Early Predictive Response to Multi-Tyrosine Kinase Inhibitors in Advanced Refractory Radioactive-Iodine Differentiated Thyroid Cancer: A New Challenge for [18F]FDG PET/CT
Abstract
:1. Introduction
2. Advanced Refractory DTC and MKIs Employment Rationale
2.1. Multi-Kinase Inhibitors in Advanced RAI-R DTCs
2.2. MKIs Related-Adverse Events
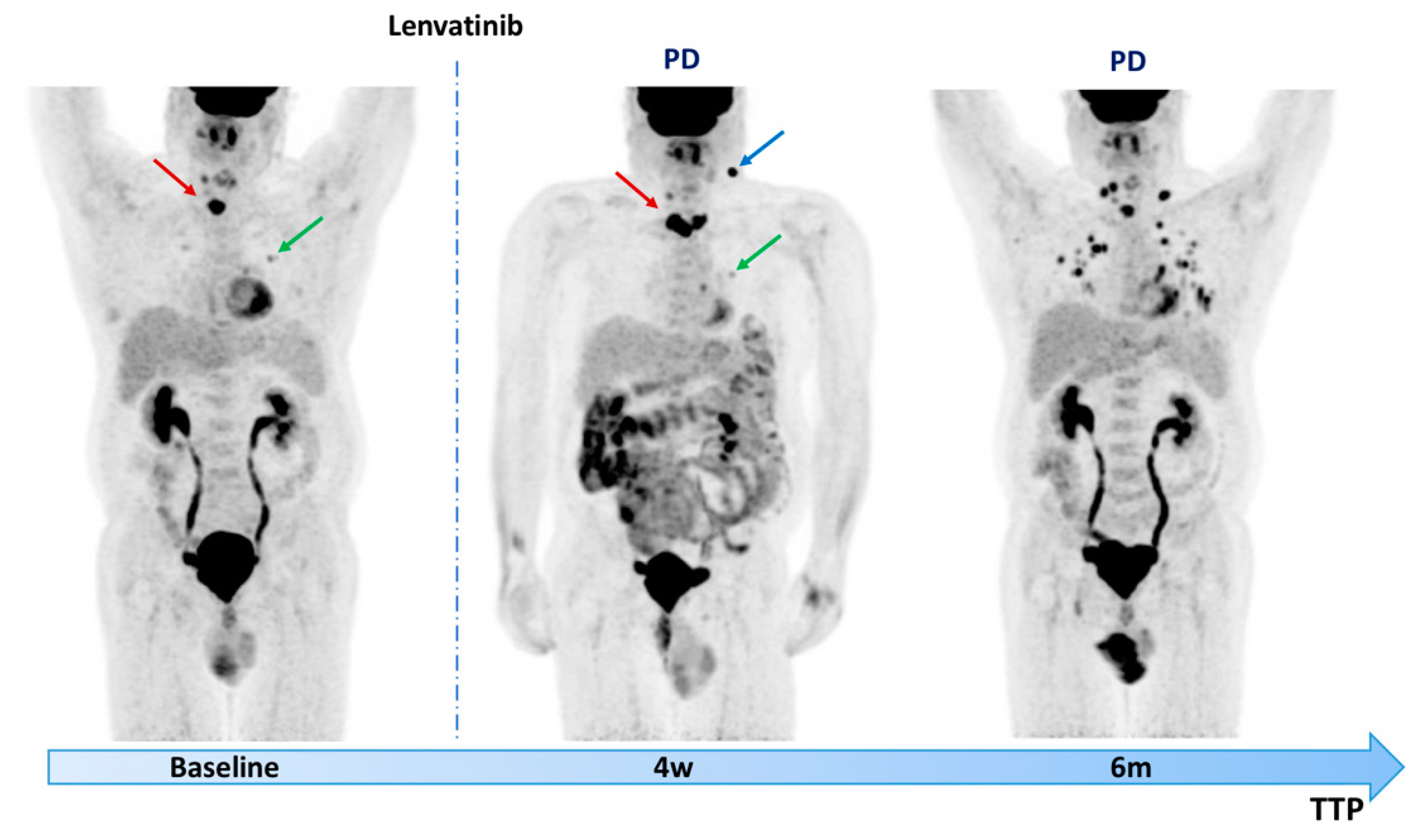
3. [18F]FDG PET/CT in Early MKIs Prediction of Response
4. Future Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PI3K | phosphatidylinositol-3 kinase |
| MAPK | mitogen activated protein kinase |
| RAS | rat sarcoma |
| PTEN | phosphatase and tensin homolog |
| AKT | protein kinase B |
| RAF | rapidly accelerated fibrosarcoma |
| FGFR | fibroblast growth factor receptors |
| FLT3 | Fms Related Receptor Tyrosine Kinase 3 |
| EGFR | Epidermal Growth Factor Receptor |
| TKR | tyrosine kinase receptor |
| VEGFR | vascular endothelial growth factor receptor |
| PDGFR | platelet derived growth factor receptor |
| RET | rearranged during transfection |
| TSH | thyroid stimulating hormone |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Iglesias, L.; Klain, M.; Pitoia, F.; Schlumberger, M.J. Radioactive iodine-refractory differentiated thyroid cancer: An uncommon but challenging situation. Arch. Endocrinol. Metab. 2017, 61, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayarangaiah, A.; Sidhu, G.; Brown, J.; Campbell, O.; McFarlane, S. Therapeutic options for advanced thyroid cancer. Int. J. Clin. Endocrinol. Metab. 2019, 5, 026–034. [Google Scholar] [CrossRef] [Green Version]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; de Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Bae, M.R.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol. 2018, 87, 158–164. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: What is new and what has changed? Cancer 2017, 123, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, L.; Cappagli, V.; Valerio, L.; Giani, C.; Viola, D.; Puleo, L.; Gambale, C.; Minaldi, E.; Campopiano, M.C.; Matrone, A.; et al. Thyroid cancers: From surgery to current and future systemic therapies through their molecular identities. Int. J. Mol. Sci. 2021, 22, 3117. [Google Scholar] [CrossRef]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on fundamental mechanisms of thyroid cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Elisei, R.; Ugolini, C.; Viola, D.; Lupi, C.; Biagini, A.; Giannini, R.; Romei, C.; Miccoli, P.; Pinchera, A.; Basolo, F. BRAFV600E Mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 3943–3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated genomic analysis of hürthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell 2018, 34, 256–270. [Google Scholar] [CrossRef] [Green Version]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.Y.; Shibru, D.; Bastian, B.; Griffin, A. The prevalence and prognostic value of braf mutation in thyroid cancer. Ann. Surg. 2007, 246, 466–470. [Google Scholar] [CrossRef]
- Ciampi, R.; Romei, C.; Pieruzzi, L.; Tacito, A.; Molinaro, E.; Agate, L.; Bottici, V.; Casella, F.; Ugolini, C.; Materazzi, G.; et al. Classical point mutations of ret, braf and ras oncogenes are not shared in papillary and medullary thyroid cancer occurring simultaneously in the same gland. J. Endocrinol. Investig. 2017, 40, 55–62. [Google Scholar] [CrossRef]
