Flap Thickness and the Risk of Complications in Mechanical Microkeratome and Femtosecond Laser In Situ Keratomileusis: A Literature Review and Statistical Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search
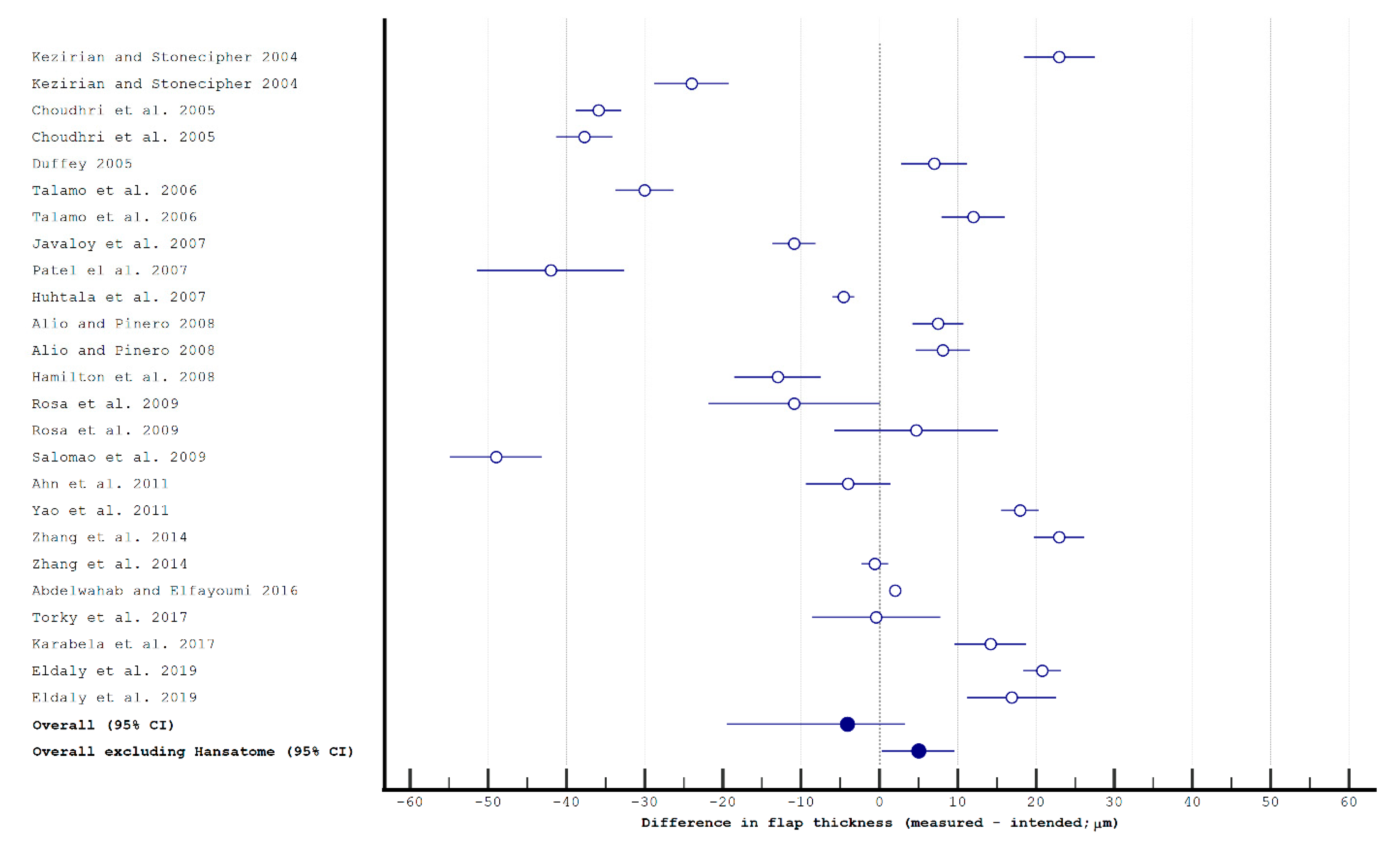
2.2. Study Selection for the Statistical Analysis
3. Results
3.1. Flap Thickness and Morphology in Manual Keratomes and Femtosecond Lasers
3.2. Risk of Intraoperative Complications Associated with Flap Creation
| Study | Method for Flap Creation | Number of Eyes * | Free Cap | Incomplete Flap | ButtonHole | Thin | Thick | Irregular Flap | Suction Loss | Epithelial Gas BreakThrough | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stulting et al., 1999 [72] | manual (Chiron Automated Corneal Shaper) | 1244 ** (M) | 0.5% | 0.1% | 0.1% | 0.1% | 0.0% | ||||
| Lin and Malloney 1999 [49] | manual (Chiron Automated Corneal Shaper) | 1019 (M) | 1.0% | 0.3% | 0.9% | ||||||
| Tham and Moloney 2000 [46] | manual (Bausch and Lomb Hansatome or Automated Corneal Shaper) | 3988 (N/A) | 0.1% | 0.2% | 0.1% | 0.2% | 0.1% | ||||
| Jacobs and Taravella 2002 [48] | manual (Bausch and Lomb Hansatome or Automated Corneal Shaper) | 84,711 (N/A) | 0.0% | 0.1% | 0.1% | 0.1% | 0.0% | ||||
| Nakano et al., 2004 [51] | manual (Nidek MK-2000, Bausch and Lomb Hansatome and Automated Corneal Shaper) | 34,182 | 0.08% | 0.23% | 0.13% | ||||||
| Carrillo et al., 2005 [53] | manual (Nidek MK-2000) | 26,600 | 0.086% | 0.049% | 0.049% | 0.019% | 0.019% | ||||
| Albeda-Vallés et al., 2007 [50] | manual (Moria LSK-1) | 34,099 (M/H) | 1.67% | 0.36% | 0.11% | 0.82% | 0.93% | ||||
| Al-Mezaine et al., 2011 [47] | manual (Bausch and Lomb Hansatome and Moria LSK2) | 4352 (M/H) | 0.1% | 0.6% | 0.2% | 0.1% | |||||
| Haft et al., 2009 [55] | femtosecond (AMO IntraLase 15 and 30 kHz) | 4772 (N/A) | 0.06%# | 0.02% | 0.06%# | 0.25% |
3.3. Risk of Postoperative Complications Associated with Flap Creation
| Study | Method for Flap Creation | Number of Eyes * | Flap Displacement | Epithelial Ingrowth | Local Keratitis (Culture Positive or Negative) | Flap Folds | DLK | TLSS |
|---|---|---|---|---|---|---|---|---|
| Stulting et al., 1999 [72] | manual (Chiron Automated Corneal Shaper) | 1062 + 182 * (only myopic) | 0.4% (partial in 0.6%) | 1.8% | 0.2% | 0.2% | ||
| Lin and Maloney 1999 [49] | manual (Chiron Automated Corneal Shaper) | 1019 | 2.0% | 2.2% | 1.1% | 1.8% | ||
| Recep et al., 2000 [101] | manual (Moria micokeratome) | 1481 | 1.42% | |||||
| Clare et al., 2011 [87] | manual (Moria One Use-Plus) | 23,997 | 0.033% ** | |||||
| Muñoz et al., 2006 [82] | femtosecond (AMO IntraLase 15 and 30 kHz) | 765 | 1.3% | |||||
| Stonecipher et al., 2006 [81] | femtosecond (IntraLase) | 5667 | 1.1% | |||||
| Sutton and Hodge 2008 [102] | femtosecond (AMO IntraLase 15 and 30 kHz) | 1000 | 0.4% | 0.0% | 0.0% | 0.2% | 0.0% | |
| Haft et al., 2009 [55] | femtosecond (AMO IntraLase 15 and 30 kHz) | 4772 | 0.42% | 0.25% | ||||
| Clare et al., 2011 [87] | femtosecond (IntraLase FS-60) | 57,241 | 0.003% ** | |||||
| de Paula et al., 2012 [78] | femtosecond (AMO IntraLase 60 kHz) | 801 | 12.4% | |||||
| Tomita et al., 2013 [76] | femtosecond (Femto LDV–IntraLase 60 kHz) | 818 | 8.17% –37.5% |
3.4. Other Considerations
| Pros | Cons |
|---|---|
|
|
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclosure
Appendix A
| Study | Number of Eyes | Method of Flap Creation | Intended Thickness [μm] | Method for Flap Thickness Measurement (Device Name) | Measured Central Flap Thickness ±SD [μm] | Timing of Flap Thickness Measurement |
|---|---|---|---|---|---|---|
| Manual microkeratomes | ||||||
| Kezirian and Stonecipher 2004 [116] | 126 | Carriazo-Barraquer (Moria) | 130 | USP (DGH Pachette 50/60 KHz) | 153 ± 26 | After lifting the flap (total thickness − thickness after flap lift) |
| Kezirian and Stonecipher 2004 [116] | 143 | Hansatome (Bausch & Lomb) | 180 | USP (DGH Pachette 50/60 KHz) | 156 ± 29 | After lifting the flap (total thickness − thickness after flap lift) |
| Choudhri et al., 2005 [32] | 138 | Hansatome (Bausch & Lomb) | 160 | USP (American Surgical Instruments Corneal Gauge) | 124.1 ± 17.4 | After lifting the flap (total thickness − thickness after flap lift) |
| Choudhri et al., 2005 [32] | 112 | Hansatome (Bausch & Lomb) | 180 | USP (American Surgical Instruments Corneal Gauge) | 142.3 ± 19.6 | After lifting the flap (total thickness − thickness after flap lift) |
| Duffey 2005 [23] | 42 | LSK-1 (Moria) | 100 | USP (DGH Pachette) | 107.0 ± 14.0 | After lifting the flap (total thickness − thickness after flap lift) |
| Talamo et al., 2006 [35] | 100 | LSK-1 (Moria) | 160 | USP (DGH Pachette II) | 130.0 ± 19.0 | After lifting the flap (total thickness − thickness after flap lift) |
| Talamo et al., 2006 [35] | 135 | M2 (Moria) | 130 | USP (DGH Pachette II) | 142.0 ± 24 | After lifting the flap (total thickness − thickness after flap lift) |
| Javaloy et al., 2007 [40] | 100 | M2 (Moria) | 160 | confocal microscopy (ASL 165a) | 149.1 ± 14.0 | 1 and 3 months postop |
| Patel el al., 2007 [26] | 21 | Hansatome (Bausch & Lomb) | 180 | confocal microscopy (Nidek ConfoScan 3 or 4) | 138.0 ± 22 | 1 month postop |
