
2. Steroidogenesis in 46,XX
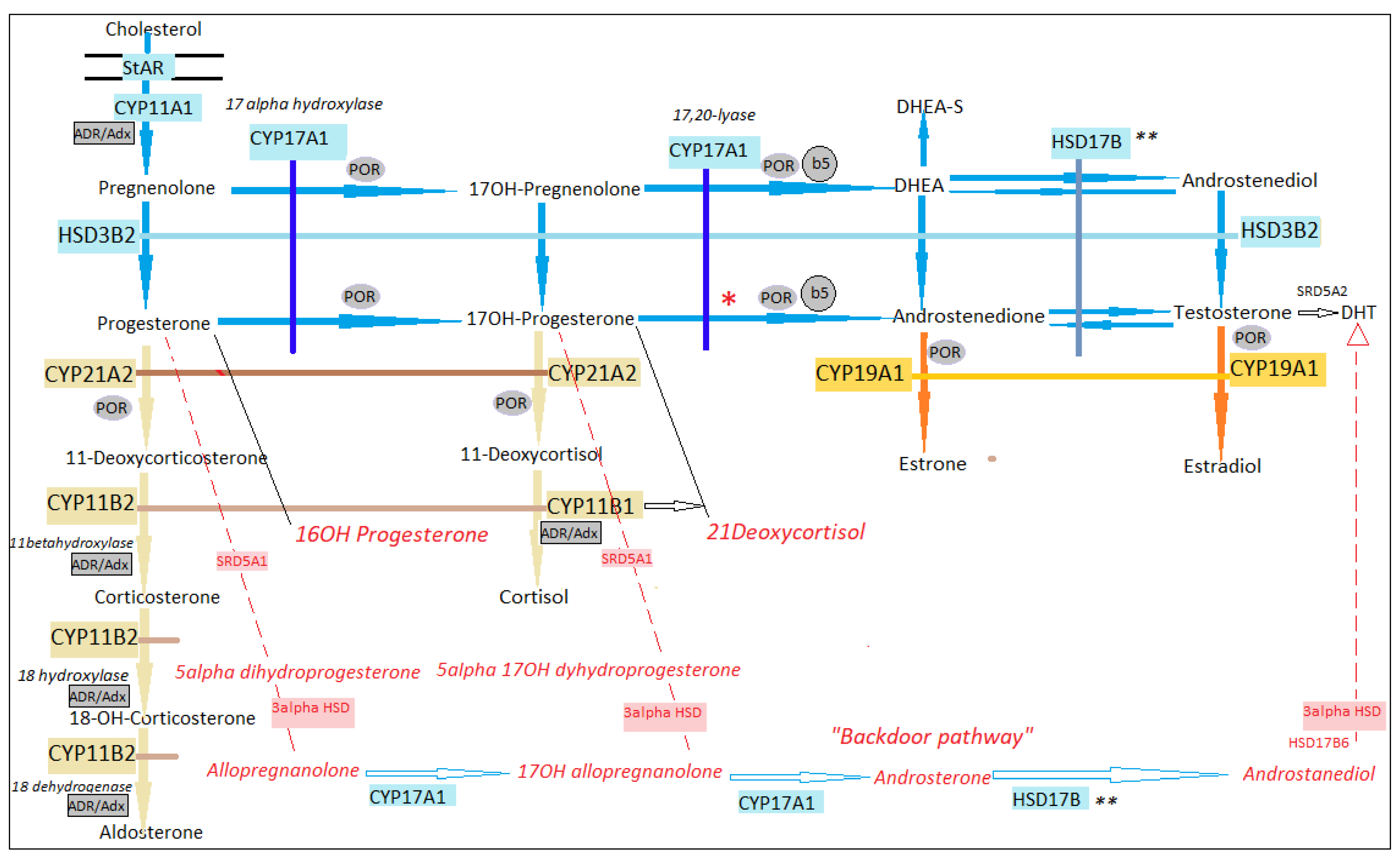
3. Sex Development in 46,XX
3.1. Gonads

3.2. Internal Genital Organs
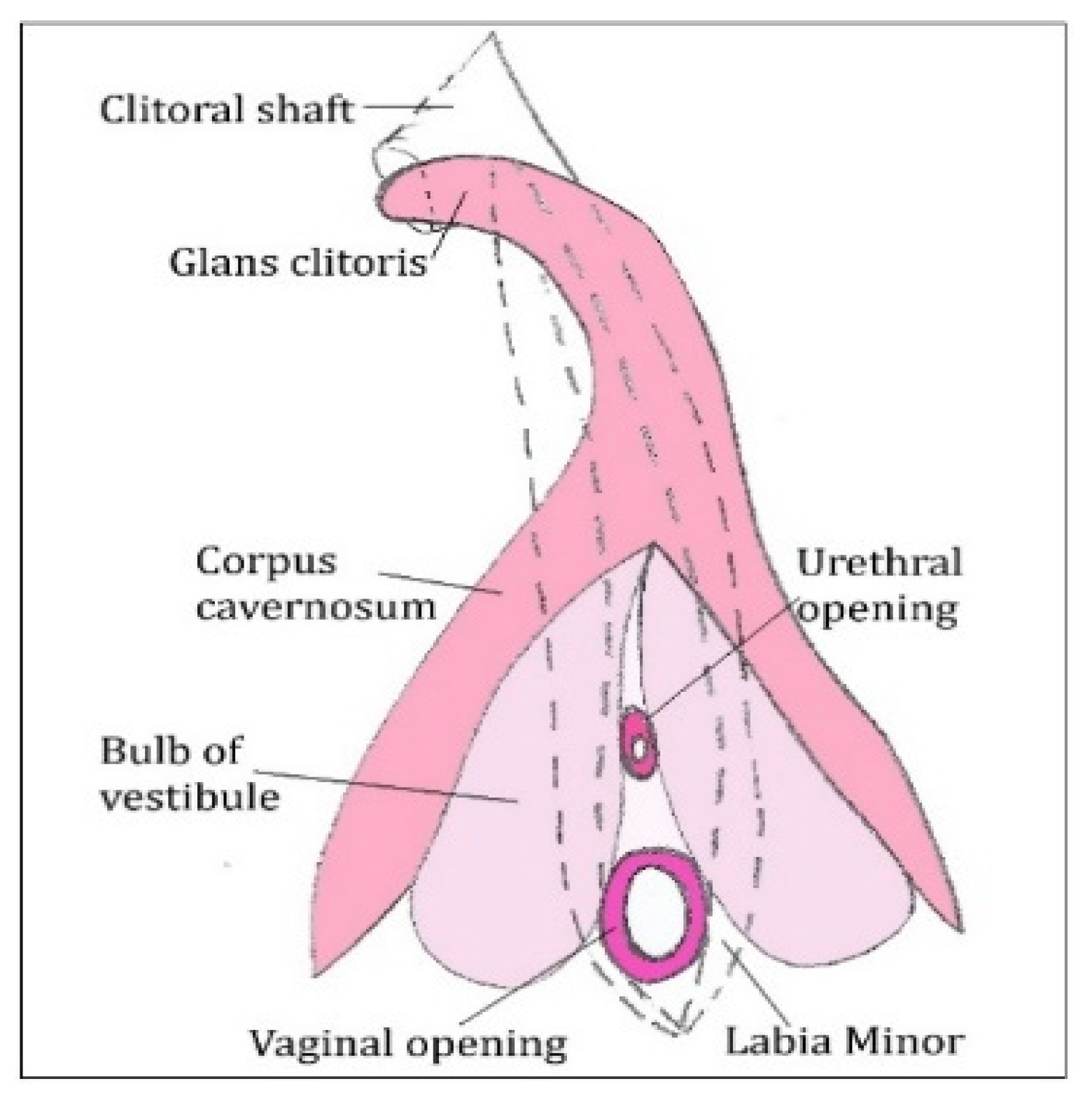
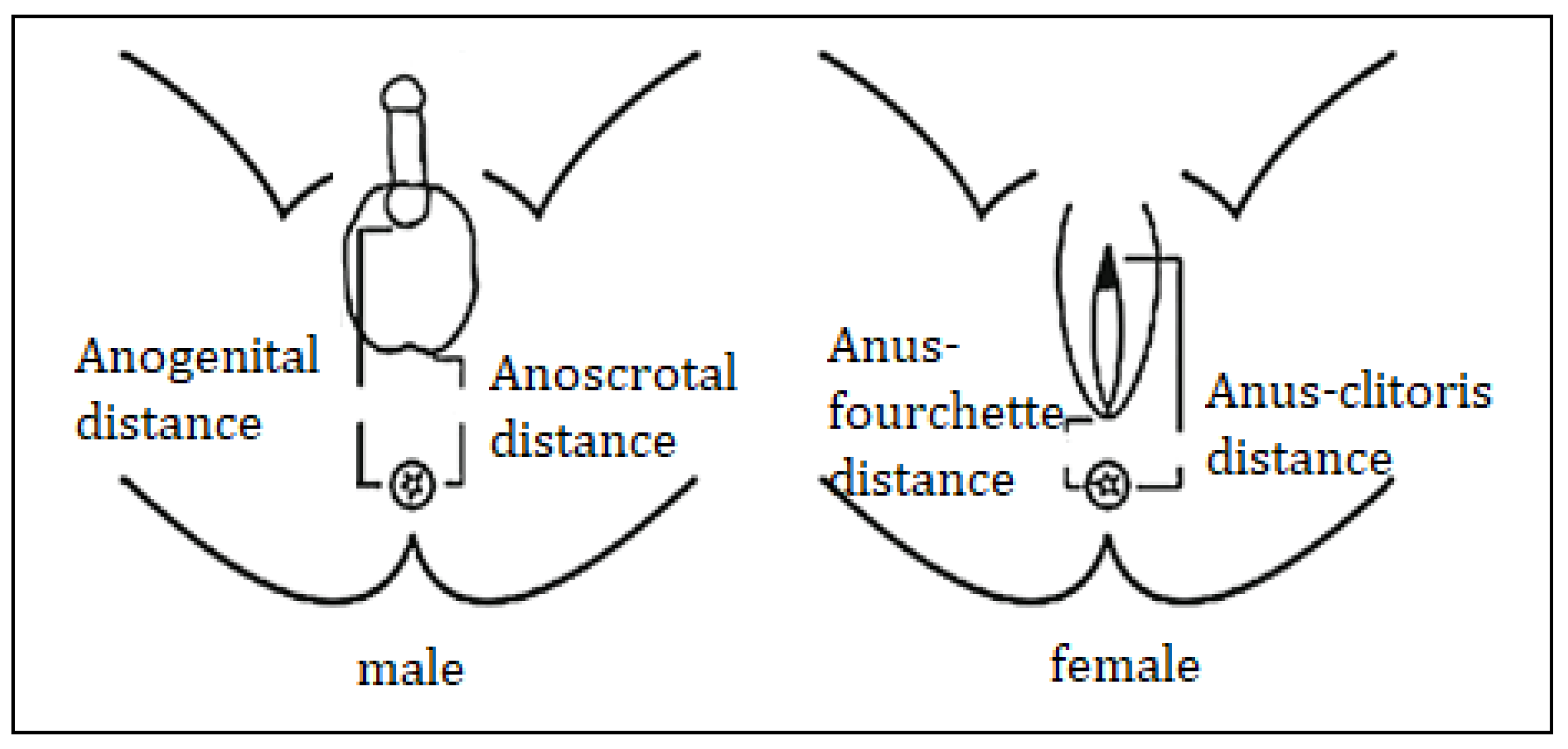
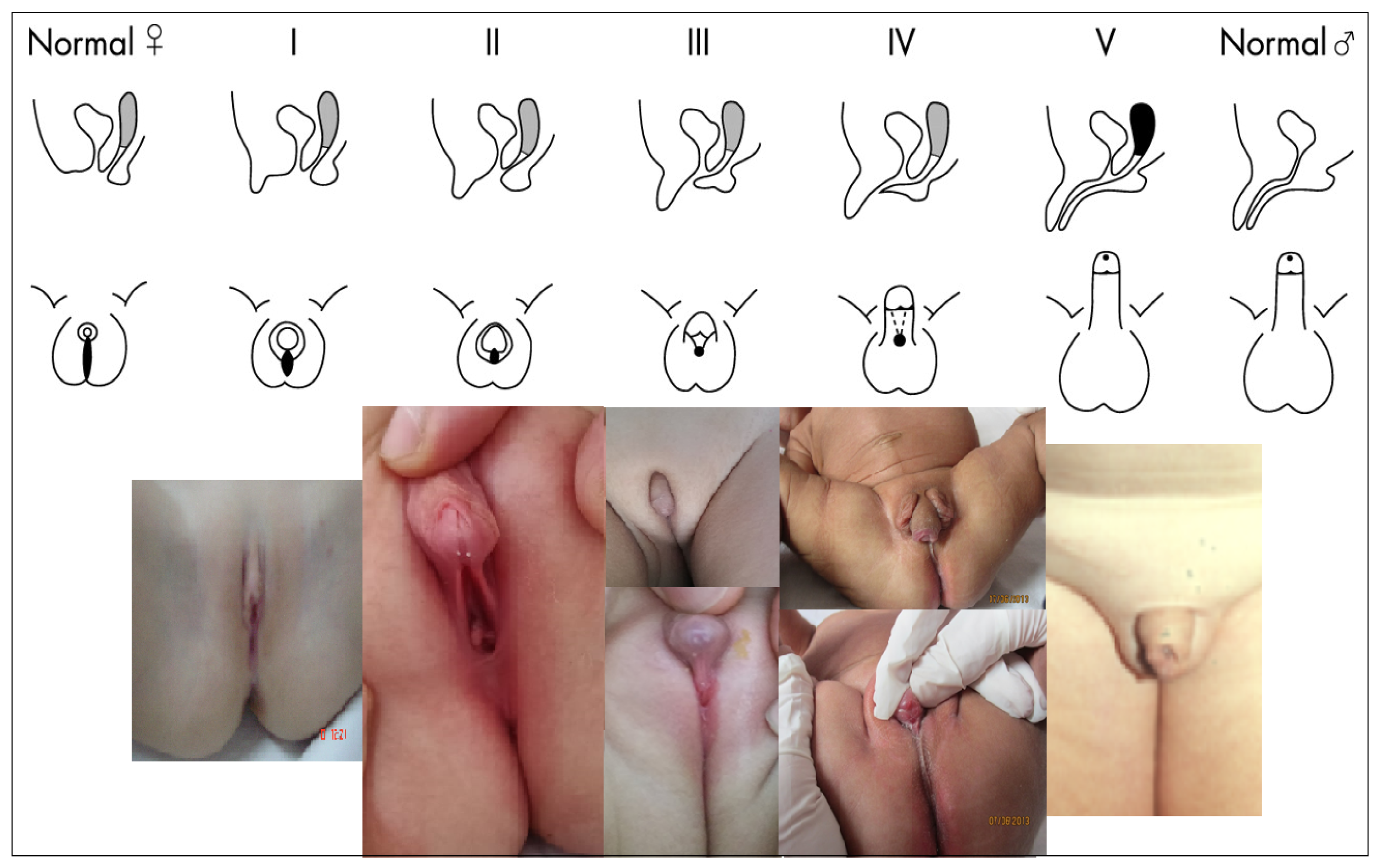
3.3. External Genital Organs
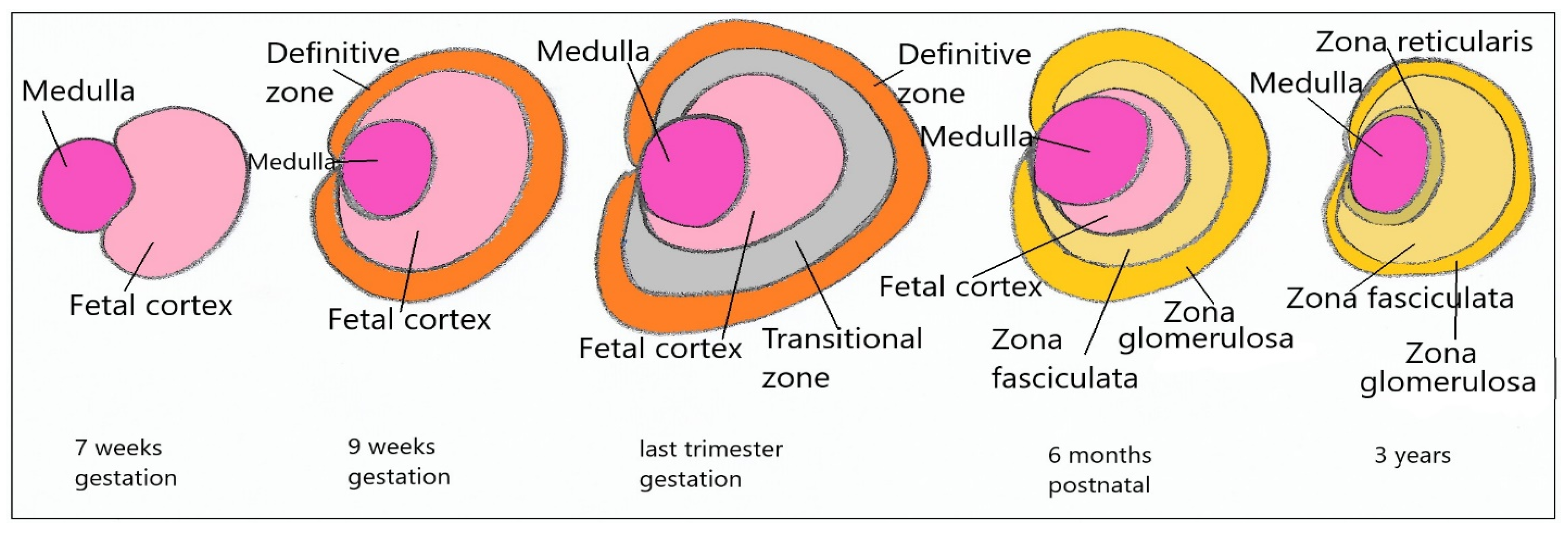
4. Adrenal Development
Differences in Sex Development in 46,XX
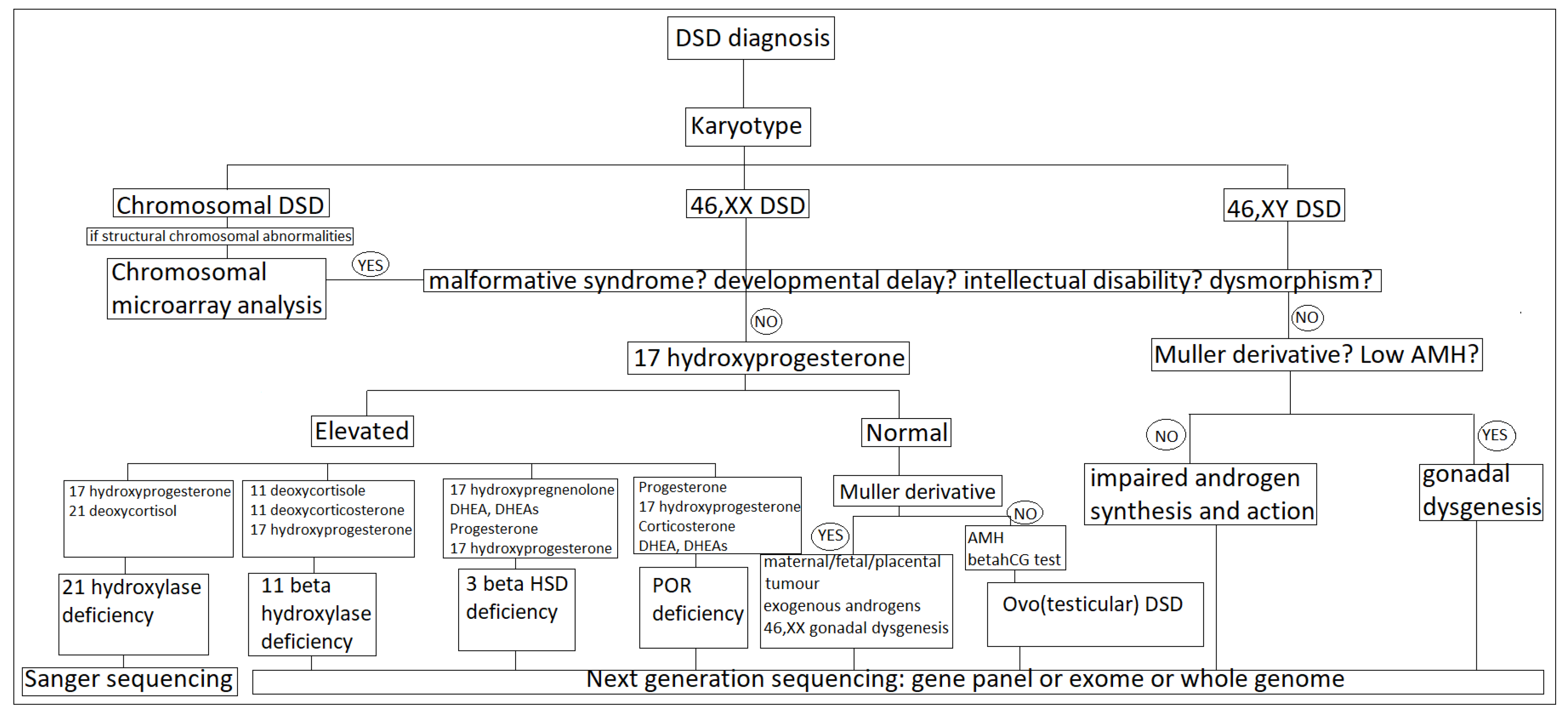
5. Clinical Assessment
6. Hormonal Assessment
7. Anatomic Assessment
8. Genetic Assessment
9. 21 Hydroxylase Deficiency
9.1. Frequency
9.2. Etiopathogenesis
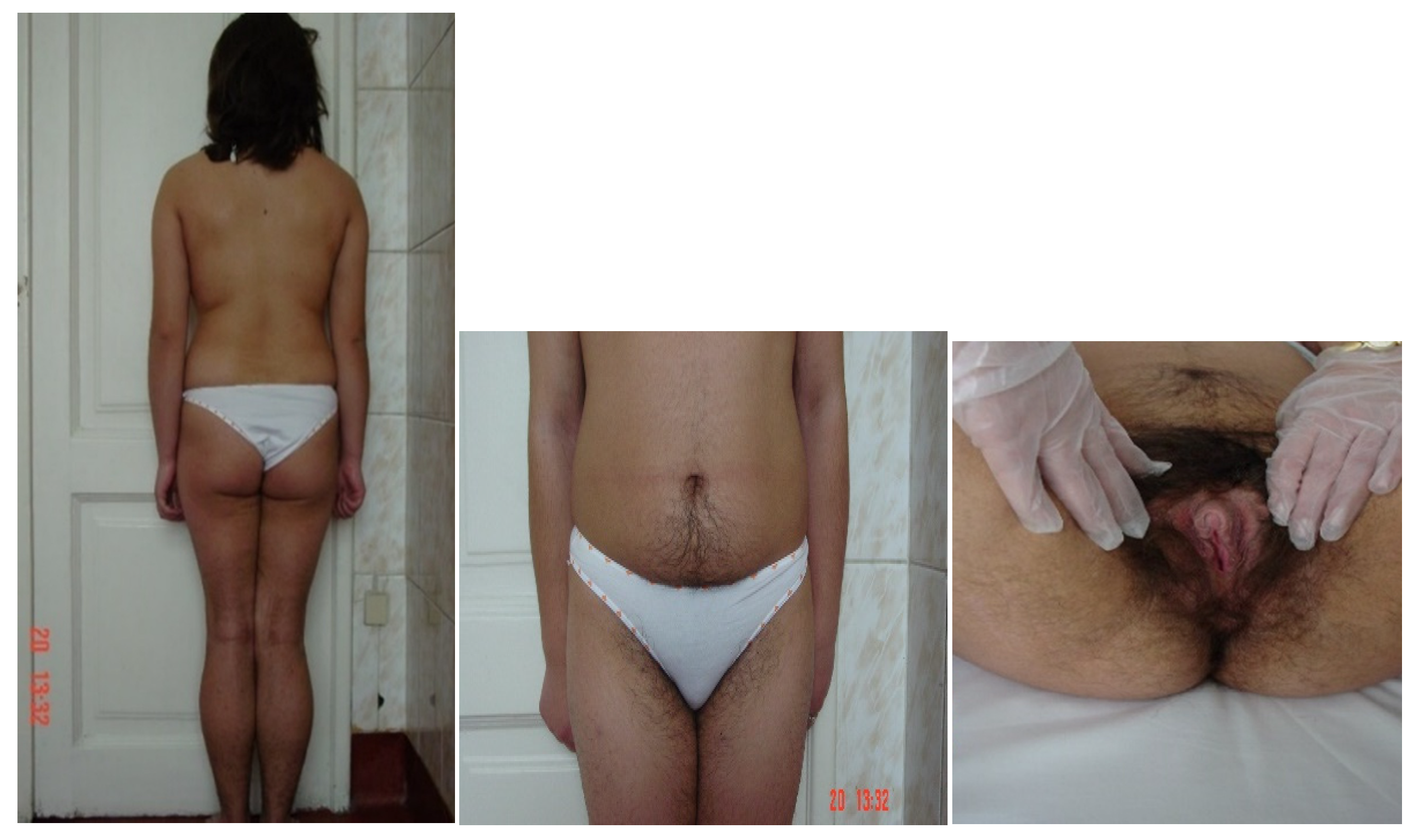
9.3. Clinical Picture
9.4. Treatment
9.5. Prenatal Diagnostic and Treatment
9.6. Neonatal Screening
10. 11 Beta Hydroxylase Deficiency
10.1. Frequency
10.2. Etiopathogenesis
10.3. Clinical Picture
10.4. Treatment
11. 3-βHSD Type 2 Deficiency
11.1. Etiopathogenesis
11.2. Clinical Picture
12. POR Deficiency
12.1. Etiopathogenesis
12.2. Clinical Picture
13. Glucocorticoid Receptor Deficiency
14. Maternal Androgens Excess
15. Pregnancy Luteoma
16. Aromatase Deficiency
16.1. Etiopathogenese
16.2. Clinical Picture
17. 46,XX DSD by Gonadal Differentiation Abnormalities
17.1. Testicular DSD
17.2. Ovotesticular DSD
17.3. 46,XX Gonadal Dysgenesis
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society* clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef]
- Auchus, R.J.; Chang, A.Y. 46,XX DSD: The masculinised female. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 219–242. [Google Scholar] [CrossRef]
- Rey, R.A.; Houk, C.P.; Witchel, S.; Lee, P.A. Disorders of Sex Development. In Greenspan’s Basic & Clinical Endocrinology, 10th ed.; Gardner, D.G., Shoback, D., Eds.; McGraw-Hill Medical: New York, NY, USA, 2018; pp. 501–547. [Google Scholar]
- Kamrath, C.; Wudy, S.A.; Krone, N. Steroid biochemistry. Endocr. Dev. 2014, 27, 41–52. [Google Scholar]
- Krone, N.; Dhir, V.; Ivison, H.E.; Arlt, W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin. Endocrinol. 2007, 66, 162–172. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, T.B.; Aron, D.C.; Findling, J.W.; Tyrrell, B. Glucocorticoids and Adrenal Androgens. In Greenspan’s Basic & Clinical Endocrinology, 10th ed.; Gardner, D.G., Shoback, D., Eds.; McGraw-Hill Medical: New York, NY, USA, 2018; pp. 299–342. [Google Scholar]