- Chakravarty, D.; Santos, E.; Ryder, M.; Knauf, J.A.; Liao, X.H.; West, B.L.; Bollag, G.; Kolesnick, R.; Thin, T.H.; Rosen, N.; et al. Small-molecule mapk inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional braf activation. J. Clin. Investig. 2011, 121, 4700–4711. [Google Scholar] [CrossRef] [Green Version]
- Tirrò, E.; Martorana, F.; Romano, C.; Vitale, S.R.; Motta, G.; di Gregorio, S.; Massimino, M.; Pennisi, M.S.; Stella, S.; Puma, A.; et al. Molecular alterations in thyroid cancer: From bench to clinical practice. Genes 2019, 10, 709. [Google Scholar] [CrossRef] [Green Version]
- Höppner, W.; Dralle, H.; Brabant, G. Duplication of 9 base pairs in the critical cysteine rich domain of the ret proto-oncogene causes multiple endocrine neoplasia type 2A. Hum. Mutat. 1998, 11. [Google Scholar] [CrossRef]
- Ciampi, R.; Romei, C.; Ramone, T.; Prete, A.; Tacito, A.; Cappagli, V.; Bottici, V.; Viola, D.; Torregrossa, L.; Ugolini, C.; et al. Genetic landscape of somatic mutations in a large cohort of sporadic medullary thyroid carcinomas studied by next-generation targeted sequencing. iScience 2019, 20, 324–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Censi, S.; Cavedon, E.; Bertazza, L.; Galuppini, F.; Watutantrige-Fernando, S.; de Lazzari, P.; Nacamulli, D.; Pennelli, G.; Fassina, A.; Iacobone, M.; et al. Frequency and significance of ras, tert promoter, and braf mutations in cytologically indeterminate thyroid nodules: A monocentric case series at a tertiary-level endocrinology unit. Front. Endocrinol. 2017, 8, 273. [Google Scholar] [CrossRef]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Marech, I.; Asabella, A.N.; di Palo, A.; Porcelli, M.; Lavelli, V.; Rubini, G.; Ferrari, C.; Gadaleta, C.D. Tyrosine-kinase inhibitors therapies with mainly anti-angiogenic activity in advanced renal cell carcinoma: Value of PET/CT in response evaluation. Int. J. Mol. Sci. 2017, 18, 1937. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.M.; Ahn, B.-C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics 2021, 11, 6251. [Google Scholar] [CrossRef] [PubMed]
- Gild, M.L.; Tsang, V.H.M.; Clifton-Bligh, R.J.; Robinson, B.G. Multikinase inhibitors in thyroid cancer: Timing of targeted therapy. Nat. Rev. Endocrinol. 2021, 17, 225–234. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de La Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic diff erentiated thyroid cancer: A randomised, double-blind, phase 3 Trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via Inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Locati, L.D.; Licitra, L.; Agate, L.; Ou, S.H.I.; Boucher, A.; Jarzab, B.; Qin, S.; Kane, M.A.; Wirth, L.J.; Chen, C.; et al. Treatment of advanced thyroid cancer with axitinib: Phase 2 study with pharmacokinetic/pharmacodynamic and quality-of-life assessments. Cancer 2014, 120, 2694–2703. [Google Scholar] [CrossRef] [Green Version]