| Huhtala et al., 2007 [34] | 300 | M2 (Moria) | 120 | USP (Cilco) | 115.4 ± 12.5 | After lifting the flap (total thickness − thickness after flap lift) |
| Alió and Piñero 2008 [6] | 22 | M2 (Moria) | 110 | high-frequency USP (Ultralink Artemis) | 117.5 ± 7.8 | 1 month postop |
| Alió and Piñero 2008 [6] | 22 | Carriazo-Pendular Microkeratome (Schwind) | 110 | high-frequency USP (Ultralink Artemis) | 118.1 ± 8.3 | 1 month postop |
| Hamilton et al., 2008 [30] | 32 | One Use (Moria) | 130 | USP (N/A) | 117.0 ± 16.0 | After lifting the flap (total thickness − thickness after flap lift) |
| Rosa et al., 2009 [20] | 20 | Hansatome Zero Compression (Bausch & Lomb) | 160 | USP (Sonogage Corneo-Gage Plus) | 149.1 ± 24.9 | After lifting the flap (total thickness − thickness after flap lift); in 1of 4 group 20 min postop |
| Rosa et al., 2009 [20] | 20 | Zyoptix XP (Bausch & Lomb) | 120 | USP (Sonogage Corneo-Gage Plus) | 124.7 ± 23.8 | After lifting the flap (total thickness − thickness after flap lift); in 1of 4 group 20 min postop |
| Salomão et al., 2009 [31] | 70 | Hansatome (Bausch & Lomb) | 180 | USP (N/A) | 131.0 ± 25 | After lifting the flap (total thickness − thickness after flap lift) |
| Ahn et al., 2011 [27] | 52 | M2 (Moria) | 130 | OCT (Optovue RTVue FD-OCT) | 126.0 ± 19.9 ** | 2 months postop |
| Yao et al., 2011 [37] | 38 | M3 (Moria) | 110 | OCT (Carl Zeiss Visante) | 112.2 ± 5.4 | 1 week–6 months postop |
| Zhang et al., 2014 [117] | 50 | M2 (Moria) | 110 | USP (DGH 550) | 133.0 ± 13.9 | After lifting the flap (total thickness − thickness after flap lift) |
| Zhang et al., 2014 [33] | 60 | One Use-Plus SBK (Moria) | 110 | USP (DGH 550) | 109.4 ± 6.8 | After lifting the flap (total thickness − thickness after flap lift) |
| Abdelwahab and Elfayoumi 2016 [52] | 500 | One Use-Plus SBK (Moria) | 100 | USP (DGH 55 Pachmate) | 102.0 ± 6.1 | After lifting the flap (total thickness − thickness after flap lift) |
| Torky et al., 2017 [118] | 23 | M2 (Moria) | N/A | USP (Tomey SP 100) | 104.6 ± 20.1 | After lifting the flap (total thickness − thickness after flap lift) |
| Karabela et al., 2017 [119] | 72 | M2 (Moria) | 120 | USP (Nidek Echoscan US-1800) | 134.2 ± 19.9 | After lifting the flap (total thickness − thickness after flap lift) |
| Eldaly et al., 2019 [28] | 22 | M2 (Moria) | 100–110 | OCT (Heidelberg Engineering Spectralis) | 125.8 ± 5.8 | 1 month postop |
| Eldaly et al., 2019 [28] | 22 | M2 (Moria) | 130 | OCT (Heidelberg Engineering Spectralis) | 146.9 ± 13.6 | 1 month postop |
| Femtosecond laser | ||||||
| Kezirian and Stonecipher 2004 [116] | 106 | IntraLase S3 (Abbott Medical Optics) | 130 | USP (DGH Pachette 50/60 KHz) | 114 ± 14 | After lifting the flap (total thickness − thickness after flap lift) |
| Binder 2004 [120] | 34 | IntraLase S3 (Abbott Medical Optics) | 110 | USP (Sonnogage Cornea Scan II 5) | 125.0 ± 12.0 | After lifting the flap (total thickness − thickness after flap lift) |
| Binder 2004 [120] | 22 | IntraLase S3 (Abbott Medical Optics) | 120 | USP (Sonnogage Cornea Scan II 5) | 122.4 ± 11.9 | After lifting the flap (total thickness − thickness after flap lift) |
| Binder 2004 [120] | 21 | IntraLase S3 (Abbott Medical Optics) | 130 | USP (Sonnogage Cornea Scan II 5) | 128.7 ± 16.6 | After lifting the flap (total thickness − thickness after flap lift) |
| Binder 2004 [120] | 26 | IntraLase S3 (Abbott Medical Optics) | 140 | USP (Sonnogage Cornea Scan II 5) | 132.5 ± 18.5 | After lifting the flap (total thickness − thickness after flap lift) |
| Talamo et al., 2006 [35] | 99 | IntraLase FS (Abbott Medical Optics) | 110 | USP (DGH Pachette II) | 119.0 ± 12 | After lifting the flap (total thickness − thickness after flap lift) |
| Javaloy et al., 2007 [40] | 100 | IntraLase FS (Abbott Medical Optics) | 120 | confocal microscopy (ASL 165a) | 130.1 ± 1.7 | 1 and 3 months postop |
| Patel el al., 2007 [26] | 21 | IntraLase 15- kHz (Abbott Medical Optics) | 120 | confocal microscopy (Nidek ConfoScan 3 or 4) | 143.0 ± 16 | 1 month postop |
| Alió and Piñero 2008 [6] | 22 | IntraLase 30- kHz (Abbott Medical Optics) | 110 | high-frequency USP (Ultralink Artemis) | 116.0 ± 6.2 | 1 month postop |
| Hamilton et al., 2008 [30] | 32 | IntraLase 60- kHz (Abbott Medical Optics) | 110–120 | USP (N/A) | 120.0 ± 13 | After lifting the flap (total thickness − thickness after flap lift) |
| Sutton and Hodge 2008 [102] | 838 | IntraLase 15- kHz (Abbott Medical Optics) | 105 | USP (Sonogage Corneo-Gage Plus) | 116.8 ± 10.8 | After lifting the flap (total thickness − thickness after flap lift) |
| Sutton and Hodge 2008 [102] | 162 | IntraLase 30- kHz (Abbott Medical Optics) | 115 | USP (Sonogage Corneo-Gage Plus) | 114.0 ± 9.8 | After lifting the flap (total thickness − thickness after flap lift) |
| Rosa et al., 2009 [20] | 20 | IntraLase FS 60 kHz (Abbott Medical Optics) | 120 | USP (Sonogage Corneo-Gage Plus) | 115.5 ± 12.5 | After lifting the flap (total thickness − thickness after flap lift); in 1of 4 group 20 min postop |
| Salomão et al., 2009 [31] | 113 | IntraLase 30- or 60- kHz (Abbott Medical Optics) | 100–110 | USP (N/A) | 131.0 ± 25 | After lifting the flap (total thickness − thickness after flap lift) |
