TEG® and ROTEM® Traces: Clinical Applications of Viscoelastic Coagulation Monitoring in Neonatal Intensive Care Unit
Abstract
1. Background
2. Methods
2.1. Thromboelastography (TEG®)
2.2. Thromboelastometry (ROTEM®)
3. Developmental Hemostasis: The Neonatal Coagulation System
4. Viscoelastic Testing Results from Healthy Neonates
4.1. TEG®/ROTEM® Results from Cord Blood Samples
4.2. TEG®/ROTEM® Results from Newborns’ Blood Samples
4.3. Viscoelastic Testing in Sick Neonatal Population
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartmann, J.; Murphy, M.; Dias, J.D. Viscoelastic Hemostatic Assays: Moving from the Laboratory to the Site of Care—A Review of Established and Emerging Technologies. Diagnostics 2020, 10, 118. [Google Scholar] [CrossRef]
- Crochemore, T.; Piza, F.M.D.T.; Rodrigues, R.D.R.; Guerra, J.C.D.C.; Ferraz, L.J.R.; Corrêa, T.D. A new era of thromboelastometry. Einstein 2017, 15, 380–385. [Google Scholar] [CrossRef]
- Görlinger, K.; Dirkmann, D.; Hanke, A. Rotational thromboelastometry (ROTEM®). In Trauma Induced Coagulopathy; Gonzalez, E., Moore, H., Moore, E., Eds.; Springer: Cham, Switzerland, 2016; pp. 267–298. [Google Scholar]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef]
- Chen, A.; Teruya, J. Global Hemostasis Testing Thromboelastography: Old Technology, New Applications. Clin. Lab. Med. 2009, 29, 391–407. [Google Scholar] [CrossRef]
- Hoffman, M.; Monroe, D.M., 3rd. A cell-based model of hemostasis. Thromb Haemost. 2001, 85, 958–965. [Google Scholar]
- Haas, T.; Fries, D.; Tanaka, K.; Asmis, L.; Curry, N.; Schöchl, H. Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding: Is there any evidence? Br. J. Anaesth. 2015, 114, 217–224. [Google Scholar] [CrossRef]
- Shen, L.; Tabaie, S.; Ivascu, N. Viscoelastic testing inside and beyond the operating room. J. Thorac. Dis. 2017, 9, S299–S308. [Google Scholar] [CrossRef]
- Dhara, S.; Moore, E.E.; Yaffe, M.B.; Moore, H.B.; Barrett, C.D. Modern Management of Bleeding, Clotting, and Coagulopathy in Trauma Patients: What Is the Role of Viscoelastic Assays? Curr. Trauma Rep. 2020, 6, 69–81. [Google Scholar] [CrossRef]
- Park, M.S.; Martini, W.Z.; Dubick, M.A.; Salinas, J.; Butenas, S.; Kheirabadi, B.S.; Pusateri, A.E.; Vos, J.A.; Guymon, C.H.; Wolf, S.; et al. Thromboelastography as a Better Indicator of Hypercoagulable State After Injury Than Prothrombin Time or Activated Partial Thromboplastin Time. J. Trauma Inj. Infect. Crit. Care 2009, 67, 266–276. [Google Scholar] [CrossRef]
- Konstantinidi, A.; Sokou, R.; Parastatidou, S.; Lampropoulou, K.; Katsaras, G.; Boutsikou, T.; Gounaris, A.K.; Tsantes, A.E.; Iacovidou, N. Clinical Application of Thromboelastography/Thromboelastometry (TEG/TEM) in the Neonatal Population: A Narrative Review. Semin. Thromb. Hemost. 2019, 45, 449–457. [Google Scholar] [CrossRef]
- Radicioni, M.; Mezzetti, D.; Del Vecchio, A.; Motta, M. Thromboelastography: Might work in neonatology too? J. Matern. Neonatal Med. 2012, 25, 10–13. [Google Scholar] [CrossRef]
- Simurda, T.; Zolkova, J.; Kolkova, Z.; Loderer, D.; Dobrotova, M.; Skornova, I.; Brunclíkova, M.; Grendar, M.; Lasabova, Z.; Stasko, J.; et al. Comparison of clinical phenotype with genetic and laboratory results in 31 patients with congenital dysfibrinogenemia in northern Slovakia. Int. J. Hematol. 2020, 111, 795–802. [Google Scholar] [CrossRef]
- Hartert, H. Blutgerinnungsstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. J. Mol. Med. 1948, 26, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Haemonetics TEG. Available online: https://teg.haemonetics.com/en/teg-5000-thrombelastograph (accessed on 10 May 2021).
- National Institute for Health and Care Excellence. Detecting, Managing and Monitoring Haemostasis: Viscoelastometric Point-of-Care Testing (ROTEM, TEG and Sonoclot Systems). NICE Diagnostics Guidance [DG13]. 2014. Available online: www.nice.org.uk/guidance/dg13 (accessed on 10 May 2021).