- Carr, L.L.; Mankoff, D.A.; Goulart, B.H.; Eaton, K.D.; Capell, P.T.; Kell, E.M.; Bauman, J.E.; Martins, R.G. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin. Cancer Res. 2010, 16, 5260–5268. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qin, S.; Xu, J.; Guo, W.; Xiong, J.; Bai, Y.; Sun, G.; Yang, Y.; Wang, L.; Xu, N.; et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: Results from a randomized, placebo-controlled, parallel-arm, phase ii trial. J. Clin. Oncol. 2013, 31, 3219–3225. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wang, C.; Gao, W.; Cui, R.; Liang, J. Overwhelming rapid metabolic and structural response to apatinib in radioiodine refractory differentiated thyroid cancer. Oncotarget 2017, 8, 42252–42261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, V.R.; Leggas, M. Sunitinib Malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin. Ther. 2007, 29, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Jarzab, B.; Cabanillas, M.E.; Robinson, B.; Pacini, F.; Ball, D.W.; McCaffrey, J.; Newbold, K.; Allison, R.; Martins, R.G.; et al. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin. Cancer Res. 2016, 22, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Haddad, R.I.; Schlumberger, M.; Wirth, L.J.; Sherman, E.J.; Shah, M.H.; Robinson, B.; Dutcus, C.E.; Teng, A.; Gianoukakis, A.G.; Sherman, S.I. Incidence and timing of common adverse events in lenvatinib-treated patients from the select trial and their association with survival outcomes. Endocrine 2017, 56, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Nervo, A.; Gallo, M.; Samà, M.T.; Felicetti, F.; Alfano, M.; Migliore, E.; Marchisio, F.; Berardelli, R.; Arvat, E.; Piovesan, A. Lenvatinib in advanced radioiodine-refractory thyroid cancer: A snapshot of real-life clinical practice. Anticancer. Res. 2018, 38, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur. Thyroid. J. 2019, 8, 227–245. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Dandekar, M.; Joshi, A.; D’Cruz, A. Defining a rational step-care algorithm for managing thyroid carcinoma patients with elevated thyroglobulin and negative on radioiodine scintigraphy (TENIS): Considerations and challenges towards developing an appropriate roadmap. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1167–1171. [Google Scholar] [CrossRef] [Green Version]
- Silberstein, E.B. The problem of the patient with thyroglobulin elevation but negative iodine scintigraphy: The tenis syndrome. Semin. Nucl. Med. 2011, 41, 113–120. [Google Scholar] [CrossRef]
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358. [Google Scholar] [CrossRef]
- Suzuki, C.; Kiyota, N.; Imamura, Y.; Goto, H.; Suto, H.; Chayahara, N.; Toyoda, M.; Ito, Y.; Miya, A.; Miyauchi, A.; et al. exploratory analysis to predict optimal tumor burden for starting lenvatinib in patients with radioiodine-refractory differentiated thyroid cancer. Front. Oncol. 2021, 11, 1. [Google Scholar] [CrossRef]
- Dohán, O.; Carrasco, N. Advances in Na+/I- Symporter (NIS) Research in the Thyroid and Beyond. In Proceedings of the Molecular and Cellular Endocrinology, Amsterdam, The Netherlands, 31 December 2003; Volume 213, pp. 59–70. [Google Scholar]
- Garcia, D.; Singh, V. Nuclear Medicine PET/CT Thyroid Cancer Assessment, Protocols, and Interpretation; [Updated 2021 May 1]. StatPearls Publishing; January 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570634/ (accessed on 20 May 2021).