| Ahn et al., 2011 [27] | 50 | IntraLase | 110 | OCT (Optovue RTVue FD-OCT) | 130.3 ± 13.2 ** | 2 months postop |
| Ahn et al., 2011 [27] | 40 | VisuMax (Carl Zeiss Meditec) | 110 | OCT (Optovue RTVue FD-OCT) | 133.9 ± 13.9 ** | 2 months postop |
| Ahn et al., 2011 [27] | 64 | Femto LDV (Ziemer) | 110 | OCT (Optovue RTVue FD-OCT) | 105.8 ± 8.2 ** | 2 months postop |
| Yao et al., 2011 [37] | 25 | VisuMax (Carl Zeiss Meditec) | 100 | OCT (Carl Zeiss Visante) | 114.2 ± 6.9 | 1 week–6 months postop |
| Kim et al., 2011 [36] | 19 | IntraLase (Abbott Medical Optics) | 110 | OCT (Carl Zeiss Visante) | 115.2 ± 5.0 | 1 week postop |
| Kim et al., 2011 [36] | 7 | IntraLase (Abbott Medical Optics) | 120 | OCT (Carl Zeiss Visante) | 121.9 ± 5.8 | 1 week postop |
| Kim et al., 2011 [36] | 9 | IntraLase (Abbott Medical Optics) | 130 | OCT (Carl Zeiss Visante) | 134.4 ± 5.0 | 1 week postop |
| Zhang et al., 2014 [117] | 72 | FS200 (Alcon) | 110 | USP (DGH 550) | 105.5 ± 5.9 | After lifting the flap (total thickness − thickness after flap lift) |
| Zhang et al., 2014 [33] | 60 | FS200 (Alcon) | 110 | USP (DGH 550) | 112.7 ± 7.2 | After lifting the flap (total thickness − thickness after flap lift) |
| Zheng et al., 2015 [24] | 200 | FS200 (Alcon) | 110 | OCT (OptoVue RTVue FD-OCT) | 105.4 ± 3.4 | 1 week postop |
| Zheng et al., 2015 [24] | 200 | VisuMax (Carl Zeiss Meditec) | 110 | OCT (OptoVue RTVue FD-OCT) | 110.8 ± 3.9 | 1 week postop |
| Liu et al., 2016 [38] | 200 | FS200 (Alcon) | 110 | OCT (OptoVue RTVue FD-OCT) | 105.4 ± 4.5 | 1 week postop |
| Liu et al., 2016 [38] | 200 | IntraLase FS60 (Abbott Medical Optics) | 110 | OCT (OptoVue RTVue FD-OCT) | 109.2 ± 11.6 | 1 week postop |
| Torky et al., 2017 [118] | 26 | Visumax FSL (Carl Zeiss Meditec) | 100 | USP (Tomey SP 100) | 100.1 ± 16.1 | After lifting the flap (total thickness − thickness after flap lift) |
| Eldaly et al., 2019 [28] | 25 | FS200 (Alcon) | 100–110 | OCT (Heidelberg Engineering Spectralis) | 114.6 ± 6.1 | 1 month postop |
| Eldaly et al., 2019 [28] | 24 | FS200 (Alcon) | 130 | OCT (Heidelberg Engineering Spectralis) | 140.8 ± 13.8 | 1 month postop |
| Parafita-Fernandez et al., 2020 [39] | 44 | FS200 (Alcon) | 120 | OCT (Zeiss Cirrus HD-OCT 5000) | 132.2 ± 8.1 | 1 day–3 months postop |
References
- Winkler von Mohrenfels, C.; Khoramnia, R.; Salgado, J.; Wüllner, C.; Donitzky, C.; Maier, M.; Lohmann, C.P. First Clinical Results with a New 200 kHz Femtosecond Laser System. Br. J. Ophthalmol. 2012, 96, 788–792. [Google Scholar] [CrossRef][Green Version]
- Tandogan, T.; Khoramnia, R.; Gye, H.; Auffarth, G.; Kim, D.; Choi, C. Einfluss Unterschiedlicher Ablations frequenzen Auf Die Klinischen Ergebnisse Bei Photorefraktiver Keratektomie Unter Verwendung Derselben Excimer-Laser-Plattform: Ein Kontralateraler Vergleich. Klin. Mon. Augenheilkd. 2016, 234, 706–712. [Google Scholar]
- Salgado, J.P.; Khoramnia, R.; Lohmann, C.P.; Winkler von Mohrenfels, C. Corneal Collagen Crosslinking in Post-LASIK Keratectasia. Br. J. Ophthalmol. 2011, 95, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Slade, S.G.; Durrie, D.S.; Binder, P.S. A Prospective, Contralateral Eye Study Comparing Thin-Flap LASIK (sub-Bowman Keratomileusis) with Photorefractive Keratectomy. Ophthalmology 2009, 116, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Khoramnia, R.; Salgado, J.P.; Wuellner, C.; Donitzky, C.; Lohmann, C.P.; Winkler von Mohrenfels, C. Safety, Efficacy, Predictability and Stability of Laser in Situ Keratomileusis (LASIK) with a 1000-Hz Scanning Spot Excimer Laser. Acta Ophthalmol. 2012, 90, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Alió, J.L.; Piñero, D.P. Very High-Frequency Digital Ultrasound Measurement of the LASIK Flap Thickness Profile Using the IntraLase Femtosecond Laser and M2 and Carriazo-Pendular Microkeratomes. J. Refract. Surg. 2008, 24, 12–23. [Google Scholar] [CrossRef]
- Holzer, M.P.; Rabsilber, T.M.; Auffarth, G.U. Femtosecond Laser-Assisted Corneal Flap Cuts: Morphology, Accuracy, and Histopathology. Invest. Ophthalmol. Vis. Sci. 2006, 47, 2828–2831. [Google Scholar] [CrossRef]
- Khoramnia, R.; Salgado, J.P.; Lohmann, C.P.; Kobuch, K.A.; von Mohrenfels, C.W. Precision, Morphology, and Histology of Corneal Flap Cuts Using a 200-kHz Femtosecond Laser. Eur. J. Ophthalmol. 2012, 22, 161–167. [Google Scholar] [CrossRef]
- Mrochen, M.; Donges, A.; Korn, G. [Femtosecond laser for refractive corneal surgery: Foundations, mode of action and clinical applications]. Ophthalmology 2006, 103, 1005–1013. [Google Scholar] [CrossRef]
- Nagy, Z.Z.; Szaflik, J.P. The Role of Femtolaser in Cataract Surgery. Klin. Ocz. 2012, 114, 324–327. [Google Scholar] [CrossRef]
- Chung, S.H.; Mazur, E. Surgical Applications of Femtosecond Lasers. J. Biophotonics 2009, 2, 557–572. [Google Scholar] [CrossRef]
- Khoramnia, R.; Holzer, M.P.; Fitting, A.; Auffarth, G.U.; Rabsilber, T.M. [Functional results after bilateral intrastromal femtosecond laser correction of presbyopia]. Ophthalmology 2013, 110, 1163–1170. [Google Scholar] [CrossRef]
- Khoramnia, R.; Fitting, A.; Rabsilber, T.M.; Thomas, B.C.; Auffarth, G.U.; Holzer, M.P. Intrastromal Femtosecond Laser Surgical Compensation of Presbyopia with Six Intrastromal Ring Cuts: 3-Year Results. Br. J. Ophthalmol. 2015, 99, 170–176. [Google Scholar] [CrossRef]