- Ganter, M.T.; Hofer, C.K. Coagulation Monitoring: Current Techniques and Clinical Use of Viscoelastic Point-of-Care Coagulation Devices. Anesth. Analg. 2008, 106, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Blombäck, B.; Bark, N. Fibrinopeptides and fibrin gel structure. Biophys. Chem. 2004, 112, 147–151. [Google Scholar] [CrossRef]
- Evans, P.A.; Hawkins, K.; Lawrence, M.; Williams, R.L.; Barrow, M.S.; Thirumalai, N.; Williams, P.R. Rheometry for blood coagulation studies. Med. Eng. Phys. 2008, 30, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.P.; Kitchen, S.; Jennings, I.; Woods, T.; Walker, I. Quality Assurance and Quality Control of Thrombelastography and Rotational Thromboelastometry: The UK NEQAS for Blood Coagulation Experience. Semin. Thromb. Hemost. 2010, 36, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Aleshnick, M.; Orfeo, T.; Brummel-Ziedins, K.; Gissel, M.; Mann, K. Interchangeability of rotational elastographic instruments and reagents. J. Trauma Acute Care Surg. 2014, 76, 107–113. [Google Scholar] [CrossRef]
- Werfen ROTEM Delta. Available online: https://www.instrumentationlaboratory.com/en/rotem-delta (accessed on 10 May 2021).
- Simurda, T.; Vilar, R.; Zolkova, J.; Ceznerova, E.; Kolkova, Z.; Loderer, D.; Neerman-Arbez, M.; Casini, A.; Brunclikova, M.; Skornova, I.; et al. A Novel Nonsense Mutation in FGB (c.1421G>A; p.Trp474Ter) in the Beta Chain of Fibrinogen Causing Hypofibrinogenemia with Bleeding Phenotype. Biomedicines 2020, 8, 605. [Google Scholar] [CrossRef]
- Andrew, M.; Paes, B.; Milner, R.; Johnston, M.; Mitchell, L.; Tollefsen, D.M.; Powers, P. Development of the human coagulation system in the full-term infant. Blood 1987, 70, 165–172. [Google Scholar] [CrossRef]
- Andrew, M.; Paes, B.; Milner, R.; Johnston, M.; Mitchell, L.; Tollefsen, D.M.; Castle, V.; Powers, P. Development of the human coagulation system in the healthy premature infant. Blood 1988, 72, 1651–1657. [Google Scholar] [CrossRef]
- Andrew, M.; Vegh, P.; Johnston, M.; Bowker, J.; Ofosu, F.; Mitchell, L. Maturation of the hemostatic system during childhood. Blood 1992, 80, 1998–2005. [Google Scholar] [CrossRef]
- Andrew, M.; Paes, B.; Johnston, M. Development of the Hemostatic System in the Neonate and Young Infant. J. Pediatr. Hematol. 1990, 12, 95–104. [Google Scholar] [CrossRef]
- Andrew, M. Developmental Hemostasis: Relevance to Hemostatic Problems During Childhood. Semin. Thromb. Hemost. 1995, 21, 341–356. [Google Scholar] [CrossRef]
- Hoffman, M. How well do we really understand coagulation? Issues Hemost Manag. 2004, 1, 4–8. [Google Scholar]
- Cade, J.F.; Hirsh, J.; Martin, M. Placental Barrier to Coagulation Factors: Its Relevance to the Coagulation Defect at Birth and to Haemorrhage in the Newborn. BMJ 1969, 2, 281–283. [Google Scholar] [CrossRef][Green Version]