- Duarte, P.S.; Marin, J.F.G.; de Carvalho, J.W.D.A.; Sapienza, M.T.; Buchpiguel, C.A. Iodine/FDG “Flip-Flop” Phenomenon Inside a Large Metastatic Thyroid Cancer Lesion Better Characterized on SPECT/CT and PET/CT Studies. Clin. Nucl. Med. 2018, 43, 436–438. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. new response evaluation criteria in solid tumours: Revised recist guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Ziai, D.; Wagner, T.; el Badaoui, A.; Hitzel, A.; Woillard, J.B.; Melloni, B.; Monteil, J. Therapy Response Evaluation with FDG-ET/CT in small cell lung cancer: A prognostic and comparison study of the PERCIST and EORTC criteria. Cancer Imaging 2013, 13, 73–80. [Google Scholar] [CrossRef]
- Takeuchi, S.; Shiga, T.; Hirata, K.; Taguchi, J.; Magota, K.; Ariga, S.; Gouda, T.; Ohhara, Y.; Homma, R.; Shimizu, Y.; et al. early prediction of lenvatinib treatment efficacy by using 18F-FDG PET/CT in patients with unresectable or advanced thyroid carcinoma that is refractory to radioiodine treatment: A protocol for a non-randomized single-arm multicenter observational study. BMJ Open 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo Hyun, O.; Lodge, M.A.; Wahl, R.L. Practical percist: A simplified guide to pet response criteria in solid tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Marotta, V.; Ramundo, V.; Camera, L.; del Prete, M.; Fonti, R.; Esposito, R.; Palmieri, G.; Salvatore, M.; Vitale, M.; Colao, A.; et al. Sorafenib in advanced iodine-refractory differentiated thyroid cancer: Efficacy, safety and exploratory analysis of role of serum thyroglobulin and FDG-PET. Clin. Endocrinol. 2013, 78, 760–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmaddy, F.; Burgard, C.; Beyer, L.; Koehler, V.; Bartenstein, P.; Fabritius, M.P.; Geyer, T.; Wenter, V.; Ilhan, H.; Spitzweg, C.; et al. 18F-FDG-PET/CT in patients with advanced, radioiodine refractory thyroid cancer treated with lenvatinib. Cancers 2021, 13, 317. [Google Scholar] [CrossRef]
- Vercellino, L.; Bousquet, G.; Baillet, G.; Barré, E.; Mathieu, O.; Just, P.-A.; Desgrandchamps, F.; Misset, J.-L.; Hindié, E.; Moretti, J.-L. 18F-FDG PET/CT imaging for an early assessment of response to sunitinib in metastatic renal carcinoma: Preliminary study. Cancer Biother. Radiopharm. 2009, 24, 137–144. Available online: https://home.liebertpub.com/cbr (accessed on 29 July 2021). [CrossRef]
- Caldarella, C.; Muoio, B.; Isgrò, M.A.; Porfiri, E.; Treglia, G.; Giovanella, L. The role of Fluorine-18-Fluorodeoxyglucose positron emission tomography in evaluating the response to tyrosine-kinase inhibitors in patients with metastatic primary renal cell carcinoma. Radiol. Oncol. 2014, 48, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhang, X.; Yang, X.; Li, H.; Cui, R.; Guan, W.; Li, X.; Zhu, Z.; Lin, Y. PET response assessment in apatinib-treated radioactive iodine-refractory thyroid cancer. Endocr. Relat. Cancer 2018, 25, 653–663. [Google Scholar] [CrossRef]
- Manohar, P.M.; Beesley, L.J.; Bellile, E.L.; Worden, F.P.; Avram, A.M. Prognostic value of FDG-PET/CT metabolic parameters in metastatic radioiodine-refractory differentiated thyroid cancer. Clin. Nucl. Med. 2018, 43, 641–647. [Google Scholar] [CrossRef]
- Valerio, L.; Guidoccio, F.; Giani, C.; Tardelli, E.; Puccini, G.; Puleo, L.; Minaldi, E.; Boni, G.; Elisei, R.; Volterrani, D. [18F]-FDG-PET/CT correlates with the response of radiorefractory thyroid cancer to lenvatinib and patient survival. J. Clin. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.H.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Na, K.J.; Choi, H. Immune Landscape of papillary thyroid cancer and immunotherapeutic implications. Endocr. Relat. Cancer 2018, 25, 523–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Joshi, A. 68Ga Dotatate Pet/Ct in Differentiated Thyroid Carcinoma with Fibular Metastasis and Mixed Response to Sorafenib. Clin. Nucl. Med. 2016, 41, e428–e435. [Google Scholar] [CrossRef] [PubMed]