- Holzer, M.P.; Knorz, M.C.; Tomalla, M.; Neuhann, T.M.; Auffarth, G.U. Intrastromal Femtosecond Laser Presbyopia Correction: 1-Year Results of a Multicenter Study. J. Refract. Surg. 2012, 28, 182–188. [Google Scholar] [CrossRef]
- Kahuam-López, N.; Navas, A.; Castillo-Salgado, C.; Graue-Hernandez, E.O.; Jimenez-Corona, A.; Ibarra, A. Laser-Assisted in-Situ Keratomileusis (LASIK) with a Mechanical Microkeratome Compared to LASIK with a Femtosecond Laser for LASIK in Adults with Myopia or Myopic Astigmatism. Cochrane Database Syst. Rev. 2020, 4, CD012946. [Google Scholar]
- Chua, D.; Htoon, H.M.; Lim, L.; Chan, C.M.; Mehta, J.S.; Tan, D.T.H.; Rosman, M. Eighteen-Year Prospective Audit of LASIK Outcomes for Myopia in 53 731 Eyes. Br. J. Ophthalmol. 2019, 103, 1228–1234. [Google Scholar] [CrossRef]
- Chen, S.; Feng, Y.; Stojanovic, A.; Jankov, M.R., 2nd; Wang, Q. IntraLase Femtosecond Laser vs. Mechanical Microkeratomes in LASIK for Myopia: A Systematic Review and Meta-Analysis. J. Refract. Surg. 2012, 28, 15–24. [Google Scholar] [CrossRef]
- Farjo, A.A.; Sugar, A.; Schallhorn, S.C.; Majmudar, P.A.; Tanzer, D.J.; Trattler, W.B.; Cason, J.B.; Donaldson, K.E.; Kymionis, G.D. Femtosecond Lasers for LASIK Flap Creation: A Report by the American Academy of Ophthalmology. Ophthalmology 2013, 120, e5–e20. [Google Scholar] [CrossRef]
- Stonecipher, K.G.; Meyer, J.J.; Stonecipher, M.; Felsted, D.J. Laser in Situ Keratomileusis Flap Complications and Complication Rates Using Mechanical Microkeratomes versus Femtosecond Laser: Retrospective Review. Med Res. Arch. 2015, 2, 1925–1933. [Google Scholar] [CrossRef]
- Rosa, A.M.; Neto Murta, J.; Quadrado, M.J.; Tavares, C.; Lobo, C.; Van Velze, R.; Castanheira-Dinis, A. Femtosecond Laser versus Mechanical Microkeratomes for Flap Creation in Laser in Situ Keratomileusis and Effect of Postoperative Measurement Interval on Estimated Femtosecond Flap Thickness. J. Cataract Refract. Surg. 2009, 35, 833–838. [Google Scholar] [CrossRef]
- Yuen, L.H.; Chan, W.K.; Koh, J.; Mehta, J.S.; Tan, D.T. A 10-Year Prospective Audit of LASIK Outcomes for Myopia in 37932 Eyes at a Single Institution in Asia. Ophthalmology 2010, 117, 1236–1244.e1. [Google Scholar] [CrossRef]
- Pokroy, R.; Mimouni, M.; Sela, T.; Munzer, G.; Kaiserman, I. Myopic Laser in Situ Keratomileusis Retreatment: Incidence and Associations. J. Cataract Refract. Surg. 2016, 42, 1408–1414. [Google Scholar] [CrossRef]
- Duffey, R.J. Thin Flap Laser in Situ Keratomileusis: Flap Dimensions with the Moria LSK-One Manual Microkeratome Using the 100-μm Head. J. Cataract Refract. Surg. 2005, 31, 1159–1162. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, Y.; Zhang, J.; Liu, Q.; Zhai, C.; Wang, Y. Comparison of Laser in Situ Keratomileusis Flaps Created by 2 Femtosecond Lasers. Cornea 2015, 34, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, H.; Prandi, B.; Diaz-Rato, A.; Morcillo, M.; Sabater, J.B. The Effect of Flap Thickness on the Visual and Refractive Outcome of Myopic Laser in Situ Keratomileusis. Eye 2005, 19, 1290–1296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, S.V.; Maguire, L.J.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. Femtosecond Laser versus Mechanical Microkeratome for LASIK. Ophthalmology 2007, 114, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, J.-K.; Kim, C.K.; Han, G.H.; Seo, K.Y.; Kim, E.K.; Kim, T.-I. Comparison of Laser in Situ Keratomileusis Flaps Created by 3 Femtosecond Lasers and a Microkeratome. J. Cataract Refract. Surg. 2011, 37, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Eldaly, Z.H.; Abdelsalam, M.A.; Hussein, M.S.; Nassr, M.A. Comparison of Laser In Situ Keratomileusis Flap Morphology and Predictability by WaveLight FS200 Femtosecond Laser and Moria Microkeratome: An Anterior Segment Optical Coherence Tomography Study. Korean J. Ophthalmol. 2019, 33, 113–121. [Google Scholar] [CrossRef]
- Von Jagow, B.; Kohnen, T. Corneal Architecture of Femtosecond Laser and Microkeratome Flaps Imaged by Anterior Segment Optical Coherence Tomography. J. Cataract Refract. Surg. 2009, 35, 35–41. [Google Scholar] [CrossRef]
- Hamilton, R.D.; Johnson, D.R.; Lee, N.; Bourla, N. Differences in the Corneal Biomechanical Effects of Surface Ablation Compared with Laser in Situ Keratomileusis Using a Microkeratome or Femtosecond Laser. J. Cataract Refract. Surg. 2008, 34, 2049–2056. [Google Scholar] [CrossRef]
- Salomão, M.Q.; Ambrósio, R., Jr.; Wilson, S.E. Dry Eye Associated with Laser in Situ Keratomileusis: Mechanical Microkeratome versus Femtosecond Laser. J. Cataract Refract. Surg. 2009, 35, 1756–1760. [Google Scholar] [CrossRef]
- Choudhri, S.A.; Feigenbaum, S.K.; Pepose, J.S. Factors Predictive of LASIK Flap Thickness with the Hansatome Zero Compression Microkeratome. J. Refract. Surg. 2005, 21, 253–259. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.-S.; Yu, Q.; Wu, J.-X.; Lian, J.-C. Comparison of Corneal Flap Thickness Using a FS200 Femtosecond Laser and a Moria SBK Microkeratome. Int. J. Ophthalmol. 2014, 7, 273–277. [Google Scholar]