- Saleh, A.; Alshameeri, R.; O’Brien, J.; Munkarah, A.; Dombrowski, M.; Bottoms, S.; Cotton, D.; Mammen, E. Maternal and neonatal primary hemostasis. Thromb. Res. 1994, 73, 125–129. [Google Scholar] [CrossRef]
- Pichler, E.; Pichler, L. The neonatal coagulation system and the vitamin K deficiency bleeding—A mini review. Wien. Med. Wochenschr. 2008, 158, 385–395. [Google Scholar] [CrossRef]
- Monagle, P.; Ignjatovic, V.; Savoia, H. Hemostasis in neonates and children: Pitfalls and dilemmas. Blood Rev. 2010, 24, 63–68. [Google Scholar] [CrossRef]
- Attard, C.; Van Der Straaten, T.; Karlaftis, V.; Monagle, P.; Ignjatovic, V. Developmental hemostasis: Age-specific differences in the levels of hemostatic proteins. J. Thromb. Haemost. 2013, 11, 1850–1854. [Google Scholar] [CrossRef]
- Ignjatovic, V.; Pelkmans, L.; Kelchtermans, H.; Al Dieri, R.; Hemker, C.; Kremers, R.; Bloemen, S.; Karlaftis, V.; Attard, C.; de Laat, B.; et al. Differences in the mechanism of blood clot formation and nanostructure in infants and children compared with adults. Thromb. Res. 2015, 136, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Toulon, P.; Rainaut, M.; Aiach, M.; Roncato, M.; Daffos, F.; Forestier, F. Antithrombin III (ATIII) and Heparin Cofactor II (HCII) in Normal Human Fetuses (21st–27th Week). Thromb. Haemost. 1986, 56, 237. [Google Scholar] [CrossRef] [PubMed]
- Reverdiau-Moalic, P.; Delahousse, B.; Body, G.; Bardos, P.; Leroy, J.; Gruel, Y. Evolution of blood coagulation activators and inhibitors in the healthy human fetus. Blood 1996, 88, 900–906. [Google Scholar] [CrossRef]
- Summaria, L. Comparison of Human Normal, Full-Term, Fetal and Adult Plasminogen by Physical and Chemical Analyses. Pathophysiol. Haemost. Thromb. 1989, 19, 266–273. [Google Scholar] [CrossRef]
- Andrew, M.; Schmidt, B.; Mitchell, L.; Paes, B.; Ofosu, F. Thrombin Generation in Newborn Plasma Is Critically Dependent on the Concentration of Prothrombin. Thromb. Haemost. 1990, 63, 27–30. [Google Scholar] [CrossRef]
- Cvirn, G.; Gallistl, S.; Muntean, W. Effects of Antithrombin and Protein C on Thrombin Generation in Newborn and Adult Plasma. Thromb. Res. 1999, 93, 183–190. [Google Scholar] [CrossRef]
- Barnes, C.; Ignjatovic, V.; Furmedge, J.; Newall, F.; Chan, A.; De Rosa, L.; Hamilton, S.; Ragg, P.; Robinson, S.; Auldist, A.; et al. Developmental haemostasis. Thromb. Haemost. 2006, 95, 362–372. [Google Scholar] [CrossRef]
- Saxonhouse, M.A.; Sola, M.C. Platelet function in term and preterm neonates. Clin. Perinatol. 2004, 31, 15–28. [Google Scholar] [CrossRef]