- Auchus, R.J. The backdoor pathway to dihydrotestosterone. Trends Endocrinol. Metab. 2004, 15, 432–438. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The “backdoor pathway” of androgen synthesis in human male sexual development. PLoS Biol. 2019, 17, e3000198. [Google Scholar] [CrossRef] [Green Version]
- Kamrath, C.; Hochberg, Z.; Hartmann, M.F.; Remer, T.; Wudy, S.A. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: Evidence from urinary steroid hormone analysis. J. Clin. Endocrinol. Metab. 2012, 97, E367–E375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arlt, W.; Walker, E.A.; Draper, N.; Ivison, H.E.; Ride, J.P.; Hammer, F.; Chalder, S.M.; Borucka-Mankiewicz, M.; Hauffa, B.P.; Malunowicz, E.M.; et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: Analytical study. Lancet 2004, 363, 2128–2135. [Google Scholar] [CrossRef]
- Rey, R.A.; Grinspon, R.P. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 221–238. [Google Scholar] [CrossRef]
- Rey, R.; Josso, N.; Racine, C. Sexual Differentiation. In Endotext -NCBI Bookshelf; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J., Hofland, H., Kalra, S., et al., Eds.; MDText.com, Inc: South Dartmouth, MA, USA, 2020; pp. 2000–2027. [Google Scholar]
- Miller, L.W.; Flück, C.E.; Breault, D.T.; Feldman, B.J. The Adrenal Cortex and Its Disorders. In Sperling Pediatric Endocrinology, 5th ed.; Sperling, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 425–490. [Google Scholar]
- Lucas-Herald, A.K.; Bashamboo, A. Gonadal development. Endocr. Dev. 2014, 27, 1–16. [Google Scholar]
- Rotgers, E.; Jørgensen, A.; Yao, H.H.C. At the crossroads of fate-Somatic cell lineage specification in the fetal gonad. Endocr. Rev. 2018, 39, 739–759. [Google Scholar] [CrossRef]
- Stévant, I.; Nef, S. Genetic Control of Gonadal Sex Determination and Development. Trends Genet. 2019, 35, 346–358. [Google Scholar] [CrossRef]
- Nef, S.; Stévant, I.; Greenfield, A. Characterizing the bipotential mammalian gonad. Curr. Top. Dev. Biol. 2019, 134, 167–194. [Google Scholar]
- Lin, Y.T.; Capel, B. Cell fate commitment during mammalian sex determination. Curr. Opin. Genet. Dev. 2015, 32, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungewitter, E.K.; Yao, H.H.C. How to make a gonad: Cellular mechanisms governing formation of the testes and ovaries. Sex. Dev. 2013, 7, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Jost, A. A new look at the mechanism controlling sex differentiation in mammals. Johns Hopkins Med. J. 1972, 130, 28–36. [Google Scholar]