- Huhtala, A.; Pietilä, J.; Mäkinen, P.; Suominen, S.; Seppänen, M.; Uusitalo, H. Corneal Flap Thickness with the Moria M2 Single-Use Head 90 Microkeratome. Acta Ophthalmol. Scand. 2007, 85, 401–406. [Google Scholar] [CrossRef]
- Talamo, J.H.; Meltzer, J.; Gardner, J. Reproducibility of Flap Thickness with IntraLase FS and Moria LSK-1 and M2 Microkeratomes. J. Refract. Surg. 2006, 22, 556–561. [Google Scholar] [CrossRef]
- Kim, C.Y.; Song, J.H.; Na, K.S.; Chung, S.-H.; Joo, C.-K. Factors Influencing Corneal Flap Thickness in Laser in Situ Keratomileusis with a Femtosecond Laser. Korean J. Ophthalmol. 2011, 25, 8–14. [Google Scholar] [CrossRef][Green Version]
- Yao, P.; Xu, Y.; Zhou, X. Comparison of the Predictability, Uniformity and Stability of a Laserin SituKeratomileusis Corneal Flap Created with a VisuMax Femtosecond Laser or a Moria Microkeratome. J. Int. Med. Res. 2011, 39, 748–758. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.-H.; Zhang, J.; Zheng, Y.; Zhai, C.-B.; Liu, J. Comparison of Corneal Flaps Created by Wavelight FS200 and Intralase FS60 Femtosecond Lasers. Int. J. Ophthalmol. 2016, 9, 1006–1010. [Google Scholar]
- Parafita-Fernandez, A.; Garcia-Gonzalez, M.; Gros-Otero, J.; Alvarez-Rementería Capelo, L.; Blázquez Sánchez, V.; Teus, M. Evolution of Visual Acuity, Flap Thickness, and Optical Density after Laser in Situ Keratomileusis Performed with a Femtosecond Laser. J. Cataract Refract. Surg. 2020, 46, 260–266. [Google Scholar] [CrossRef]
- Javaloy, J.; Vidal, M.T.; Abdelrahman, A.M.; Artola, A.; Alió, J.L. Confocal Microscopy Comparison of IntraLase Femtosecond Laser and Moria M2 Microkeratome in LASIK. J. Refract. Surg. 2006, 23, 178–187. [Google Scholar] [CrossRef]
- Moshirfar, M.; Duong, A.; Ronquillo, Y. Corneal Imaging. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kanclerz, P.; Khoramnia, R.; Wang, X. Current Developments in Corneal Topography and Tomography. Diagnostics 2021, 11, 1466. [Google Scholar] [CrossRef]
- Tang, M.; Li, Y.; Huang, D. Corneal Epithelial Remodeling after LASIK Measured by Fourier-Domain Optical Coherence Tomography. J. Ophthalmol. 2015, 2015, 860313. [Google Scholar] [CrossRef]
- Huang, D.; Tang, M.; Shekhar, R. Mathematical Model of Corneal Surface Smoothing after Laser Refractive Surgery. Am. J. Ophthalmol. 2003, 135, 267–278. [Google Scholar] [CrossRef]
- Moshirfar, M.; Brown, T.W.; Heiland, M.B.; Rosen, D.B.; Ronquillo, Y.C.; Hoopes, P.C. Comparative Analysis of LASIK Flap Diameter and Its Centration Using Two Different Femtosecond Lasers. Med. Hypothesis Discov. Innov. Ophthalmol 2019, 8, 241–249. [Google Scholar]
- Tham, V.M.; Maloney, R.K. Microkeratome Complications of Laser in Situ Keratomileusis. Ophthalmology 2000, 107, 920–924. [Google Scholar] [CrossRef]
- Al-Mezaine, H.S.; Al-Amro, S.A.; Al-Obeidan, S. Intraoperative Flap Complications in Laser in Situ Keratomileusis with Two Types of Microkeratomes. Saudi J. Ophthalmol. 2011, 25, 239–243. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Taravella, M.J. Incidence of Intraoperative Flap Complications in Laser in Situ Keratomileusis. J. Cataract Refract. Surg. 2002, 28, 23–28. [Google Scholar] [CrossRef]
- Lin, R.T.; Maloney, R.K. Flap Complications Associated with Lamellar Refractive Surgery. Am. J. Ophthalmol. 1999, 127, 129–136. [Google Scholar] [CrossRef]
- Albelda-Vallés, J.C.; Martin-Reyes, C.; Ramos, F.; Beltran, J.; Llovet, F.; Baviera, J. Effect of Preoperative Keratometric Power on Intraoperative Complications in LASIK in 34,099 Eyes. J. Refract. Surg. 2007, 23, 592–597. [Google Scholar] [CrossRef][Green Version]
- Nakano, K.; Nakano, E.; Oliveira, M.; Portellinha, W.; Alvarenga, L. Intraoperative Microkeratome Complications in 47,094 Laser in Situ Keratomileusis Surgeries. J. Refract. Surg. 2004, 20, S723–S726. [Google Scholar] [CrossRef]
- Abdelwahab, S.; Elfayoumi, M. Moria One-Use Plus Sub-Bowman’s Keratomileusis Head: A Useful Tool in the Refractive Surgeon’s Armamentarium. J. Egypt. Ophthalmol. Soc. 2016, 109, 105–108. [Google Scholar] [CrossRef]
- Carrillo, C.; Chayet, A.S.; Dougherty, P.J.; Montes, M.; Magallanes, R.; Najman, J.; Fleitman, J.; Morales, A. Incidence of Complications during Flap Creation in LASIK Using the NIDEK MK-2000 Microkeratome in 26,600 Cases. J. Refract. Surg. 2005, 21, 655–657. [Google Scholar] [CrossRef]
- Melki, S. Intraoperative Complications: Free Cap in Femtosecond LASIK. In Difficult and Complicated Cases in Refractive Surgery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 121–122. [Google Scholar]
- Haft, P.; Yoo, S.H.; Kymionis, G.D.; Ide, T.; O’Brien, T.P.; Culbertson, W.W. Complications of LASIK Flaps Made by the IntraLase 15- and 30-kHz Femtosecond Lasers. J. Refract. Surg. 2009, 25, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Watabe, M.; Nakamura, T.; Nakamura, N.; Tsuru, T.; Waring, G.O., IV. Management and Outcomes of Suction Loss during LASIK Flap Creation with a Femtosecond Laser. J. Refract. Surg. 2012, 28, 32–36. [Google Scholar] [CrossRef]
- Salz, J.J. Suction Break after Complete Raster Pattern and Incomplete Side Cut. In Difficult and Complicated Cases in Refractive Surgery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 137–139. [Google Scholar]
