Liquid Biopsy: A Family of Possible Diagnostic Tools
Abstract
:1. Introduction
2. Components of Biological Fluids
3. CTCs
4. ctDNA
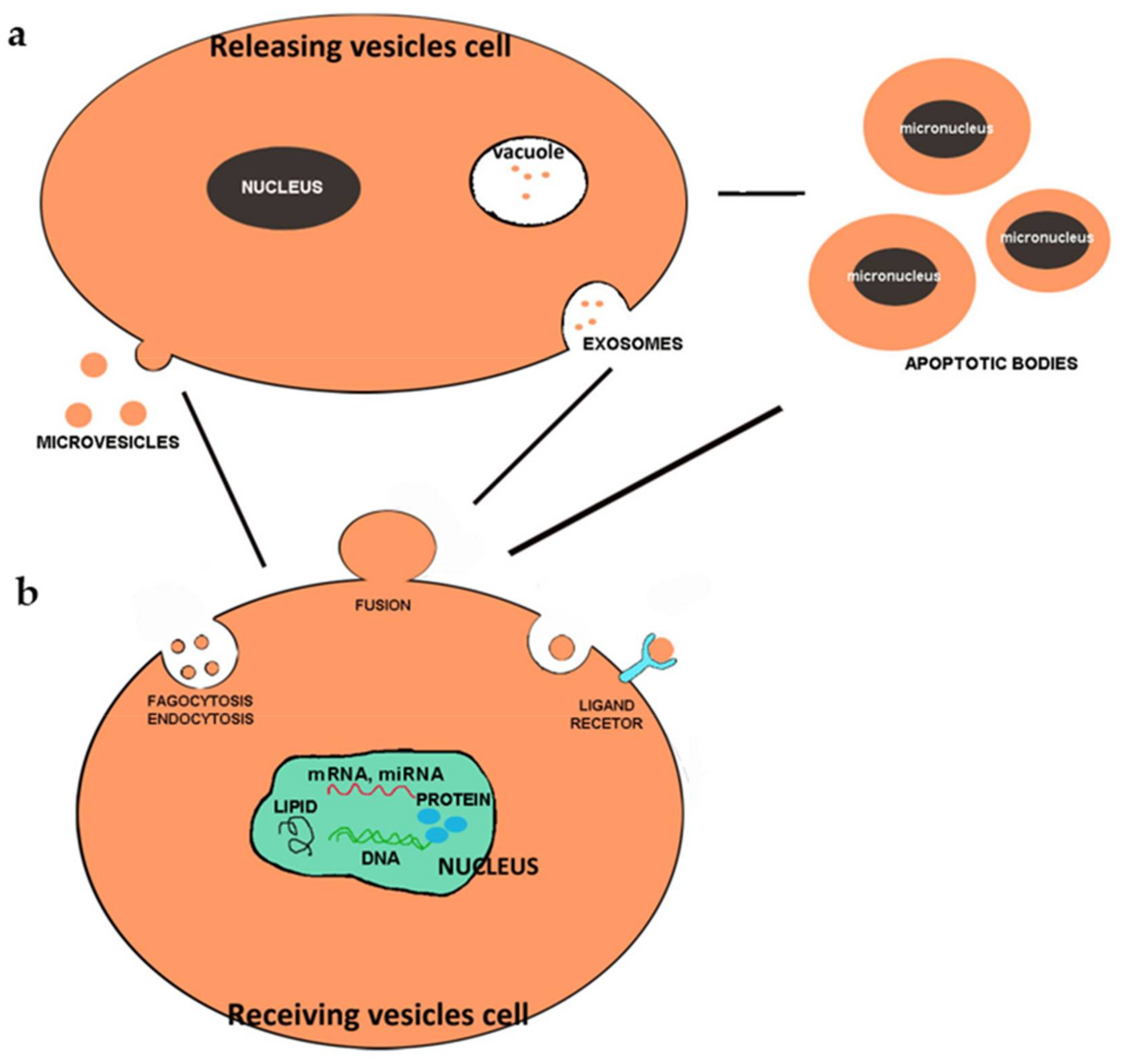
5. Extracellular Vesicles
6. Liquid Biopsies
7. Blood
8. Urine Fluid
9. Saliva
10. Breast Milk
11. Synovial Fluid
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Mader, S.; Pantel, K. Liquid Biopsy: Current Status and Future Perspectives. Oncol. Res. Treat. 2017, 40, 404–408. [Google Scholar] [CrossRef]
- Kilgour, E.; Rothwell, D.G.; Brady, G.; Dive, C. Liquid Biopsy-Based Biomarkers of Treatment Response and Resistance. Cancer Cell 2020, 37, 485–495. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Xue, V.W.; Wong, C.S.C.; Cho, W.C.S. Early detection and monitoring of cancer in liquid biopsy: Advances and challenges. Expert Rev. Mol. Diagn 2019, 19, 273–276. [Google Scholar] [CrossRef] [Green Version]
- Nazarenko, I. Extracellular Vesicles: Recent Developments in Technology and Perspectives for Cancer Liquid Biopsy. Recent Results Cancer Res. 2020, 215, 319–344. [Google Scholar]
- Ozawa, P.M.M.; Jucoski, T.S.; Vieira, E.; Carvalho, T.M.; Malheiros, D.; Ribeiro, E.M.S.F. Liquid biopsy for breast cancer using extracellular vesicles and cell-free microRNAs as biomarkers. Transl. Res. 2020, 223, 40–60. [Google Scholar] [CrossRef]
- Roy, D.; Pascher, A.; Juratli, M.A.; Sporn, J.C. The Potential of Aptamer-Mediated Liquid Biopsy for Early Detection of Cancer. Int. J. Mol. Sci. 2021, 22, 5601. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Taggart, D.; Zheng, L.; Liu, D.; Li, G.; Li, M.; Zhang, K.; Etten, R.A.V. Abstract 837: Circulating cell-free DNA methylation assay: Towards early detection of multiple cancer types. Cancer Res. 2019, 79, 837. [Google Scholar]
- Fabisiewicz, A.; Grzybowska, E. CTC clusters in cancer progression and metastasis. Med. Oncol. 2017, 34, 12. [Google Scholar] [CrossRef]
- Geethadevi, A.; Parashar, D.; Bishop, E.; Pradeep, S.; Chaluvally-Raghavan, P. ERBB signaling in CTCs of ovarian cancer and glioblastoma. Genes Cancer 2017, 8, 746–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ju, S.; Wang, X.; Cong, H. Advances in liquid biopsy using circulating tumor cells and circulating cell-free tumor DNA for detection and monitoring of breast cancer. Clin. Exp. Med. 2019, 19, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Riegelbauer, L.J.; Oppermann, E.; Kostantin, M.; Ackermann, H.; Trzmiel, A.; Stein, S.; Eichler, K.; Zharov, V.P.; Roy, D.; et al. Early dynamic changes in circulating tumor cells and prognostic relevance following interventional radiological treatments in patients with hepatocellular carcinoma. PLoS ONE 2021, 16, e0246527. [Google Scholar] [CrossRef]
