Sequential Application of Lugol’s Iodine Test after Acetic Acid for Detecting Cervical Dysplasia: A Prospective Cohort Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design, Setting, and Participants
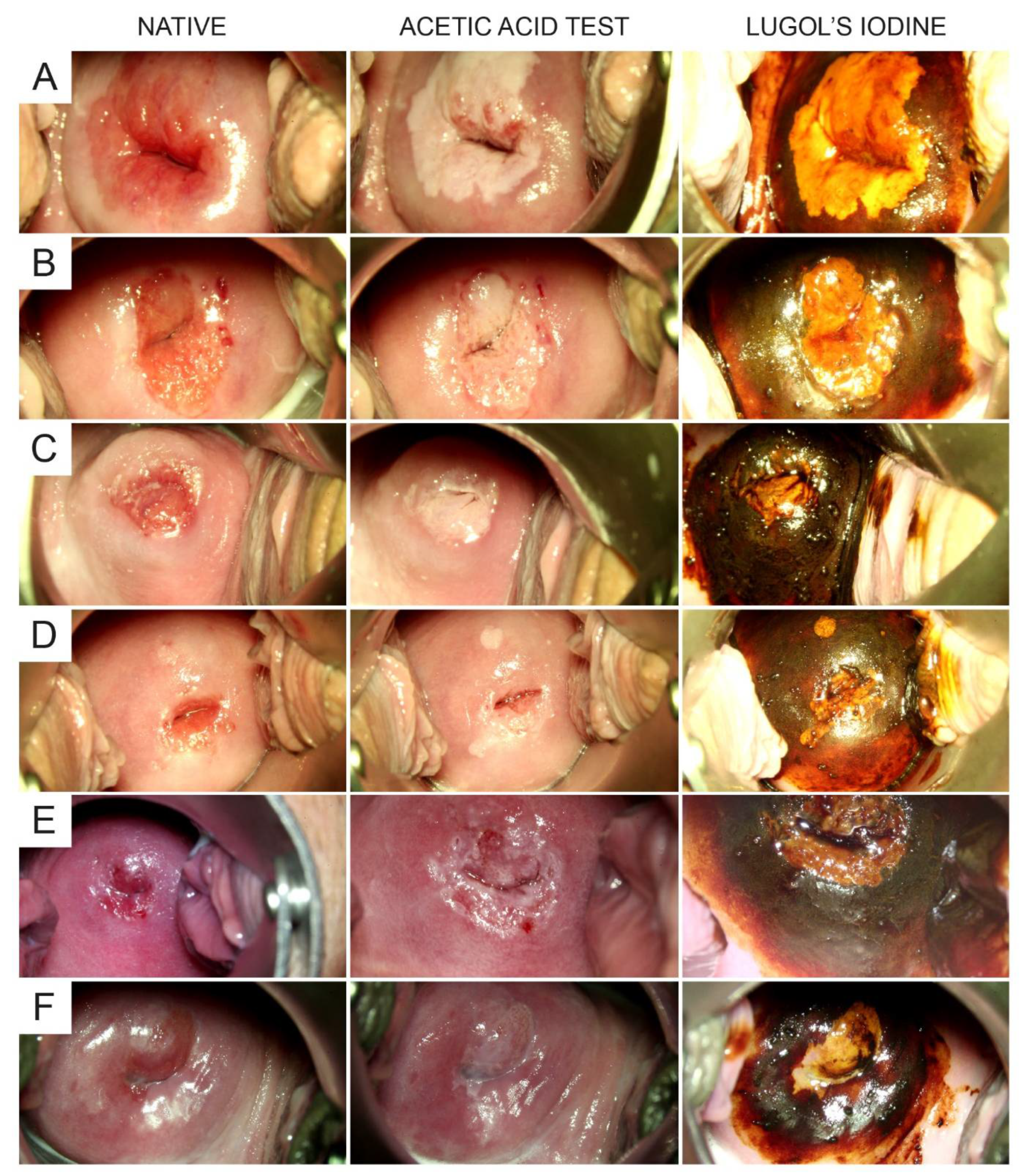
2.2. Test Methods and Variables
2.3. Analysis
2.4. Study Size
2.5. Data Management and Statistical Methods
3. Results
3.1. Patients, Colposcopies, and Biopsies
3.2. Sensitivity and Specificity of Lugol’s Iodine to Detect LSIL/HSIL
3.3. Optimal Time Point for the Assessment of Iodine Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, M.A.; Limaiem, F. Cervical Intraepithelial Squamous Cell Lesion; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cooper, D.B.; McCathran, C.E. Cervical Dysplasia; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wentzensen, N.; Schiffman, M. Filling a gap in cervical cancer screening programmes. Lancet Oncol. 2014, 15, 249–251. [Google Scholar] [CrossRef][Green Version]
- Shin, M.B.; Liu, G.; Mugo, N.; Garcia, P.J.; Rao, D.W.; Bayer, C.J.; Eckert, L.O.; Pinder, L.F.; Wasserheit, J.N.; Barnabas, R.V. A Framework for Cervical Cancer Elimination in Low-and-Middle-Income Countries: A Scoping Review and Roadmap for Interventions and Research Priorities. Front. Public Health 2021, 9, 670032. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Yang, J.; Cao, D.; Lang, J.; Shen, K. A systematic review of quality of life and sexual function of patients with cervical cancer after treatment. Int. J. Gynecol. Cancer 2014, 24, 1146–1157. [Google Scholar] [CrossRef]
- Vistad, I.; Fosså, S.D.; Dahl, A.A. A critical review of patient-rated quality of life studies of long-term survivors of cervical cancer. Gynecol. Oncol. 2006, 102, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, E.P.; Vesco, K.K.; Eder, M.; Lin, J.S.; Senger, C.A.; Burda, B.U. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011, 155, 687–697. [Google Scholar] [CrossRef]
- Soheili, M.; Keyvani, H.; Soheili, M.; Nasseri, S. Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med. J. Islam. Repub. Iran 2021, 35, 65. [Google Scholar] [CrossRef]