- Levy-Shraga, Y.; Maayan-Metzger, A.; Lubetsky, A.; Shenkman, B.; Kuint, J.; Martinowitz, U.; Kenet, G. Platelet Function of Newborns as Tested by Cone and Plate(let) Analyzer Correlates with Gestational Age. Acta Haematol. 2006, 115, 152–156. [Google Scholar] [CrossRef]
- Rajasekhar, D.; Barnard, M.R.; Bednarek, F.J.; Michelson, A.D. Platelet Hyporeactivity in Very Low Birth Weight Neonates. Thromb. Haemost. 1997, 77, 1002–1007. [Google Scholar] [CrossRef]
- Israels, S.J.; Odaibo, F.S.; Robertson, C.; McMillan, E.M.; McNicol, A. Deficient Thromboxane Synthesis and Response in Platelets from Premature Infants. Pediatr. Res. 1997, 41, 218–223. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Latini, G.; Henry, E.; Christensen, R.D. Template bleeding times of 240 neonates born at 24 to 41 weeks gestation. J. Perinatol. 2008, 28, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Roschitz, B.; Sudi, K.; Köstenberger, M.; Muntean, W. Shorter PFA-100 closure times in neonates than in adults: Role of red cells, white cells, platelets and von Willebrand factor. Acta Paediatr. 2001, 90, 664–670. [Google Scholar] [CrossRef]
- Bednarek, F.J.; Bean, S.; Barnard, M.R.; Frelinger, A.; Michelson, A.D. The platelet hyporeactivity of extremely low birth weight neonates is age-dependent. Thromb. Res. 2009, 124, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Sola-Visner, M. Platelets in the neonatal period: Developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. Hematology 2012, 2012, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, M.C. Nucleated red blood cells in the fetus and newborn. Arch. Dis. Child.-Fetal Neonatal Ed. 2001, 84, F211–F215. [Google Scholar] [CrossRef]
- Katz, J.A.; Moake, J.L.; McPherson, P.D.; Weinstein, M.J.; Moise, K.J.; Carpenter, R.J.; Sala, D.J. Relationship between human development and disappearance of unusually large von Willebrand factor multimers from plasma. Blood 1989, 73, 1851–1858. [Google Scholar] [CrossRef]
- Edwards, R.M.; Naik-Mathuria, B.J.; Gay, A.N.; Olutoye, O.O.; Teruya, J. Parameters of Thromboelastography in Healthy Newborns. Am. J. Clin. Pathol. 2008, 130, 99–102. [Google Scholar] [CrossRef]
- Sidlik, R.; Strauss, T.; Morag, I.; Shenkman, B.; Tamarin, I.; Lubetsky, A.; Livnat, T.; Kenet, G. Assessment of Functional Fibrinolysis in Cord Blood Using Modified Thromboelastography. Pediatr. Blood Cancer 2016, 63, 839–843. [Google Scholar] [CrossRef]
- Strauss, T.; Levy-Shraga, Y.; Ravid, B.; Schushan-Eisen, I.; Maayan-Metzger, A.; Kuint, J.; Kenet, G. Clot formation of neonates tested by thromboelastography correlates with gestational age. Thromb. Haemost. 2010, 103, 344–350. [Google Scholar] [CrossRef]
