Immune-Mediated Desquamative Gingivitis and Optical Coherence Tomography Diagnostic Patterns: Clinical Implication from a Systematic Review
Abstract
:1. Introduction
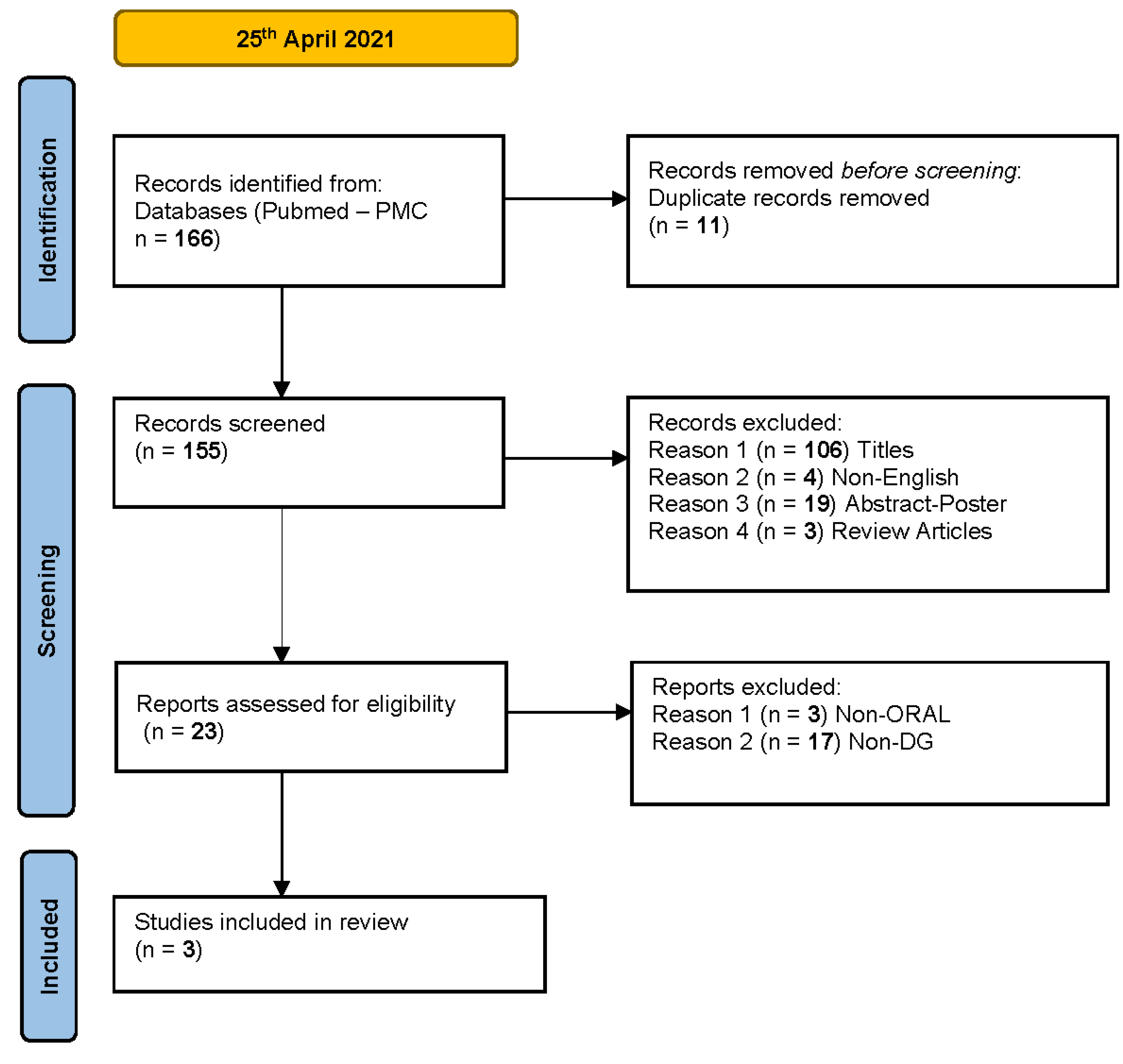
2. Materials and Methods
3. Results
4. Discussion
- Presence of multilocular subepithelial blister
- Normal stratified epithelial layer and epithelial thickness
- Altered/indistinguishable basal membrane and lamina propria
- Presence of inflammatory infiltrate
- Presence of unilocular intraepithelial blister
- Reduced stratified epithelial layer and epithelial thickness
- Normal basal membrane and lamina propria
- Presence of acantholytic cells in the blister
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo Russo, L.; Fedele, S.; Guiglia, R.; Ciavarella, D.; Lo Muzio, L.; Gallo, P.; Di Liberto, C.; Campisi, G. Diagnostic Pathways and Clinical Significance of Desquamative Gingivitis. J. Periodontol. 2008, 79, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Lamberts, A.; Diercks, G.F.H.; Pas, H.H.; Meijer, J.M.; Bolling, M.C.; Horváth, B. Oral Lesions in Autoimmune Bullous Diseases: An Overview of Clinical Characteristics and Diagnostic Algorithm. Am. J. Clin. Dermatol. 2019, 20, 847–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassona, Y.; Cirillo, N.; Taimeh, D.; Al Khawaldeh, H.; Sawair, F. Diagnostic patterns and delays in autoimmune blistering diseases of the mouth: A cross-sectional study. Oral Dis. 2018, 24, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Maderal, A.D.; Lee Salisbury, P.; Jorizzo, J.L. Desquamative gingivitis: Diagnosis and treatment. J. Am. Acad. Dermatol. 2018, 78, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.; Joshi, S.; Abdelghani, A.; Mee, J.; Andiappan, M.; Setterfield, J. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br. J. Dermatol. 2020, 182, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, J.; Woo, S.B.; Ahmed, A.R. Oral involvement in autoimmune blistering diseases. Clin. Dermatol. 2001, 19, 737–741. [Google Scholar] [CrossRef]
- Kridin, K. Subepidermal autoimmune bullous diseases: Overview, epidemiology, and associations. Immunol. Res. 2018, 66, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Bresler, S.C.; Bavarian, R.; Granter, S.R.; Woo, S. Bin Direct immunofluorescence is of limited utility in patients with low clinical suspicion for an oral autoimmune bullous disorder. Oral Dis. 2019, 26, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, P.H.; Wang, R.K. Theory, developments and applications of optical coherence tomography. J. Phys. D Appl. Phys. 2005, 38, 2519–2535. [Google Scholar] [CrossRef]
- Di Stasio, D.; Lauritano, D.; Romano, A.; Salerno, C.; Minervini, G.; Minervini, G.; Gentile, E.; Serpico, R.; Lucchese, A. In vivo characterization of oral pemphigus vulgaris by optical coherence tomography. J. Biol. Regul. Homeost. Agents 2015, 29, 39–41. [Google Scholar] [PubMed]