- Arbyn, M.; Simon, M.; Peeters, E.; Xu, L.; Meijer, C.J.L.M.; Berkhof, J.; Cuschieri, K.; Bonde, J.; Vanlencak, A.O.; Zhao, F.-H.; et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Khan, M.J.; Werner, C.L.; Darragh, T.M.; Guido, R.S.; Mathews, C.; Moscicki, A.-B.; Mitchell, M.M.; Schiffman, M.; Wentzensen, N.; Massad, L.S.; et al. ASCCP Colposcopy Standards: Role of Colposcopy, Benefits, Potential Harms, and Terminology for Colposcopic Practice. J. Low. Genit. Tract Dis. 2017, 21, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.B.; Goyal, M. Colposcopy; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Redman, C.W.E.; Kesic, V.; Cruickshank, M.E.; Gultekin, M.; Carcopino, X.; Sanchez, M.C.; Grigore, M.; Jakobsson, M.; Kuppers, V.; Pedro, A.; et al. European consensus statement on essential colposcopy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 57–62. [Google Scholar] [CrossRef]
- Mayeaux, E.J.; Novetsky, A.P.; Chelmow, D.; Choma, K.; Garcia, F.; Liu, A.H.; Papasozomenos, T.; Einstein, M.H. Systematic Review of International Colposcopy Quality Improvement Guidelines. J. Low. Genit. Tract Dis. 2017, 21, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Reich, O.; Pickel, H. 200 years of diagnosis and treatment of cervical precancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 165–171. [Google Scholar] [CrossRef]
- Waxman, A.G.; Conageski, C.; Silver, M.I.; Tedeschi, C.; Stier, E.A.; Apgar, B.; Huh, W.K.; Wentzensen, N.; Massad, L.S.; Khan, M.J.; et al. ASCCP Colposcopy Standards: How Do We Perform Colposcopy? Implications for Establishing Standards. J. Low. Genit. Tract Dis. 2017, 21, 235–241. [Google Scholar] [CrossRef]
- Catarino, R.; Schäfer, S.; Vassilakos, P.; Petignat, P.; Arbyn, M. Accuracy of combinations of visual inspection using acetic acid or lugol iodine to detect cervical precancer: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Hilal, Z.; Tempfer, C.B.; Burgard, L.; Rehman, S.; Rezniczek, G.A. How long is too long? Application of acetic acid during colposcopy: A prospective study. Am. J. Obstet. Gynecol. 2020, 223, 101.e1–101.e8. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Bentley, J.; Bösze, P.; Girardi, F.; Haefner, H.; Menton, M.; Perrotta, M.; Prendiville, W.; Russell, P.; Sideri, M.; et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet. Gynecol. 2012, 120, 166–172. [Google Scholar] [CrossRef]
- OnkoZert. Erhebungsbogen “Dysplasie-Einheit”. Available online: https://www.onkozert.de/organ/gyn/ (accessed on 30 May 2021).