- Cvirn, G.; Gallistl, S.; Kutschera, J.; Wagner, T.; Ferstl, U.; Jurgens, G.; Koestenberger, M. Clot Strength: A Comparison Between Cord and Adult Blood by Means of Thrombelastometry. J. Pediatr. Hematol. 2008, 30, 210–213. [Google Scholar] [CrossRef]
- Wiegele, M.; Kimberger, O.; Schaden, E.; Marhofer, P.; Baierl, A.; Willschke, H.; Triffterer, L. Establishing reference ranges of cord blood: Point-of-care hemostatic function assessment in preterm and term neonates. Pediatr. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Mirabella, L.; Cotoia, A.; Colacicco, G.; Tullo, L.; Salatto, P.; Mollica, G.; Mariano, K.; Dambrosio, M.; Cinnella, G. Reference values for coagulation assessment in full-term newborns. Minerva Anestesiol 2017, 83, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Schott, N.J.; Emery, S.P.; Garbee, C.; Waters, J. Thromboelastography in term neonates. J. Matern. Neonatal Med. 2018, 31, 2599–2604. [Google Scholar] [CrossRef]
- Motta, M.; Guaragni, B.; Pezzotti, E.; Rodriguez-Perez, C.; Chirico, G. Reference intervals of citrated-native whole blood thromboelastography in premature neonates. Early Hum. Dev. 2017, 115, 60–63. [Google Scholar] [CrossRef]
- Miller, B.E.; Bailey, J.M.; Mancuso, T.J.; Weinstein, M.S.; Holbrook, G.W.; Silvey, E.M.; Tosone, S.R.; Levy, J.H. Functional Maturity of the Coagulation System in Children. Anesth. Analg. 1997, 84, 745–748. [Google Scholar] [CrossRef]
- Sewell, E.K.; Forman, K.R.; Wong, E.C.C.; Gallagher, M.; Luban, N.L.C.; Massaro, A. Thromboelastography in term neonates: An alternative approach to evaluating coagulopathy. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 102, F79–F84. [Google Scholar] [CrossRef]
- Sokou, R.; Foudoulaki-Paparizos, L.; Lytras, T.; Konstantinidi, A.; Theodoraki, M.; Lambadaridis, I.; Gounaris, A.; Valsami, S.; Politou, M.; Gialeraki, A.; et al. Reference ranges of thromboelastometry in healthy full-term and pre-term neonates. Clin. Chem. Lab. Med. 2017, 55, 1592–1597. [Google Scholar] [CrossRef]
- Sokou, R.; Konstantinidi, A.; Stefanaki, C.; Tsantes, A.G.; Parastatidou, S.; Lampropoulou, K.; Katsaras, G.; Tavoulari, E.; Iacovidou, N.; Kyriakou, E.; et al. Thromboelastometry: Studying hemostatic profile in small for gestational age neonates—a pilot observational study. Eur. J. Nucl. Med. Mol. Imaging 2019, 178, 551–557. [Google Scholar] [CrossRef]
- Ravn, H.B.; Andreasen, J.B.; Hvas, A.-M. Does whole blood coagulation analysis reflect developmental haemostasis? Blood Coagul. Fibrinolysis 2017, 28, 218–223. [Google Scholar] [CrossRef]
- Theodoraki, M.; Sokou, R.; Valsami, S.; Iliodromiti, Z.; Pouliakis, A.; Parastatidou, S.; Karavana, G.; Ioakeimidis, G.; Georgiadou, P.; Iacovidou, N.; et al. Reference Values of Thrombolastometry Parameters in Healthy Term Neonates. Children 2020, 7, 259. [Google Scholar] [CrossRef]