- Guigon, C.J.; Cohen-Tannoudji, M. Reconsidering the roles of female germ cells in ovarian development and folliculogenesis. Biol. Aujourdhui 2011, 205, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, K.; Cortvrindt, R.; Verlinde, F.; De Schepper, J.; Bourgain, C.; Smitz, J. Number of ovarian follicles in human fetuses with the 45,X karyotype. Fertil. Steril. 2004, 81, 1112–1119. [Google Scholar] [CrossRef]
- Chassot, A.A.; Ranc, F.; Gregoire, E.P.; Roepers-Gajadien, H.L.; Taketo, M.M.; Camerino, G.; de Rooij, D.G.; Schedl, A.; Chaboissier, M.C. Activation of β-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum. Mol. Genet. 2008, 17, 1264–1277. [Google Scholar] [CrossRef] [Green Version]
- Lindeman, R.E.; Gearhart, M.D.; Minkina, A.; Krentz, A.D.; Bardwell, V.J.; Zarkower, D. Sexual cell-fate reprogramming in the ovary by DMRT1. Curr. Biol. 2015, 25, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Ye, L.; Chen, H. Sex determination and maintenance: The role of DMRT1 and FOXL2. Asian J. Androl. 2017, 19, 619–624. [Google Scholar] [PubMed]
- Loke, J.; Pearlman, A.; Radi, O.; Zuffardi, O.; Giussani, U.; Pallotta, R.; Camerino, G.; Ostrer, H. Mutations in MAP3K1 tilt the balance from SOX9/FGF9 to WNT/β-catenin signaling. Hum. Mol. Genet. 2014, 23, 1073–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCabe, E.R.B. DAX1: Increasing complexity in the roles of this novel nuclear receptor. Mol. Cell. Endocrinol. 2007, 265–266, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Rudigier, L.J.; Dame, C.; Scholz, H.; Kirschner, K.M. Ex vivo cultures combined with vivomorpholino induced gene knockdown provide a system to assess the role of WT1 and GATA4 during gonad differentiation. PLoS ONE 2017, 12, e0176296. [Google Scholar]
- Welsh, M.; Suzuki, H.; Yamada, G. The masculinization programming window. Endocr Dev. 2014, 27, 17–27. [Google Scholar] [PubMed]
- Jost, A.; Vigier, B.; Prepin, J.; Perchellet, J.P. Studies on sex differentiation in mammals. Recent Prog. Horm. Res. 1973, 29, 1–41. [Google Scholar] [PubMed]