- Beckmann, M.W.; Quaas, J.; Bischofberger, A.; Kämmerle, A.; Lux, M.P.; Wesselmann, S. Establishment of the Certification System “Gynaecological Dysplasia” in Germany. Geburtshilfe Frauenheilkd. 2014, 74, 860–867. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Reich, O.; Pickel, H. 100 years of iodine testing of the cervix: A critical review and implications for the future. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 261, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dou, Y.; Wang, M.; Li, Y.; Wang, F.; Xie, X.; Wang, X. A retrospective analysis on 1901 women with high grade cervical intraepithelial neoplasia by colposcopic biopsy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 217, 53–58. [Google Scholar] [CrossRef]
- Nayak, P.K.; Mitra, S.; Agrawal, S.; Hussain, N.; Thakur, P.; Mishra, B. Role of various screening techniques in detecting preinvasive lesions of the cervix among symptomatic women and women having unhealthy cervix. J. Cancer Res. Ther. 2021, 17, 180–185. [Google Scholar] [CrossRef]
- Ferris, D.G.; Ho, T.H.; Guijon, F.; Macfee, M.S.; Guerra, D.M.; Barrasso, R.; Duncan, I.D.; Litaker, M.S. A comparison of colposcopy using optical and video colposcopes. J. Low. Genit. Tract Dis. 2000, 4, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sabrina, F.; Ilkka, K.; Mervi, H.-N.; Charles, R.; Simon, L.; Ameli, T.; Esther, M.; Maria, K.; Eeva, P.; Pekka, N. Fostering Prevention of Cervical Cancer by a Correct Diagnosis of Precursors: A Structured Case-Based Colposcopy Course in Finland, Norway and UK. Cancers 2020, 12, 3201. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, N.; Lavitola, G.; Della Corte, L.; Bifulco, G. Diagnostic Accuracy of Endocervicoscopy in Identifying and Grading Cervical Intraepithelial Neoplasia Lesion. Gynecol. Obstet. Invest. 2020, 85, 196–205. [Google Scholar] [CrossRef]
- De Franciscis, P.; La Manna, V.; Schiattarella, A.; Ambrosio, D.; Labriola, D.; Procaccianti, R.; Ammaturo, F.P.; Morlando, M.; Torella, M. Diagnostic accuracy of endocervicoscopy as preoperative tool to improve the excisional treatment of cervical preneoplastic lesions. Minerva Ginecol. 2018, 70, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Marani, C.; Gardner, F.; Yeoh, C.C.; Akaev, I.; Votano, S. Endocervicoscopy and Biopsy to Detect Cervical Intraepithelial Squamous Neoplasia in Nonvisible Squamocolumnar Junction With Unsatisfactory Colposcopy: A Pilot Study. Technol. Cancer Res. Treat. 2018, 17, 1533034617753811. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristic | Value |
|---|---|
| Number of patients | 320 |
| Age (years) | 37.6 (32.1–45.5), range 20.4–83.6 |
| Body mass index (kg/m2) | 23.8 (21.4–28.0), range 16.0–48.6 (m * = 4) |
| Parity | 1 (0–2), range 0–6 (m = 3) |
| Allergies (yes/no) | 102 (32.0%)/217 (68.0%) (m = 1) |
| Currently smoking (yes/no) | 145 (45.9%)/171 (54.1%) (m = 4) |
| Ever smoked (yes/no) | 178 (56.3%)/138 (43.7%) (m = 4) |
| Alcohol abuse (yes/no) | 6 (1.9%)/312 (98.1%) (m = 2) |
| Drug abuse (yes/no) | 13 (4.1%)/306 (95.9%) (m = 1) |
| Concomitant disease (yes/no) | 131 (41.1%)/188 (58.9%) (m = 1) |
| Number of concomitant diseases | 0 (0–1), range 0–4 (m = 1) |
| Prescription drug use (yes/no) | 177 (55.5%)/142 (44.5%) (m = 1) |
| Number of prescription drugs | 1 (0–1), range 0–12 (m = 1) |
| Immunosuppressive conditions (yes/no) | 18 (5.7%)/299 (94.3%) (m = 3) |
| Type of transformation zone | |
| Types 1/2/3 | 182 (57.6%)/43 (13.6%)/91(28.8%) (m = 4) |
| Indication for colposcopy | |
| ASC-H | 21 (6.6%) |
| AGC endocervical favor neoplastic | 5 (1.6%) |
| LSIL | 121 (37.8%) |
| HSIL | 173 (54.1%) |
| HPV status (pos/neg/unknown) | 197 (61.5%)/20 (6.3%)/103 (32.2%) |
| Colposcopic findings (after acetic acid test; RIO classification) | |
| Normal | 84 (26.3%) |
| Minor changes | 137 (42.8%) |
| Major changes | 97 (30.3%) |
| Suspicious for invasion | 1 (0.3%) |
| Non-specific | 1 (0.3%) |
| Colposcopic findings (after Lugol’s iodine) | |