- Kang, Y.G.; Martin, D.J.; Marquez, J.; Lewis, J.H.; Bontempo, F.A.; Shaw, B.W.; Starzl, T.E.; Winter, P.M. Intraoperative Changes in Blood Coagulation and Thrombelastographic Monitoring in Liver Transplantation. Anesth. Analg. 1985, 64, 888–896. [Google Scholar] [CrossRef]
- Mpaili, E.; Tsilimigras, D.I.; Moris, D.; Sigala, F.; Frank, S.M.; Hartmann, J.; Pawlik, T.M. Utility of viscoelastic coagulation testing in liver surgery: A systematic review. HPB 2021, 23, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Mallett, S.V. Clinical Utility of Viscoelastic Tests of Coagulation (TEG/ROTEM) in Patients with Liver Disease and during Liver Transplantation. Semin. Thromb. Hemost. 2015, 41, 527–537. [Google Scholar] [CrossRef]
- Agarwal, S.; Abdelmotieleb, M. Viscoelastic testing in cardiac surgery. Transfusion 2020, 60, S52–S60. [Google Scholar] [CrossRef]
- Gilbert, B.W.; Bissell, B.D.; Santiago, R.D.; Rech, M.A. Tracing the Lines: A Review of Viscoelastography for Emergency Medicine Clinicians. J. Emerg. Med. 2020, 59, 201–215. [Google Scholar] [CrossRef]
- Wikkelsø, A.; Wetterslev, J.; Møller, A.M.; Afshari, A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst. Rev. 2016, 2016, CD007871. [Google Scholar] [CrossRef]
- Amgalan, A.; Allen, T.; Othman, M.; Ahmadzia, H.K. Systematic review of viscoelastic testing (TEG/ROTEM) in obstetrics and recommendations from the women’s SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 1813–1838. [Google Scholar] [CrossRef]
- Gonzalez, E.; Moore, E.E.; Moore, H.B. Management of Trauma-Induced Coagulopathy with Thrombelastography. Crit. Care Clin. 2017, 33, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Drumheller, B.C.; Stein, D.; Moore, L.J.; Rizoli, S.B.; Cohen, M.J. Thromboelastography and rotational thromboelastometry for the surgical intensivist: A narrative review. J. Trauma Acute Care Surg. 2019, 86, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.; Stanworth, S.; Curry, N.; Woolley, T.; Cooper, C.; Ukoumunne, O.C.; Zhelev, Z.; Hyde, C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst. Rev. 2015, 2, CD010438. [Google Scholar] [CrossRef]
- Da Luz, L.T.; Nascimento, B.; Shankarakutty, A.K.; Rizoli, S.; Adhikari, N.K. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: Descriptive systematic review. Crit. Care 2014, 18, 1–26. [Google Scholar] [CrossRef]
- Müller, M.C.; Meijers, J.C.; Vroom, M.B.; Juffermans, N.P. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: A systematic review. Crit. Care 2014, 18, 1–11. [Google Scholar] [CrossRef]
- Bareille, M.; Hardy, M.; Douxfils, J.; Roullet, S.; Lasne, D.; Levy, J.; Stépanian, A.; Susen, S.; Frère, C.; Lecompte, T.; et al. Viscoelastometric Testing to Assess Hemostasis of COVID-19: A Systematic Review. J. Clin. Med. 2021, 10, 1740. [Google Scholar] [CrossRef]