- Capocasale, G.; Panzarella, V.; Rodolico, V.; Di Fede, O.; Campisi, G. In vivo optical coherence tomography imaging in a case of mucous membrane pemphigoid and a negative Nikolsky’s sign. J. Dermatol. 2018, 45, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, D.; Lauritano, D.; Loffredo, F.; Gentile, E.; Della Vella, F.; Petruzzi, M.; Lucchese, A. Optical coherence tomography imaging of oral mucosa bullous diseases: A preliminary study. Dentomaxillofacial Radiol. 2019, 48. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaqman, M.; Hamdan, A.; Karadsheh, O.; Sawair, F.; Hassona, Y. Desquamative gingivitis: A challenging diagnosis for clinicians. Br. Dent. J. 2020, 229, 26–30. [Google Scholar] [CrossRef] [PubMed]

| First Author (Year) | Population (Disease) | Intervention (Type, Model, Brand, Device) | Outcomes | Study Design | OCT Diagnostic Parameters | Reference Diagnosis |
|---|---|---|---|---|---|---|
| Di Stasio (2015) | 1 case (PV) | NS | Evaluation of feasibility to image epithelial architecture of oral PV in vivo | Case report | − Localization of blister (intraepithelial vs. subepithelial) − Status of the basal membrane (MB) (normal/homogeneous vs. alterated/indistinguishable) | None |
| Capocasale (2018) | 1 case (MMP) | SS -OCT VivoSight® Michelson Diagnosis Ltd, version 2.0, Orpington, Kent, UK | Evaluation of tissue microstructure in a patient with oral MMP | Case report | − Localization of blister (intraepithelial vs. subepithelial | Histopathology |
| Di Stasio (2020) | 3 cases (1 PV, 2 MMP) | SS- OCT (IVS- 300 by Santec) | Examination of epithelial and subepithelial layer, and distinction between intra-and sub-epithelial detachment in oral PV and MMP lesions | Case series | − Localization of blister (intraepithelial vs. subepithelial) − Morphology of blister (unilocular or multilocular) − Epithelial thickness (normal vs. reduced) − Acantholytic cells into blister (present vs. absent). Only for PV case − Inflammatory infiltrate (present vs. absent). Only for MMP cases | Histopathology and DIF |
| Observational Studies | Bias Due to Confounding | Bias in Participant Selection | Bias in Classification of Interventions | Bias Due to Deviation from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Di Stasio (2015) | UR | SR | LR | NI | LR | MR | LR | SR |
| Capocasale (2018) | NI | SR | LR | NI | LR | MR | LR | SR |
| Di Stasio (2020) | NI | SR | LR | NI | LR | MR | LR | SR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzarella, V.; Bartolone, A.; Rodolico, V.; Capocasale, G.; Maniscalco, L.; Matranga, D.; Di Fede, O.; Campisi, G. Immune-Mediated Desquamative Gingivitis and Optical Coherence Tomography Diagnostic Patterns: Clinical Implication from a Systematic Review. Diagnostics 2021, 11, 1453. https://doi.org/10.3390/diagnostics11081453
Panzarella V, Bartolone A, Rodolico V, Capocasale G, Maniscalco L, Matranga D, Di Fede O, Campisi G. Immune-Mediated Desquamative Gingivitis and Optical Coherence Tomography Diagnostic Patterns: Clinical Implication from a Systematic Review. Diagnostics. 2021; 11(8):1453. https://doi.org/10.3390/diagnostics11081453
Chicago/Turabian StylePanzarella, Vera, Alessia Bartolone, Vito Rodolico, Giorgia Capocasale, Laura Maniscalco, Domenica Matranga, Olga Di Fede, and Giuseppina Campisi. 2021. "Immune-Mediated Desquamative Gingivitis and Optical Coherence Tomography Diagnostic Patterns: Clinical Implication from a Systematic Review" Diagnostics 11, no. 8: 1453. https://doi.org/10.3390/diagnostics11081453