| Iodine positive (dark brown staining only) | 73 (22.8%) |
| Iodine negative (yellow staining **) | 247 (77.2%) |
| Histological results | |
| Negative for dysplasia | 77 (24.1%) |
| LSIL | 88 (27.5%) |
| HSIL | 151 (47.2%) |
| AIS | 3 (0.9%) |
| Invasive cancer | 1 (0.3%) |
| Item | Value |
|---|---|
| Number of patients | 320 |
| With 0 cervical biopsies (ECC only) | 10 (3.1%) |
| With 1 cervical biopsy | 76 (23.8%) |
| With 2 cervical biopsies | 107 (33.4%) |
| With 3 cervical biopsies | 119 (37.2%) |
| With 4 cervical biopsies | 8 (2.5%) |
| Cervical biopsies per patient | 2 (1–3); range 0–4 |
| Number of cervical biopsies | 679 |
| Acetic acid test positive | 469 (69.1%) |
| Lugol’s iodine test positive * | 524 (77.2%) |
| Both tests positive | 428 (63.0%) |
| Both tests negative | 114 (16.8%) |
| With pathologic finding | 415 (61.1%) |
| Acetic acid test positive | 316 (76.1%) |
| Lugol’s iodine test positive* | 338 (81.4%) |
| Both tests positive | 288 (69.4%) |
| Both tests negative | 49 (11.8%) |
| Outcome Item | Value |
|---|---|
| Sensitivity and specificity of Lugol’s iodine to identify LSIL/HSIL | |
| Sensitivity, % | 81.4 (77.3–85.0) |
| Specificity, % | 29.5 (24.2–35.5) |
| PPV, % | 64.5 (60.2–68.6) |
| NPV, % | 50.3 (42.2–58.4) |
| Accuracy, % | 61.3 |
| Additional lesions identified by Lugol’s iodine test * | 96 |
| No pathology | 46 (47.9%) |
| LSIL | 29 (30.2%) |
| HSIL ** | 21 (21.9%) |
| Number needed to biopsy | 1.92 |
| Number of patients with at least one additionally identified lesion by Lugol’s iodine test* | 66/320 (20.6%) |
| Clinical management change based on Lugol’s iodine *** | 17/320 (5.3%) |
| Sensitivity and specificity of Lugol’s iodine to identify healthy epithelium | |
| Sensitivity, % | 29.5 (24.2–35.5) |
| Specificity, % | 81.4 (77.3–85.0) |
| Item | Value | |
|---|---|---|
| Number of videos analysed, n | 233 | |
| Grading of Lugol’s iodine test | Rater 1 | Rater 2 |
| No iodine-negativity (dark brown staining only), n (%) | 42 (18.0%) | 32 (13.7%) |
| Iodine negative (yellow staining), * n (%) | 191 (82.0%) | 201 (86.3%) |
| Minimal yellow staining (grade 1) | 50 (26.2%) | 14 (7.0%) |
| Intermediate yellow staining (grade 2) | 40 (20.9%) | 52 (25.9%) |
| Intense yellow staining (grade 3) | 101 (52.9%) | 135 (67.2%) |
| Inter-rater agreement ** | ||
| Exact, % | 60.1 | |
| Grades 0/1 vs. grades 2/3, % | 76.8 | |
| Max. difference of 1 grade, % | 88.4 | |
| Fleiss’s kappa | 0.47 (95%-CI: 0.38–0.55) [moderate] | |
| Cronbach’s alpha | 0.83 [good] | |
| Correlation of grading and lesion severity | Rater 1 | Rater 2 |
| Spearman rank order correlation, *** CC; P | 0.300; <0.00001 | 0.308; <0.00001 |
| Chi square test ****, P | 0.295 | 0.078 |
| Fading of MSINL (yes/no), n (%) | 0 (0%)/191 | 0 (0%)/201 |
| Time to first appearance of MSINL within 10 seconds (yes/no), n (%) | 191 (100%)/0 | 201 (100%)/0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezniczek, G.A.; Ertan, S.; Rehman, S.; Tempfer, C.B. Sequential Application of Lugol’s Iodine Test after Acetic Acid for Detecting Cervical Dysplasia: A Prospective Cohort Study. Diagnostics 2021, 11, 1598. https://doi.org/10.3390/diagnostics11091598
Rezniczek GA, Ertan S, Rehman S, Tempfer CB. Sequential Application of Lugol’s Iodine Test after Acetic Acid for Detecting Cervical Dysplasia: A Prospective Cohort Study. Diagnostics. 2021; 11(9):1598. https://doi.org/10.3390/diagnostics11091598
Chicago/Turabian StyleRezniczek, Günther A., Samira Ertan, Sadia Rehman, and Clemens B. Tempfer. 2021. "Sequential Application of Lugol’s Iodine Test after Acetic Acid for Detecting Cervical Dysplasia: A Prospective Cohort Study" Diagnostics 11, no. 9: 1598. https://doi.org/10.3390/diagnostics11091598
APA StyleRezniczek, G. A., Ertan, S., Rehman, S., & Tempfer, C. B. (2021). Sequential Application of Lugol’s Iodine Test after Acetic Acid for Detecting Cervical Dysplasia: A Prospective Cohort Study. Diagnostics, 11(9), 1598. https://doi.org/10.3390/diagnostics11091598