- Phillips, R.C.; Shahi, N.; Leopold, D.; Levek, C.; Shirek, G.; Hilton, S.; Hyslop, R.; Gien, J.; Kinsella, J.P.; Buckvold, S.; et al. Thromboelastography-guided management of coagulopathy in neonates with congenital diaphragmatic hernia supported by extracorporeal membrane oxygenation. Pediatr. Surg. Int. 2020, 36, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.; Sullivan, J.E.; Myers, J.; Wells, T.; Calhoun, A.; Berkenbosch, J.; Tzanetos, D.T. Use of Thromboelastography to Predict Thrombotic Complications in Pediatric and Neonatal Extracorporeal Membranous Oxygenation. J. Extra-Corpor. Technol. 2018, 50, 149–154. [Google Scholar]
- Lissitchkov, T.; Madan, B.; Khayat, C.D.; Zozulya, N.; Ross, C.; Karimi, M.; Kavakli, K.; De Angulo, G.R.; Almomen, A.; Schwartz, B.A.; et al. Efficacy and safety of a new human fibrinogen concentrate in patients with congenital fibrinogen deficiency: An interim analysis of a Phase III trial. Transfusion 2018, 58, 413–422. [Google Scholar] [CrossRef]
- Simurda, T.; Casini, A.; Stasko, J.; Hudecek, J.; Skornova, I.; Vilar, R.; Neerman-Arbez, M.; Kubisz, P. Perioperative management of a severe congenital hypofibrinogenemia with thrombotic phenotype. Thromb. Res. 2020, 188, 1–4. [Google Scholar] [CrossRef]
- Cai, H.; Liang, M.; Yang, J.; Zhang, X. Congenital hypofibrinogenemia in pregnancy. Blood Coagul. Fibrinolysis 2018, 29, 155–159. [Google Scholar] [CrossRef]
- Peyvandi, F.; Haertel, S.; Knaub, S.; Mannucci, P.M. Incidence of bleeding symptoms in 100 patients with inherited afibrinogenemia or hypofibrinogenemia. J. Thromb. Haemost. 2006, 4, 1634–1637. [Google Scholar] [CrossRef]
- Nakayama, Y.; Nakajima, Y.; Tanaka, K.A.; Sessler, D.I.; Maeda, S.; Iida, J.; Ogawa, S.; Mizobe, T. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br. J. Anaesth. 2015, 114, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, D.; Willems, A.; Romlin, B.S.; Belisle, S.; Van der Linden, P. Development of a specific algorithm to guide haemostatic therapy in children undergoing cardiac surgery. Eur. J. Anaesthesiol. 2015, 32, 320–329. [Google Scholar] [CrossRef]
- Scott, J.P.; Niebler, R.A.; Stuth, E.A.E.; Newman, D.K.; Tweddell, J.S.; Bercovitz, R.; Benson, D.W.; Cole, R.; Simpson, P.M.; Yan, K.; et al. Rotational Thromboelastometry Rapidly Predicts Thrombocytopenia and Hypofibrinogenemia During Neonatal Cardiopulmonary Bypass. World J. Pediatr. Congenit. Hear. Surg. 2018, 9, 424–433. [Google Scholar] [CrossRef]
- De Moerloose, P.; Neerman-Arbez, M.; Casini, A. Clinical Features and Management of Congenital Fibrinogen Deficiencies. Semin. Thromb. Hemost. 2016, 42, 366–374. [Google Scholar] [CrossRef]