- Razgulyaeva, E.A. Rescue of Primary Incomplete Microkeratome Flap with Secondary Femtosecond Laser Flap in LASIK. Case Rep. Ophthalmol. Med. 2014, 2014, 289354. [Google Scholar] [CrossRef]
- Courtin, R.; Saad, A.; Guilbert, E.; Grise-Dulac, A.; Gatinel, D. Opaque Bubble Layer Risk Factors in Femtosecond Laser-Assisted LASIK. J. Refract. Surg. 2015, 31, 608–612. [Google Scholar] [CrossRef]
- Srinivasan, S.; Herzig, S. Sub-Epithelial Gas Breakthrough during Femtosecond Laser Flap Creation for LASIK. Br. J. Ophthalmol. 2007, 91, 1373. [Google Scholar] [CrossRef]
- Alió, J.L.; Wróbel, D.; Abbouda, A. Vertical Gas Breakthrough during Femtosecond Laser Flap. In Difficult and Complicated Cases in Refractive Surgery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 117–119. [Google Scholar]
- Seider, M.I.; Ide, T.; Kymionis, G.D.; Culbertson, W.W.; O’Brien, T.P.; Yoo, S.H. Epithelial Breakthrough during IntraLase Flap Creation for Laser in Situ Keratomileusis. J. Cataract Refract. Surg. 2008, 34, 859–863. [Google Scholar] [CrossRef]
- Ribeiro, G.C.; Krueger, R.R. Management of Bilateral Gas-Bubble Breakthrough during Femtosecond LASIK in the Presence of Anterior Basement Membrane Dystrophy. J. Cataract Refract. Surg. 2014, 40, 1736–1739. [Google Scholar] [CrossRef]
- Kaiserman, I.; Maresky, H.S.; Bahar, I.; Rootman, D.S. Incidence, Possible Risk Factors, and Potential Effects of an Opaque Bubble Layer Created by a Femtosecond Laser. J. Cataract Refract. Surg. 2008, 34, 417–423. [Google Scholar] [CrossRef]
- Liu, C.-H.; Sun, C.-C.; Hui-Kang Ma, D.; Chien-Chieh Huang, J.; Liu, C.-F.; Chen, H.-F.; Hsiao, C.-H. Opaque Bubble Layer: Incidence, Risk Factors, and Clinical Relevance. J. Cataract Refract. Surg. 2014, 40, 435–440. [Google Scholar] [CrossRef]
- Soong, H.K.; de Melo Franco, R. Anterior Chamber Gas Bubbles during Femtosecond Laser Flap Creation in LASIK: Video Evidence of Entry via Trabecular Meshwork. J. Cataract Refract. Surg. 2012, 38, 2184–2185. [Google Scholar] [CrossRef]
- Utine, C.A.; Altunsoy, M.; Basar, D. Visante Anterior Segment OCT in a Patient with Gas Bubbles in the Anterior Chamber after Femtosecond Laser Corneal Flap Formation. Int. Ophthalmol. 2010, 30, 81–84. [Google Scholar] [CrossRef]
- Kanclerz, P.; Grzybowski, A. Does Corneal Refractive Surgery Increase the Risk of Retinal Detachment? A Literature Review and Statistical Analysis. J. Refract. Surg. 2019, 35, 517–524. [Google Scholar] [CrossRef]
- Grzybowski, A.; Kanclerz, P. Early Postoperative Intraocular Pressure Elevation Following Cataract Surgery. Curr. Opin. Ophthalmol. 2019, 30, 56–62. [Google Scholar] [CrossRef]
- Hernández-Verdejo, J.L.; Teus, M.A.; Román, J.M.; Bolívar, G. Porcine Model to Compare Real-Time Intraocular Pressure during LASIK with a Mechanical Microkeratome and Femtosecond Laser. Invest. Ophthalmol. Vis. Sci. 2007, 48, 68–72. [Google Scholar] [CrossRef]
- Winkler von Mohrenfels, C.; Khoramnia, R.; Maier, M.M.; Pfäffl, W.; Hölzlwimmer, G.; Lohmann, C. [Cut quality of a new femtosecond laser system]. Klin. Monbl. Augenheilkd. 2009, 226, 470–474. [Google Scholar] [CrossRef]
- Stulting, R.D.; Carr, J.D.; Thompson, K.P.; Waring, G.O., III; Wiley, W.M.; Walker, J.G. Complications of Laser in Situ Keratomileusis for the Correction of Myopia. Ophthalmology 1999, 106, 13–20. [Google Scholar] [CrossRef]
- Chan, A.; Ou, J.; Manche, E.E. Comparison of the Femtosecond Laser and Mechanical Keratome for Laser in Situ Keratomileusis. Arch. Ophthalmol. 2008, 126, 1484–1490. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Jin, H.-Y.; Suo, Y.; Patel, S.V.; Montés-Micó, R.; Manche, E.E.; Xu, X. Femtosecond Laser versus Mechanical Microkeratome Laser in Situ Keratomileusis for Myopia: Metaanalysis of Randomized Controlled Trials. J. Cataract Refract. Surg. 2011, 37, 2151–2159. [Google Scholar] [CrossRef]
- Moshirfar, M.; Gardiner, J.P.; Schliesser, J.A.; Espandar, L.; Feiz, V.; Mifflin, M.D.; Chang, J.C. Laser in Situ Keratomileusis Flap Complications Using Mechanical Microkeratome versus Femtosecond Laser: Retrospective Comparison. J. Cataract Refract. Surg. 2010, 36, 1925–1933. [Google Scholar] [CrossRef]
- Tomita, M.; Sotoyama, Y.; Yukawa, S.; Nakamura, T. Comparison of DLK Incidence after Laser in Situ Keratomileusis Associated with Two Femtosecond Lasers: Femto LDV and IntraLase FS60. Clin. Ophthalmol. 2013, 7, 1365–1371. [Google Scholar] [CrossRef]
- Santhiago, M.R.; Kara-Junior, N.; Waring, G.O., 4th. Microkeratome versus Femtosecond Flaps: Accuracy and Complications. Curr. Opin. Ophthalmol. 2014, 25, 270–274. [Google Scholar] [CrossRef]
- De Paula, F.H.; Khairallah, C.G.; Niziol, L.M.; Musch, D.C.; Shtein, R.M. Diffuse Lamellar Keratitis after Laser in Situ Keratomileusis with Femtosecond Laser Flap Creation. J. Cataract Refract. Surg. 2012, 38, 1014–1019. [Google Scholar] [CrossRef]
- Dos Santos, A.M.; Torricelli, A.A.M.; Marino, G.K.; Garcia, R.; Netto, M.V.; Bechara, S.J.; Wilson, S.E. Femtosecond Laser-Assisted LASIK Flap Complications. J. Refract. Surg. 2016, 32, 52–59. [Google Scholar] [CrossRef]
- Ng, E.Y.J.; Thinagaran, S.; Kinsella, F.; O’Keefe, M. Prophylaxis of Diffuse Lamellar Keratitis with Intraoperative Interface Steroids in LASIK. J. Refract. Surg. 2009, 25, 306–311. [Google Scholar]