- Roy, D.; Tiirikainen, M. Diagnostic Power of DNA Methylation Classifiers for Early Detection of Cancer. Trends Cancer 2020, 6, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Gangoda, L.; Boukouris, S.; Liem, M.; Kalra, H.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics 2015, 15, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Fraser, K.; Ghaddar, B.; Yang, K.; Kim, E.; Balaj, L.; Chiocca, E.A.; Breakefield, X.O.; Lee, H.; Weissleder, R. Multiplexed Profiling of Single Extracellular Vesicles. ACS Nano 2018, 12, 494–503. [Google Scholar] [CrossRef]
- Maas, S.L.; Breakefiel, X.O.; Weaver, A.M. Extracellular vesicles: Unique intercellular delivery vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Nieuwland, R.; Falcon-Perez, J.M.; Soekmadji, C.; Boilard, E.; Carter, D.; Buzas, E.I. Essentials of extracellular vesicles: Posters on basic and clinical aspects of extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1548234. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Hauser, P.; Wang, S.; Didenko, V.V. Apoptotic Bodies: Selective Detection in Extracellular Vesicles. Methods Mol. Biol. 2017, 1554, 193–200. [Google Scholar]
- Ihara, T.; Yamamoto, T.; Sugamata, M.; Okumura, H.; Ueno, Y. The process of ultrastructural changes from nuclei to apoptotic body. Virchows Arch. 1998, 433, 443–447. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More than Just Debri. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communicatio. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Xu, T.; Wang, S.; Chang, H.; Yu, T.; Zhu, Y.; Chen, J. Liquid Biopsy Applications in the Clinic. Mol. Diagn. Ther. 2020, 1–8. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Macías, M.; Alegre, E.; Díaz-Lagares, A.; Patiño, A.; Pérez-Gracia, J.L.; Sanmamed, M.; López-López, R.; Varo, N.; González, A. Liquid Biopsy: From Basic Research to Clinical Practice. Adv. Clin. Chem. 2018, 83, 73–119. [Google Scholar] [PubMed]
- Junqueira-Neto, S.; Batista, I.A.; Costa, J.L.; Melo, S.A. Liquid Biopsy beyond Circulating. Tumor Cells and Cell-Free DNA. Acta Cytol. 2019, 63, 479–488. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; García Hernández, J.L.; García, A.C.; Córdova Martínez, A.; Mielgo-Ayuso, J.; Cruz-Hernández, J.J. Liquid Biopsy as Novel Tool in Precision Medicine: Origins, Properties, identification and clinical perspective of cancer’s biomarkers. Diagnostics 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenemo, M.; Teleman, J.; Sjöström, M.; Grubb, G.; Malmström, E.; Malmström, J.; Niméus, E. Cancer associated proteins in blood plasma: Determining normal variation. Proteomics 2016, 16, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Gabriel, M.; Heymann, M.F.; Heymann, D. Circulating Tumor Cells as a Tool for Assessing Tumor Heterogeneity. Theranostics 2019, 9, 4580–4594. [Google Scholar] [CrossRef]
- Tong, B.M. Circulating tumor cells in patients with lung cancer: Developments and applications for precision medicine. Future Oncol. 2019, 15, 2531–2542. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early detection of cancer: Past, present, and future. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhawal, R.; Oberg, A.L.; Zhang, S.; Kohli, M. Challenges and Opportunities in Clinical Applications of Blood-Based Proteomics in Cancer. Cancers 2020, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Craig, A.F.M.; Borchmann, S.; Kurtz, D.M. Liquid biopsy in lymphoma: Molecular methods and clinical applications. Cancer Treat. Rev. 2020, 91, 102106. [Google Scholar] [CrossRef]