| Author-PMID | Journal- Year | MKIs | PET Timing | PET Parameters | Main Findings |
|---|---|---|---|---|---|
| Ahmaddy et al. 33467085 | Cancers 2021 | lenvatinib | 3 v 6 mo | mPERCIST; SUVmax; SUVmean; MTV; TLG; SUVpeak | All RECIST responders showed a decline in PET-parameters. Non-responders according to mPERCIST showed significantly lower median PFS and DSS, whereas according to RECIST, only DSS was significantly lower. |
| Wang et al. 29618578 | Endocrine-Rel Canc. 2018 | apatinib | baseline; 4 weeks; 8 weeks | EORTC; ΔMTV; ΔTLG | A strong correlation between the best change in ΔCT% and [18F]FDG PET/CT parameters after the first treatment cycle (ΔMTV%, ΔTLG%, and ΔSUVmax%). A significant difference in PFS was observed between patients with ΔMTV% < −45% and ≥−45% and between patients with ΔTLG% < −80% and ≥−80%. |
| Lin et al. 28178685 | Oncotarget 2017 | apatinib | baseline; 4 weeks 8 weeks | SUVmax | A significant decrease in SUVmax after 8 weeks of treatment was found. Linear correlations of change in diameter after 8 w of therapy with changes in SUVmax after 4/8 w of therapy were observed in the target lesions. |
| Carr et al. 20847059 | Clin Cancer Res. 2010 | sunitinib | baseline; 7 days | SUVmax | A statistically significant association of TTP with increases in average % SUV was found. The risk of tumor progression increased by 4% for each 1% increase in average SUV from baseline. |
| Marotta et al. 23009688 | Clin Endocrinol 2013 | sorafenib | baseline; 15 days | SUVmax | Baseline average SUVmax was significantly higher in patients who showed DP, but no significant correlation with PFS was found. Early [18F]FDG-PET scans showed a reduction in average SUVmax in all responders. |
| Manohar et al. 30015659 | Clin Nucl Med. 2018 | N.D. | baseline | log-MTV; log-TLG | The log-MTV values for patients on MKIs therapy tended to be higher than with other therapies. MKIs therapy was associated with significantly higher OS rates. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, C.; Santo, G.; Ruta, R.; Lavelli, V.; Rubini, D.; Mammucci, P.; Sardaro, A.; Rubini, G. Early Predictive Response to Multi-Tyrosine Kinase Inhibitors in Advanced Refractory Radioactive-Iodine Differentiated Thyroid Cancer: A New Challenge for [18F]FDG PET/CT. Diagnostics 2021, 11, 1417. https://doi.org/10.3390/diagnostics11081417
Ferrari C, Santo G, Ruta R, Lavelli V, Rubini D, Mammucci P, Sardaro A, Rubini G. Early Predictive Response to Multi-Tyrosine Kinase Inhibitors in Advanced Refractory Radioactive-Iodine Differentiated Thyroid Cancer: A New Challenge for [18F]FDG PET/CT. Diagnostics. 2021; 11(8):1417. https://doi.org/10.3390/diagnostics11081417
Chicago/Turabian StyleFerrari, Cristina, Giulia Santo, Rossella Ruta, Valentina Lavelli, Dino Rubini, Paolo Mammucci, Angela Sardaro, and Giuseppe Rubini. 2021. "Early Predictive Response to Multi-Tyrosine Kinase Inhibitors in Advanced Refractory Radioactive-Iodine Differentiated Thyroid Cancer: A New Challenge for [18F]FDG PET/CT" Diagnostics 11, no. 8: 1417. https://doi.org/10.3390/diagnostics11081417
APA StyleFerrari, C., Santo, G., Ruta, R., Lavelli, V., Rubini, D., Mammucci, P., Sardaro, A., & Rubini, G. (2021). Early Predictive Response to Multi-Tyrosine Kinase Inhibitors in Advanced Refractory Radioactive-Iodine Differentiated Thyroid Cancer: A New Challenge for [18F]FDG PET/CT. Diagnostics, 11(8), 1417. https://doi.org/10.3390/diagnostics11081417