- Stonecipher, K.G.; Dishler, J.G.; Ignacio, T.S.; Binder, P.S. Transient Light Sensitivity after Femtosecond Laser Flap Creation: Clinical Findings and Management. J. Cataract Refract. Surg. 2006, 32, 91–94. [Google Scholar] [CrossRef]
- Muñoz, G.; Albarrán-Diego, C.; Sakla, H.F.; Javaloy, J.; Alió, J.L. Transient Light-Sensitivity Syndrome after Laser in Situ Keratomileusis with the Femtosecond Laser Incidence and Prevention. J. Cataract Refract. Surg. 2006, 32, 2075–2079. [Google Scholar] [CrossRef]
- Riau, A.K.; Liu, Y.-C.; Lwin, N.C.; Ang, H.P.; Tan, N.Y.S.; Yam, G.H.F.; Tan, D.T.; Mehta, J.S. Comparative Study of nJ- and μJ-Energy Level Femtosecond Lasers: Evaluation of Flap Adhesion Strength, Stromal Bed Quality, and Tissue Responses. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3186–3194. [Google Scholar] [CrossRef]
- Weisberg, M. GAPP Syndrome. In Difficult and Complicated Cases in Refractive Surgery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 167–169. [Google Scholar]
- Knorz, M.C.; Vossmerbaeumer, U. Comparison of Flap Adhesion Strength Using the Amadeus Microkeratome and the IntraLase iFS Femtosecond Laser in Rabbits. J. Refract. Surg. 2008, 24, 875–878. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, M.J.; Kim, T.-I.; Choi, H.-J.; Pak, J.H.; Tchah, H. A Femtosecond Laser Creates a Stronger Flap than a Mechanical Microkeratome. Invest. Ophthalmol. Vis. Sci. 2006, 47, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Clare, G.; Moore, T.C.B.; Grills, C.; Leccisotti, A.; Moore, J.E.; Schallhorn, S. Early Flap Displacement after LASIK. Ophthalmology 2011, 118, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.S.; Leung, A.T.; Wu, J.T.; Cheng, A.C.; Fan, D.S.; Rao, S.K.; Talamo, J.H.; Barraquer, C. Management of Severe Flap Wrinkling or Dislodgment after Laser in Situ Keratomileusis. J. Cataract Refract. Surg. 1999, 25, 1441–1447. [Google Scholar] [CrossRef]
- Lee, K.-W.; Joo, C.-K. Clinical Results of Laser in Situ Keratomileusis with Superior and Nasal Hinges. J. Cataract Refract. Surg. 2003, 29, 457–461. [Google Scholar] [CrossRef]
- Li, H.; Sun, T.; Wang, M.; Zhao, J. Safety and Effectiveness of Thin-Flap LASIK Using a Femtosecond Laser and Microkeratome in the Correction of High Myopia in Chinese Patients. J. Refract. Surg. 2010, 26, 99–106. [Google Scholar] [CrossRef]
- Tanna, M.; Schallhorn, S.C.; Hettinger, K.A. Femtosecond Laser versus Mechanical Microkeratome: A Retrospective Comparison of Visual Outcomes at 3 Months. J. Refract. Surg. 2009, 25, S668–S671. [Google Scholar] [CrossRef]
- Moshirfar, M.; Desautels, J.D.; Quist, T.S.; Skanchy, D.F.; Williams, M.T.; Wallace, R.T. Rainbow Glare after Laser-Assisted in Situ Keratomileusis: A Review of Literature. Clin. Ophthalmol. 2016, 10, 2245–2249. [Google Scholar] [CrossRef]
- Bamba, S.; Rocha, K.M.; Ramos-Esteban, J.C.; Krueger, R.R. Incidence of Rainbow Glare after Laser in Situ Keratomileusis Flap Creation with a 60 kHz Femtosecond Laser. J. Cataract Refract. Surg. 2009, 35, 1082–1086. [Google Scholar] [CrossRef]
- Kymionis, G.D.; Kontadakis, G.A.; Naoumidi, I.; Kankariya, V.P.; Panagopoulou, S.; Manousaki, A.; Grentzelos, M.A.; Pallikaris, I.G. Comparative Study of Stromal Bed of LASIK Flaps Created with Femtosecond Lasers (IntraLase FS150, WaveLight FS200) and Mechanical Microkeratome. Br. J. Ophthalmol. 2014, 98, 133–137. [Google Scholar] [CrossRef]
- Sarayba, M.A.; Ignacio, T.S.; Binder, P.S.; Tran, D.B. Comparative Study of Stromal Bed Quality by Using Mechanical, IntraLase Femtosecond Laser 15- and 30-kHz Microkeratomes. Cornea 2007, 26, 446–451. [Google Scholar] [CrossRef]
- Sarayba, M.A.; Ignacio, T.S.; Tran, D.B.; Binder, P.S. A 60 kHz IntraLase Femtosecond Laser Creates a Smoother LASIK Stromal Bed Surface Compared to a Zyoptix XP Mechanical Microkeratome in Human Donor Eyes. J. Refract. Surg. 2007, 23, 331–337. [Google Scholar] [CrossRef]
- Krueger, R.R.; Thornton, I.L.; Xu, M.; Bor, Z.; van den Berg, T.J.T.P. Rainbow Glare as an Optical Side Effect of IntraLASIK. Ophthalmology 2008, 115, 1187–1195.e1. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.-G. High Incidence of Rainbow Glare after Femtosecond Laser Assisted-LASIK Using the Upgraded FS200 Femtosecond Laser. BMC Ophthalmol. 2018, 18, 71. [Google Scholar] [CrossRef]
- Gatinel, D.; Saad, A.; Guilbert, E.; Rouger, H. Unilateral Rainbow Glare after Uncomplicated Femto-LASIK Using the FS-200 Femtosecond Laser. J. Refract. Surg. 2013, 29, 498–501. [Google Scholar] [CrossRef]
- Gatinel, D.; Saad, A.; Guilbert, E.; Rouger, H. Simultaneous Correction of Unilateral Rainbow Glare and Residual Astigmatism by Undersurface Flap Photoablation after Femtosecond Laser-Assisted LASIK. J. Refract. Surg. 2015, 31, 406–410. [Google Scholar] [CrossRef][Green Version]
- Recep, O.F.; Cağil, N.; Hasiripi, H. Outcome of Flap Subluxation after Laser in Situ Keratomileusis: Results of 6 Month Follow-Up. J. Cataract Refract. Surg. 2000, 26, 1158–1162. [Google Scholar] [CrossRef]
- Sutton, G.; Hodge, C. Accuracy and Precision of LASIK Flap Thickness Using the IntraLase Femtosecond Laser in 1000 Consecutive Cases. J. Refract. Surg. 2008, 24, 802–806. [Google Scholar] [CrossRef]