- Lousada-Fernandez, F.; Rapado-Gonzalez, O.; Lopez-Cedrun, J.L.; Lopez-Lopez, R.; Muinelo-Romay, L.; Suarez-Cunqueiro, M.M. Liquid Biopsy in Oral Cancer. Int. J. Mol. Sci. 2018, 19, 1704. [Google Scholar] [CrossRef] [Green Version]
- Johann, D.J., Jr.; Steliga, M.; Shin, I.J.; Yoon, D.; Arnaoutakis, K.; Hutchins, L.; Liu, M.; Liem, J.; Walker, K.; Pereira, A.; et al. Liquid biopsy and its role in an advanced clinical trial for lung cancer. Exp. Biol. Med. 2018, 243, 262–271. [Google Scholar] [CrossRef]
- Bidard, F.C.; Ferrand, F.R.; Huguet, F.; Hammel, P.; Louvet, C.; Malka, D. Disseminated and circulating tumor cells in gastrointestinal oncology. Crit. Rev. Oncol. Hematol. 2012, 82, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Gorges, T.M.; Stein, A.; Quidde, J.; Hauch, S.; Röck, K.; Riethdorf, S.; Joosse, S.A.; Pantel, K. Improved detection of circulating tumor cells in metastatic colorectal cancer by the combination of the cell Search(R) System and the AdnaTest(R). PLoS ONE 2016, 11, e0155126. [Google Scholar] [CrossRef]
- Crowley, E.; di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2003, 10, 472–484. [Google Scholar] [CrossRef]
- Giannopoulou, L.; Zavridou, M.; Kasimir-Bauer, S.; Lianidou, E.S. Liquid biopsy in ovarian cancer: The potential of circulating miRNAs and exosomes. Transl. Res. 2019, 205, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Puche-Sanz, I.; Rodríguez-Martínez, A.; Garrido-Navas, M.C.; Robles-Fernández, I.; Vázquez-Alonso, F.; Álvarez Cubero, M.J.; Lorente-Acosta, J.A.; Serrano-Fernández, M.J.; Cózar-Olmo, J.M. Liquid biopsy and prostate cancer. Current evidence applied to clinical practice. Actas Urol. Esp. Engl. Ed. 2020, 44, 139–147. [Google Scholar] [CrossRef]
- Alimirzaie, S.; Bagherzadeh, M.; Akbari, M.R. Liquid biopsy in breast cancer: A comprehensive review. Clin. Genet. 2019, 95, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Stefancu, A.; Badarinza, M.; Moisoiu, V.; Stefania, D.; Serban, O.; Leopold, N.; Fodo, D. SERS-based liquid biopsy of saliva and serum from patients with Sjögren’s syndrome. Anal. Bioanal. Chem. 2019, 411, 5877–5883. [Google Scholar] [CrossRef] [PubMed]
- Aravanis, A.M.; Lee, M.; Klausner, R.D. Next-Generation Sequencing of Circulating Tumor DNA for Early Cancer Detection. Cell 2017, 168, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Lin, S.Y.; Song, W.; Su, Y.-H. Urine-based liquid biopsy for non-urological cancers. Genet. Test. Mol. Biomark. 2019, 23, 277–283. [Google Scholar]
- Suzuki, S.; Suzuki, J.; Kume, K.; Yoshida, K.; Suyama, H.; Kawasaki, Y.; Nozawa, R.; Suzuki, H.; Fujiki, T.; Kamiyama, S.; et al. Poor renal accumulation of 99mTc-DMSA in idiopathic tubular proteinuria. Nephron 1999, 81, 49–54. [Google Scholar] [CrossRef]