- Siiteri, P.K.; Wilson, J.D. Testosterone formation and metabolism during male sexual diflferentiation in the human embryo. J. Clin. Endocrinol. Metab. 1974, 38, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.; Saunders, P.T.K.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Smith, L.B.; Sharpe, R.M. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Invest. 2008, 118, 1479–1490. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, M.L.; Shy, M.; Chanc, W.R.; Lipshultz, L.I. The relationship between anogenital distance and azoospermia in adult men. Int. J. Androl. 2012, 35, 726–730. [Google Scholar] [CrossRef]
- Lee, P.A.; Houk, C.P. Outcome studies among men with micropenis. J. Pediatr. Endocrinol. Metab. 2004, 17, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Blaschko, S.D.; Cunha, G.R.; Baskin, L.S. Molecular mechanisms of external genitalia development. Differentiation 2012, 84, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Xu, X.; Huo, X. Anogenital distance and its application in environmental health research. Environ. Sci. Pollut. Res. Int. 2014, 21, 5457–5464. [Google Scholar] [CrossRef]
- Adrenal Gland Embryology, Anatomy, and Physiology | Oncohema Key. Available online: https://oncohemakey.com/adrenal-gland-embryology-anatomy-and-physiology/ (accessed on 24 May 2021).
- Willenberg, H.S.; Bornstein, S.R. Adrenal Cortex; Development, Anatomy, Physiology. Endotext. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278945/ (accessed on 25 May 2021).
- Hughes, I.A.; Houk, C.; Ahmed, S.F.; Lee, P.A. Consensus statement on management of intersex disorders. Arch. Dis. Child. 2006, 91, 554–563. [Google Scholar] [CrossRef]
- Cools, M.; Nordenström, A.; Robeva, R.; Hall, J.; Westerveld, P.; Flück, C.; Köhler, B.; Berra, M.; Springer, A.; Schweizer, K.; et al. Caring for individuals with a difference of sex development (DSD): A Consensus Statement. Nat. Rev. Endocrinol. 2018, 14, 415–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouvattier, C. Disorders of Sex Development: Endocrine Aspects. In Pediatric Urology; Gearhart, J.P., Rink, R.C., Mouriquand, P.D.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 459–475. [Google Scholar]