- Mostafaie, A.; Ahari, A.M.; Ghyassi, F.S.; Hajebrahimi, S.; Yousefi, M. Femtosecond Laser Versus Mechanical Microkeratome in Thin-Flap Laser in Situ Keratomileusis (Lasik) for Correction of Refractive Errors an Evidence-Based Effectiveness and Cost Analysis. J. Lasers Med. Sci 2011, 2, 6–11. [Google Scholar]
- Kanclerz, P.; Alio, J.L. The Benefits and Drawbacks of Femtosecond Laser-Assisted Cataract Surgery. Eur. J. Ophthalmol. 2020, 1120672120922448. [Google Scholar] [CrossRef]
- Krueger, R.R.; Meister, C.S. A Review of Small Incision Lenticule Extraction Complications. Curr. Opin. Ophthalmol. 2018, 29, 292–298. [Google Scholar] [CrossRef]
- Chan, C.; Lawless, M.; Sutton, G.; Versace, P.; Hodge, C. Small Incision Lenticule Extraction (SMILE) in 2015. Clin. Exp. Optom. 2016, 99, 204–212. [Google Scholar] [CrossRef]
- Alió Del Barrio, J.L.; El Zarif, M.; de Miguel, M.P.; Azaar, A.; Makdissy, N.; Harb, W.; El Achkar, I.; Arnalich-Montiel, F.; Alió, J.L. Cellular Therapy with Human Autologous Adipose-Derived Adult Stem Cells for Advanced Keratoconus. Cornea 2017, 36, 952–960. [Google Scholar] [CrossRef]
- Aristeidou, A.; Taniguchi, E.V.; Tsatsos, M.; Muller, R.; McAlinden, C.; Pineda, R.; Paschalis, E.I. The Evolution of Corneal and Refractive Surgery with the Femtosecond Laser. Eye Vis. 2015, 2, 12. [Google Scholar] [CrossRef]
- Santhiago, M.R.; Giacomin, N.T.; Smadja, D.; Bechara, S.J. Ectasia Risk Factors in Refractive Surgery. Clin. Ophthalmol. 2016, 10, 713–720. [Google Scholar] [CrossRef]
- Abdolahian, M.; Moalem, M.A.; Jahady Hoseiny, M.; Noorizadeh, F.; Zareei, A. Keratorefractive Surgery Outcomes in Keratoconus Suspect Patients. J. Ophthalmol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Moshirfar, M.; Smedley, J.G.; Muthappan, V.; Jarsted, A.; Ostler, E.M. Rate of Ectasia and Incidence of Irregular Topography in Patients with Unidentified Preoperative Risk Factors Undergoing Femtosecond Laser-Assisted LASIK. Clin. Ophthalmol. 2014, 8, 35–42. [Google Scholar] [CrossRef]
- Said, A.; Hamade, I.H.; Tabbara, K.F. Late Onset Corneal Ectasia after LASIK Surgery. Saudi J. Ophthalmol 2011, 25, 225–230. [Google Scholar] [CrossRef]
- Hersh, P.S.; Fry, K.L.; Bishop, D.S. Incidence and Associations of Retreatment after LASIK. Ophthalmology 2003, 110, 748–754. [Google Scholar] [CrossRef]
- Randleman, J.B.; White, A.J., Jr.; Lynn, M.J.; Hu, M.H.; Stulting, R.D. Incidence, Outcomes, and Risk Factors for Retreatment after Wavefront-Optimized Ablations with PRK and LASIK. J. Refract. Surg. 2009, 25, 273–276. [Google Scholar] [CrossRef]
- Kruh, J.N.; Garrett, K.A.; Huntington, B.; Robinson, S.; Melki, S.A. Risk Factors for Retreatment Following Myopic LASIK with Femtosecond Laser and Custom Ablation for the Treatment of Myopia. Semin. Ophthalmol. 2017, 32, 316–320. [Google Scholar] [CrossRef]
- Kezirian, G.M.; Stonecipher, K.G. Comparison of the IntraLase Femtosecond Laser and Mechanical Keratomes for Laser in Situ Keratomileusis. J. Cataract Refract. Surg. 2004, 30, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.-G.; Xia, Y.-J. Comparison of Corneal Flap Morphology Using AS-OCT in LASIK with the WaveLight FS200 Femtosecond Laser versus a Mechanical Microkeratome. J. Refract. Surg. 2013, 29, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Torky, M.A.; Al Zafiri, Y.A.; Khattab, A.M.; Farag, R.K.; Awad, E.A. Visumax Femtolasik versus Moria M2 Microkeratome in Mild to Moderate Myopia: Efficacy, Safety, Predictability, Aberrometric Changes and Flap Thickness Predictability. BMC Ophthalmol. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Karabela, Y.; Muftuoglu, O.; Kaya, F. Corneal Flap Thickness with the Moria M2 Single-Use Head 90 Microkeratome in 72 Consecutive LASIK Procedures. Clin. Ophthalmol. 2017, 11, 487–492. [Google Scholar] [CrossRef][Green Version]
- Binder, P.S. Flap Dimensions Created with the IntraLase FS Laser. J. Cataract Refract. Surg. 2004, 30, 26–32. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanclerz, P.; Khoramnia, R. Flap Thickness and the Risk of Complications in Mechanical Microkeratome and Femtosecond Laser In Situ Keratomileusis: A Literature Review and Statistical Analysis. Diagnostics 2021, 11, 1588. https://doi.org/10.3390/diagnostics11091588
Kanclerz P, Khoramnia R. Flap Thickness and the Risk of Complications in Mechanical Microkeratome and Femtosecond Laser In Situ Keratomileusis: A Literature Review and Statistical Analysis. Diagnostics. 2021; 11(9):1588. https://doi.org/10.3390/diagnostics11091588
Chicago/Turabian StyleKanclerz, Piotr, and Ramin Khoramnia. 2021. "Flap Thickness and the Risk of Complications in Mechanical Microkeratome and Femtosecond Laser In Situ Keratomileusis: A Literature Review and Statistical Analysis" Diagnostics 11, no. 9: 1588. https://doi.org/10.3390/diagnostics11091588
APA StyleKanclerz, P., & Khoramnia, R. (2021). Flap Thickness and the Risk of Complications in Mechanical Microkeratome and Femtosecond Laser In Situ Keratomileusis: A Literature Review and Statistical Analysis. Diagnostics, 11(9), 1588. https://doi.org/10.3390/diagnostics11091588