- Goldstein, S.L. Urine Output Assessment in Acute Kidney Injury: The Cheapest and Most Impactful Biomarker. Front. Pediatr. 2020, 7, 565. [Google Scholar] [CrossRef] [Green Version]
- Manga, M.; Bartram, J.; Evans, B.E. Economic cost analysis of low-cost sanitation technology options in informal settlement areas (case study: Soweto, Johannesburg). Int. J. Hyg. Environ. Health 2020, 223, 289–298. [Google Scholar] [CrossRef]
- La Thangue, N.; Kerr, D.J. Predictive biomarkers: A paradigm shift towards personalized cancer medicine. Nat. Rev. Clin. Oncol. 2011, 8, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Murthy, V.; Takahashi, H.; Huyser, M.; Okano, M.; Tokumaru, Y.; Rashid, O.M.; Matsuyama, R.; Endo, I.; Takabe, K. Urine as a Source of Liquid Biopsy for Cancer. Cancers 2021, 13, 2652. [Google Scholar] [CrossRef]
- Micanovic, R.; LaFavers, K.; Garimella, P.S.; Wu, X.-R.; El-Achkar, T.M. Uromodulin (Tamm–Horsfall protein): Guardian of urinary and systemic homeostasis. Nephrol. Dial. Transplant. 2019, 35, 33–43. [Google Scholar] [CrossRef]
- Salvi, S.; Bandini, E.; Carloni, S.; Casadio, V.; Battistelli, M.; Salucci, S.; Erani, I.; Scarpi, E.; Gunelli, R.; Cicchetti, G.; et al. Detection and Investigation of Extracellular Vesicles in Serum and Urine Supernatant of Prostate Cancer Patients. Diagnostics 2021, 11, 466. [Google Scholar] [CrossRef]
- Sato, Y.; Matoba, R.; Kato, K. Recent Advances in Liquid Biopsy in Precision Oncology. Res. Biol. Pharm. Bull. 2019, 43, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacomello, G.; Scholten, A.; Parr, M.K. Current methods for stress marker detection in saliva. J. Pharm. Biomed. Anal. 2020, 30, 113604. [Google Scholar] [CrossRef] [PubMed]
- Sosnin, D.Y.; Gileva, O.S.; Mozgovaia, L.A.; Sivak, E.Y.; Beleva, N.S.; Krivtsov, A.V.; Pozdin, N.V. The NT-proBNP in saliva and blood serum in norm and under periodontitis. Klin. Lab. Diagn. 2018, 63, 164–168. [Google Scholar] [PubMed]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary biomarkers for oral cancer and pre-cancer screening: A review. Clin. Oral Investig. 2018, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Mashberg, A. Diagnosis of early oral and oropharyngeal squamous carcinoma: Obstacles and their amelioration. Oral Oncol. 2000, 36, 253–255. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Fedele, S.; Lo, R.L.; Ruoppo, E.; Lo, M.L. Oral and pharyngeal cancer: Lack of prevention and early detection by health care providers. Eur. J. Cancer Prev. 2001, 10, 381–383. [Google Scholar] [CrossRef]