- Ahmed, S.F.; Achermann, J.C.; Arlt, W.; Balen, A.H.; Conway, G.; Edwards, Z.L.; Elford, S.; Hughes, I.A.; Izatt, L.; Krone, N.; et al. UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development. Clin. Endocrinol. 2011, 75, 12–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makiyan, Z. Systematization of ambiguous genitalia. Organogenesis 2016, 12, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Ogilvy-Stuart, A.L.; Brain, C.E. Early assessment of ambiguous genitalia. Arch. Dis. Child. 2004, 89, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Honour, J.W. 17-Hydroxyprogesterone in children, adolescents and adults. Ann. Clin. Biochem. 2014, 51 Pt 4, 424–440. [Google Scholar] [CrossRef]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H.L. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Rodie, M. Investigation and initial management of ambiguous genitalia. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 197–218. [Google Scholar] [CrossRef]
- Mansour, S.M.; Hamed, S.T.; Adel, L.; Kamal, R.M.; Ahmed, D.M. Does MRI add to ultrasound in the assessment of disorders of sex development? Eur. J. Radiol. 2012, 81, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, G.B.; Parra, D.A.; Oudjhane, K.; Miller, S.F.; Babyn, P.S.; Salle, J.L.P. Imaging of ambiguous genitalia: Classification and diagnostic approach. Radiographics 2008, 28, 1891–1904. [Google Scholar] [CrossRef]
- Guerrero-Fernández, J.; Azcona San Julián, C.; Barreiro Conde, J.; Bermúdez de la Vega, J.A.; Carcavilla Urquí, A.; Castaño González, L.A.; Tello, J.M.M.; Estévez, A.R.; Fernández, D.Y.; Martínez, L.M.; et al. Management guidelines for disorders/different sex development (DSD). An. Pediatr. 2018, 89, e1–e315. [Google Scholar] [CrossRef]
- Croft, B.; Ohnesorg, T.; Sinclair, A.H. The Role of Copy Number Variants in Disorders of Sex Development. Sex. Dev. 2018, 12, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Therrell, B.L.; Berenbaum, S.A.; Manter-Kapanke, V.; Simmank, J.; Korman, K.; Prentice, L.; Gonzalez, J.; Gunn, S. Results of screening 1.9 million Texas newborns for 21-hydroxylase- deficient congenital adrenal hyperplasia. Pediatrics 1998, 101, 583–590. [Google Scholar] [CrossRef]