- Rosin, M.P.; Poh, C.F.; Guillard, M.; Williams, M.; Zhang, L.; MacaUlay, C. Visualization and other emerging technologies as change makers for oral cancer prevention. Ann. N. Y. Acad. Sci. 2007, 1098, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Patton, L.L.; Epstein, J.B.; Kerr, A.R. Adjunctive techniques for oral cancer examination and lesion diagnosis. J. Am. Dent. Assoc. 2008, 139, 896–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, B.G.; Wong, D.T. Salivary mRNA targets for cancer diagnostics. Oral Oncol. 2008, 44, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Sun, H.; Wang, X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl. Biochem. Biotechnol. 2012, 168, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Bandhakavi, S.; Stone, M.D.; Onsongo, G.; Van Riper, S.K.; Griffin, T.J. A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J. Proteome Res. 2009, 8, 5590–5600. [Google Scholar] [CrossRef] [Green Version]
- Rai, B. Salivary levels vitamin E and C in different histological grading of oral cancer. Pesqui. Bras. Odontopediatria Clin. Integr. 2008, 8, 123–125. [Google Scholar] [CrossRef]
- Palmeira, P.; Carneiro-Sampaio, M. Immunology of breast milk. Rev. Assoc. Med. Bras. 2016, 62, 584–593. [Google Scholar] [CrossRef]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: The potentiality of blood samples. J. Exp. Clin. Cancer Res. 2020, 39, 95. [Google Scholar]
- Channaveerappa, D.; Arcaro, K.F.; Darie, C. Proteomics analysis of human breast milk to assess breast cancer risk. Electrophoresis 2018, 39, 653–665. [Google Scholar]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 2018, 42, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Kim, J.S.; Shin, J.H.; Moon, K.W.; Park, J.K.; Park, K.S.; Lee, E.Y. Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis. Cells 2021, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Zanutel, R.; Caponnetto, F.; Toffoletto, B.; Cifù, A.; Pistis, C.; Di Benedetto, P.; Causero, A.; Pozzi, M.; Bassini, F.; et al. Characterization of the Proinflammatory Profile of Synovial Fluid-Derived Exosomes of Patients with Osteoarthritis. Mediat. Inflamm. 2017, 2017, 4814987. [Google Scholar] [CrossRef] [PubMed]


| Liquid Biopsy | Possible Application |
|---|---|
| Blood | Oral cancer |
| Lung cancer | |
| Gastrointestinal cancer | |
| Colorectal cancer | |
| Pancreatic cancer | |
| Liver cancer | |
| Ovarian cancer | |
| Prostate cancer | |
| Breast cancer | |
| Sjogren’s syndrome | |
| Urine | Prostate cancer |
| Urologic cancer | |
| Colorectal cancer | |
| Saliva | Urinary inflammatory diseases |
| Oral cancer | |
| Sjogren’s syndrome | |
| Breast milk | Breast cancer |
| Synovial fluid | Osteoarthritis |
| Rheumatoid arthritis | |
| Joint inflammatory diseases |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michela, B. Liquid Biopsy: A Family of Possible Diagnostic Tools. Diagnostics 2021, 11, 1391. https://doi.org/10.3390/diagnostics11081391
Michela B. Liquid Biopsy: A Family of Possible Diagnostic Tools. Diagnostics. 2021; 11(8):1391. https://doi.org/10.3390/diagnostics11081391
Chicago/Turabian StyleMichela, Battistelli. 2021. "Liquid Biopsy: A Family of Possible Diagnostic Tools" Diagnostics 11, no. 8: 1391. https://doi.org/10.3390/diagnostics11081391
APA StyleMichela, B. (2021). Liquid Biopsy: A Family of Possible Diagnostic Tools. Diagnostics, 11(8), 1391. https://doi.org/10.3390/diagnostics11081391