- Krone, N.; Braun, A.; Weinert, S.; Peter, M.; Roscher, A.A.; Partsch, C.J.; Sippell, W.G. Multiplex minisequencing of the 21-hydroxylase gene as a rapid strategy to confirm congenital adrenal hyperplasia. Clin. Chem. 2002, 48, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu, A.P.; Dauber, A.; Macedo, D.B.; Noel, S.D.; Brito, V.N.; Gill, J.C.; Cukier, P.; Thompson, I.R.; Navarro, V.M.; Gagliardi, P.C.; et al. Central Precocious Puberty Caused by Mutations in the Imprinted Gene MKRN3. N. Engl. J. Med. 2013, 368, 2467–2475. [Google Scholar] [CrossRef] [Green Version]
- Nordenström, A.; Falhammar, H. Management of endocrine disease: Diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur. J. Endocrinol. 2019, 180, R127–R145. [Google Scholar] [CrossRef]
- Hannah-Shmouni, F.; Morissette, R.; Sinaii, N.; Elman, M.; Prezant, T.R.; Chen, W.; Pulver, A.; Merke, D.P. Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians. Genet. Med. 2017, 19, 1276–1279. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.L. Congenital Adrenal Hyperplasia: Time to Replace 17OHP with 21-Deoxycortisol. Horm. Res. Paediatr. 2019, 91, 416–420. [Google Scholar] [CrossRef]
- Verma, S.; Vanryzin, C.; Sinaii, N.; Kim, M.S.; Nieman, L.K.; Ravindran, S.; Calis, K.A.; Arlt, W.; Ross, R.J.; Merke, D.P. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin. Endocrinol. 2010, 72, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Mooij, C.F.; Parajes, S.; Pijnenburg-Kleizen, K.J.; Arlt, W.; Krone, N.; Van Der Grinten, H.L.C. Influence of 17-Hydroxyprogesterone, Progesterone and Sex Steroids on Mineralocorticoid Receptor Transactivation in Congenital Adrenal Hyperplasia. Horm. Res. Paediatr. 2015. [Google Scholar] [CrossRef]
- Almasri, J.; Zaiem, F.; Rodriguez-Gutierrez, R.; Tamhane, S.U.; Iqbal, A.M.; Prokop, L.J.; Speiser, P.W.; Baskin, L.S.; Bancos, I.; Murad, M.H. Genital Reconstructive Surgery in Females With Congenital Adrenal Hyperplasia: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2018, 103, 4089–4096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röhle, R.; Gehrmann, K.; Szarras-Czapnik, M.; Claahsen-van der Grinten, H.; Pienkowski, C.; Bouvattier, C.; Cohen-Kettenis, P.; Nordenström, A.; Thyen, U.; Köhler, B.; et al. Participation of adults with disorders/differences of sex development (DSD) in the clinical study dsd-LIFE: Design, methodology, recruitment, data quality and study population. BMC Endocr. Disord. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Mueller-Godeffroy, E.; Lee, P.; Roehle, R.; Kreukels, B.P.C.; Köhler, B.; Nordenström, A.; Bouvattier, C.; Thyen, U.; dsd-LIFE group. Multicentre cross-sectional clinical evaluation study about quality of life in adults with disorders/differences of sex development (DSD) compared to country specific reference populations (dsd-LIFE). Health Qual Life Outcomes 2018, 16, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreukels, B.P.C.; Köhler, B.; Nordenström, A.; Roehle, R.; Thyen, U.; Bouvattier, C.; de Vries, A.L.C.; Cohen-Kettenis, P.T.; dsd-LIFE group. Gender Dysphoria and Gender Change in Disorders of Sex Development/Intersex Conditions: Results from the dsd-LIFE Study. J. Sex. Med. 2018, 15, 777–785. [Google Scholar] [CrossRef]
- Kreukels, B.P.C.; Cohen-Kettenis, P.T.; Roehle, R.; van de Grift, T.C.; Slowikowska-Hilczer, J.; Claahsen-van der Grinten, H.; Hirschberg, A.L.; de Vries, A.L.C.; Reisch, N.; Bouvattier, C.; et al. Sexuality in Adults with Differences/Disorders of Sex Development (DSD): Findings from the dsd-LIFE Study. J. Sex. Marital Ther. 2019, 45, 688–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speiser, P.W.; White, P.C. Congenital Adrenal Hyperplasia. N. Engl. J. Med. 2003, 349, 776–788. [Google Scholar] [CrossRef] [Green Version]
- Khattab, A.; Haider, S.; Kumar, A.; Dhawan, S.; Alam, D.; Romero, R.; Burns, J.; Li, D.; Estatico, J.; Rahi, S.; et al. Clinical, genetic, and structural basis of congenital adrenal hyperplasia due to 11β-hydroxylase deficiency. Proc. Natl. Acad. Sci. USA 2017, 114, E1933–E1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menabò, S.; Polat, S.; Baldazzi, L.; Kulle, A.E.; Holterhus, P.M.; Grötzinger, J.; Fanelli, F.; Balsamo, A.; Riepe, F.G. Congenital adrenal hyperplasia due to 11-beta-hydroxylase deficiency: Functional consequences of four CYP11B1 mutations. Eur. J. Hum. Genet. 2014, 22, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Al Alawi, A.M.; Nordenström, A.; Falhammar, H. Clinical perspectives in congenital adrenal hyperplasia due to 3β-hydroxysteroid dehydrogenase type 2 deficiency. Endocrine 2019, 63, 407–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idkowiak, J.; Cragun, D.; Hopkin, R.J.; Arlt, W. Cytochrome P450 Oxidoreductase Deficiency. GeneReviews. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20301592 (accessed on 25 May 2021).
- Fukami, M.; Ogata, T. Cytochrome P450 oxidoreductase deficiency: Rare congenital disorder leading to skeletal malformations and steroidogenic defects. Pediatr. Int. 2014, 56, 805–808. [Google Scholar] [CrossRef]
- Reisch, N.; Idkowiak, J.; Hughes, B.A.; Ivison, H.E.; Abdul-Rahman, O.A.; Hendon, L.G.; Olney, A.H.; Nielsen, S.; Harrison, R.; Blair, E.M.; et al. Prenatal diagnosis of congenital adrenal hyperplasia caused by P450 oxidoreductase deficiency. J. Clin. Endocrinol. Metab. 2013, 98, E528–E536. [Google Scholar] [CrossRef] [PubMed]
- Masarie, K.; Katz, V.; Balderston, K. Pregnancy luteomas: Clinical presentations and management strategies. Obstet. Gynecol. Surv. 2010, 65, 575–582. [Google Scholar] [CrossRef]
- Délot, E.C.; Vilain, E.J. Nonsyndromic 46,XX Testicular Disorders of Sex Development. GeneReviews. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20301589 (accessed on 25 May 2021).
- McElreavey, K.; Achermann, J.C. Steroidogenic Factor-1 (SF-1, NR5A1) and 46,XX Ovotesticular Disorders of Sex Development: One Factor, Many Phenotype. Horm. Res. Paediatr. 2017, 87, 189–190. [Google Scholar] [CrossRef] [Green Version]
- Eozenou, C.; Gonen, N.; Touzon, M.S.; Jorgensen, A.; Yatsenko, S.A.; Fusee, L.; Kamel, A.K.; Gellen, B.; Guercio, G.; Singh, P.; et al. Testis formation in XX individuals resulting from novel pathogenic variants in Wilms’ tumor 1 (WT1) gene. Proc. Natl. Acad. Sci. USA 2020, 117, 13680–13688. [Google Scholar] [CrossRef] [PubMed]
- Bramble, M.S.; Goldstein, E.H.; Lipson, A.; Ngun, T.; Eskin, A.; Gosschalk, J.E.; Roach, L.; Vashist, N.; Barseghyan, H.; Lee, E.; et al. A novel follicle-stimulating hormone receptor mutation causing primary ovarian failure: A fertility application of whole exome sequencing. Hum. Reprod. 2016, 31, 905–914. [Google Scholar] [CrossRef]
- Breehl, L.; Caban, O. Genetics, Gonadal Dysgenesis. StatPearls. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30969708 (accessed on 25 May 2021).
- França, M.M.; Mendonca, B.B. Genetics of primary ovarian insufficiency in the next-generation sequencing era. J. Endocr. Soc. 2019, 4, bvz037. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Disorder | Etiology | Serum/Plasma Steroids | Urinary Steroids |
|---|---|---|---|
| 21-hydroxylase deficiency | CYP21A2 | ↑17-hydroxyprogesterone ↑21-desoxycorticosterol ↑DHEAs, ↑androstenedione, ↑testosterone ↓aldosterone, ↑ plasma renin activity, ↓deoxycorticosterone ↓11-deoxycortisol, ↓corticosterone, ↓cortisol | ↑pregnanetriol (17-hydroxyprogesterone metabolite) ↑pregnanetriolone (21-desoxycorticosterol metabolite) ↑pregnanetriolone/tetrahydrocompoundF (cortisol metabolite) ↑17-ketosteroid/24 h |
| 11β-hydroxylase deficiency | CYP11B1 | ↑11-Deoxycorticosterol ↑11-Deoxycorticosterone ↓plasma renin activity ↑androsteondione ↑testosterone N/↑17-hydroxyprogesterone | ↑tetrahydrocompoundS (11-deoxycortisol metabolite) ↓tetrahydrocompoundF ↑tetrahydrocompoundS/tetrahydrocompoundF ↑17-ketosteroid/24 h |
| 3-βHSD deficiency | HSD3B2 | ↑Δ5-steroids-17-hydroxypregnenolone,DHEA, DHEAs ↑17-hydroxypregnenolone/17-hydroxyprogesterone ↑DHEA/androstendione ↑17-hydroxyprogesterone (synthesised from 17-hydroxypregnenolone via HSD3B1) | ↑5-pregnenetriol (17-hydroxypregnenolone metabolite) ↑androstenetriol (DHEA metabolite) ↑5-pregnenetriol/tetrahydrocompound F ↑androstenetriol/tetrahydrocompound F ↑pregnanetriol, 17α-hydroxypregnanolone (17-hydroxyprogesterone metabolites) |
| POR deficiency | POR | ↑progesterone ↑17hydroxyprogesterone ↑21desoxycorticosterol ↑corticosterone, ↑deoxycorticosterone ↓DHEA ↓DHEAs | ↑pregnanetriol, 17α-hydroxypregnanolone ↑pregnanetriolone ↑pregnanediol (pregnenolone, progesterone, deoxycorticosterone metabolites) ↓androsterone, ↓etiocholanolone (DHEA, androstendione, testosterone metabolites) |
| Aromatase deficiency | CYP19A1 | ↓estriol, estrone, estradiol ↑androstendione,testosterone,DHT | - |
| Ovotesticular or testicular 46,XX DSD | SRY, SOX9, SOX3, SOX10 NR5A1, RSPO1, WNT4 | ↑AMH ↑testosterone | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhzouz, C.; Bucerzan, S.; Miclaus, M.; Mirea, A.-M.; Miclea, D. 46,XX DSD: Developmental, Clinical and Genetic Aspects. Diagnostics 2021, 11, 1379. https://doi.org/10.3390/diagnostics11081379
Alkhzouz C, Bucerzan S, Miclaus M, Mirea A-M, Miclea D. 46,XX DSD: Developmental, Clinical and Genetic Aspects. Diagnostics. 2021; 11(8):1379. https://doi.org/10.3390/diagnostics11081379
Chicago/Turabian StyleAlkhzouz, Camelia, Simona Bucerzan, Maria Miclaus, Andreea-Manuela Mirea, and Diana Miclea. 2021. "46,XX DSD: Developmental, Clinical and Genetic Aspects" Diagnostics 11, no. 8: 1379. https://doi.org/10.3390/diagnostics11081379
APA StyleAlkhzouz, C., Bucerzan, S., Miclaus, M., Mirea, A.-M., & Miclea, D. (2021). 46,XX DSD: Developmental, Clinical and Genetic Aspects. Diagnostics, 11(8), 1379. https://doi.org/10.3390/diagnostics11081379