- Rahe-Meyer, N.; Solomon, C.; Winterhalter, M.; Piepenbrock, S.; Tanaka, K.; Haverich, A.; Pichlmaier, M. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 694–702. [Google Scholar] [CrossRef]
- Sokou, R.; Giallouros, G.; Konstantinidi, A.; Pantavou, K.; Nikolopoulos, G.; Bonovas, S.; Lytras, T.; Kyriakou, E.; Lambadaridis, I.; Gounaris, A.; et al. Thromboelastometry for diagnosis of neonatal sepsis-associated coagulopathy: An observational study. Eur. J. Nucl. Med. Mol. Imaging 2018, 177, 355–362. [Google Scholar] [CrossRef]
- Bauman, M.E.; Cheung, P.-Y.; Massicotte, M.P. Hemostasis and Platelet Dysfunction in Asphyxiated Neonates. J. Pediatr. 2011, 158, e35–e39. [Google Scholar] [CrossRef]
- Konstantinidi, A.; Sokou, R.; Tsantes, A.G.; Parastatidou, S.; Bonovas, S.; Kouskouni, E.; Gounaris, A.K.; Tsantes, A.E.; Iacovidou, N. Erratum: Thromboelastometry Variables in Neonates with Perinatal Hypoxia. Semin. Thromb. Hemost. 2020, 46, e1. [Google Scholar] [CrossRef]
- Pakvasa, M.A.; Winkler, A.M.; Hamrick, S.E.; Josephson, C.D.; Patel, R.M. Observational study of haemostatic dysfunction and bleeding in neonates with hypoxic–ischaemic encephalopathy. BMJ Open 2017, 7, e013787. [Google Scholar] [CrossRef]
- Raffaeli, G.; Tripodi, A.; Manzoni, F.; Scalambrino, E.; Pesenti, N.; Amodeo, I.; Cavallaro, G.; Villamor, E.; Peyvandi, F.; Mosca, F.; et al. Is placental blood a reliable source for the evaluation of neonatal hemostasis at birth? Transfusion 2020, 60, 1069–1077. [Google Scholar] [CrossRef]
- Raffaeli, G.; Tripodi, A.; Cavallaro, G.; Cortesi, V.; Scalambrino, E.; Pesenti, N.; Artoni, A.; Mosca, F.; Ghirardello, S. Thromboelastographic profiles of healthy very low birthweight infants serially during their first month. Arch. Dis. Child.-Fetal Neonatal Ed. 2019, 105, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, C.; Chen, X.; Wang, J.; Ke, Z.; Hu, H. Establishing a reference range for thromboelastograph parameters in the neonatal period. Int. J. Lab. Hematol. 2019, 41, 530–535. [Google Scholar] [CrossRef]
- Oswald, E.; Stalzer, B.; Heitz, E.; Weiss, M.; Schmugge, M.; Strasak, A.; Innerhofer, P.; Haas, T. Thromboelastometry (ROTEM®) in children: Age-related reference ranges and correlations with standard coagulation tests. Br. J. Anaesth. 2010, 105, 827–835. [Google Scholar] [CrossRef]
- Kettner, S.C.; Pollak, A.; Zimpfer, M.; Seybold, T.; Prusa, A.R.; Herkner, K.; Kuhle, S. Heparinase-Modified Thrombelastography in Term and Preterm Neonates. Anesth. Analg. 2004, 98, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
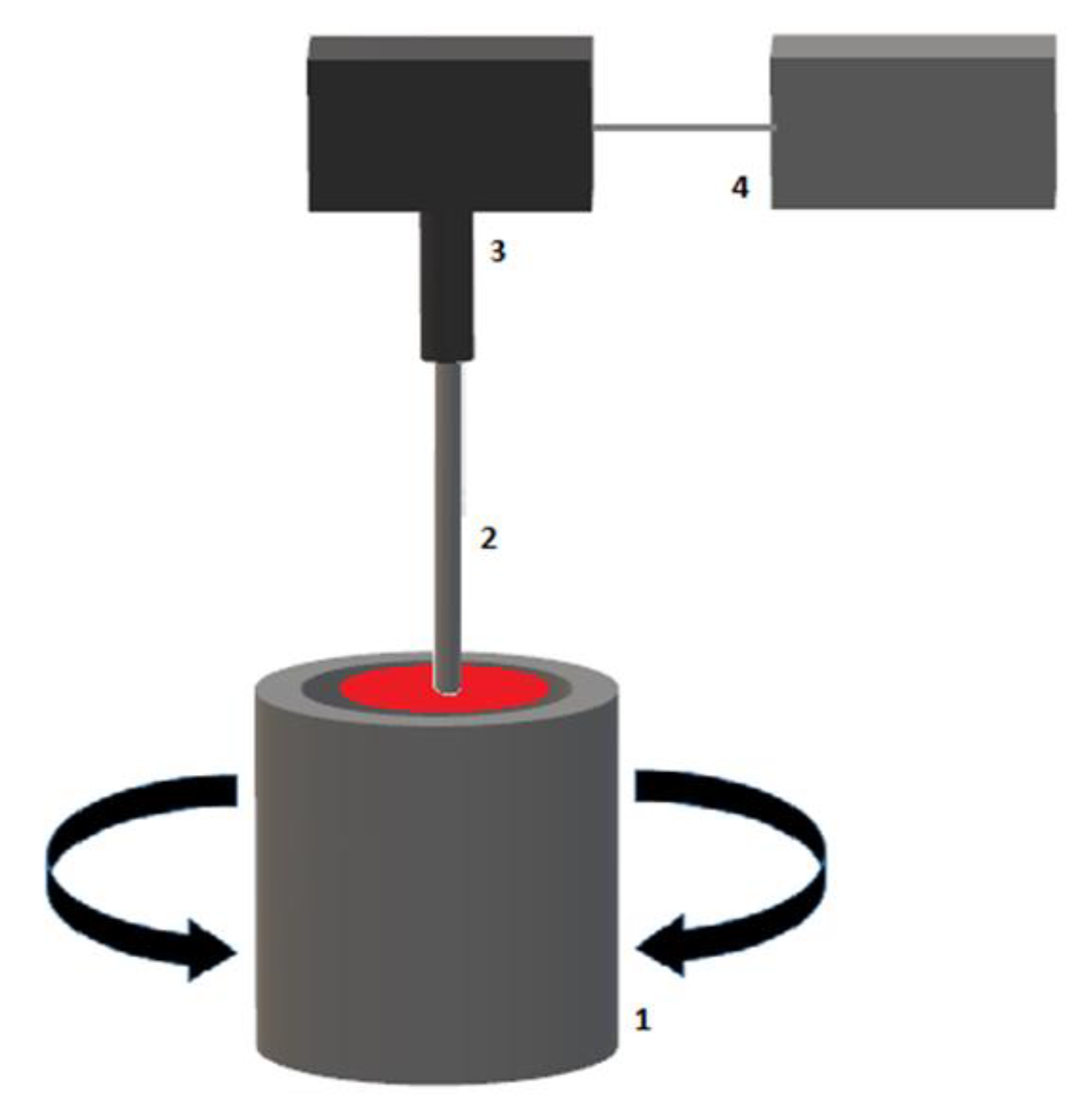
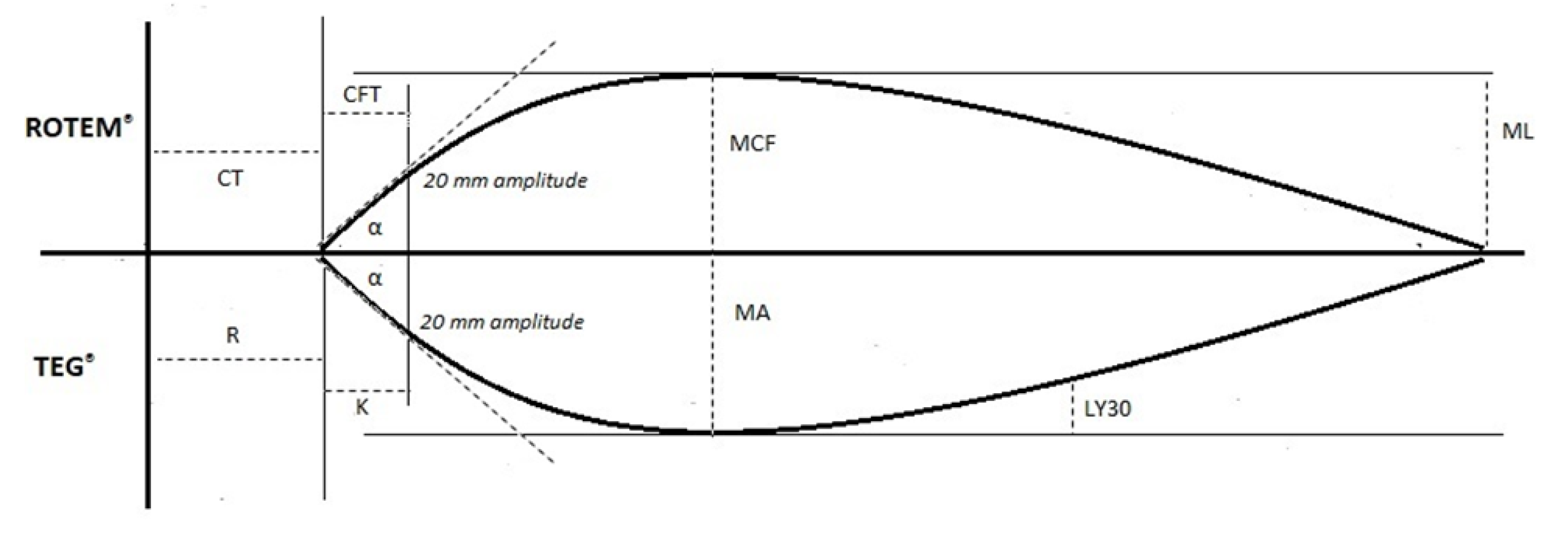
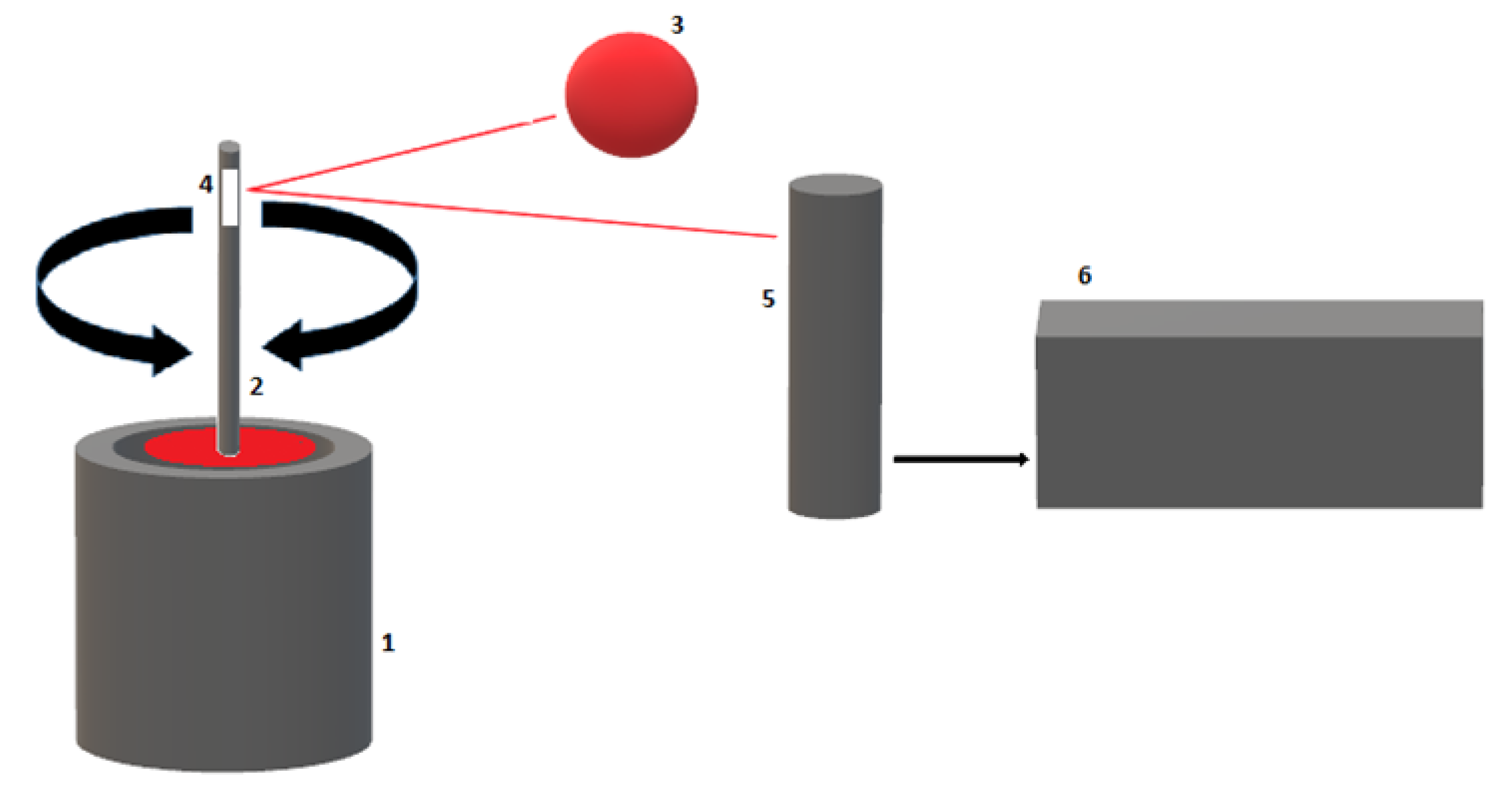
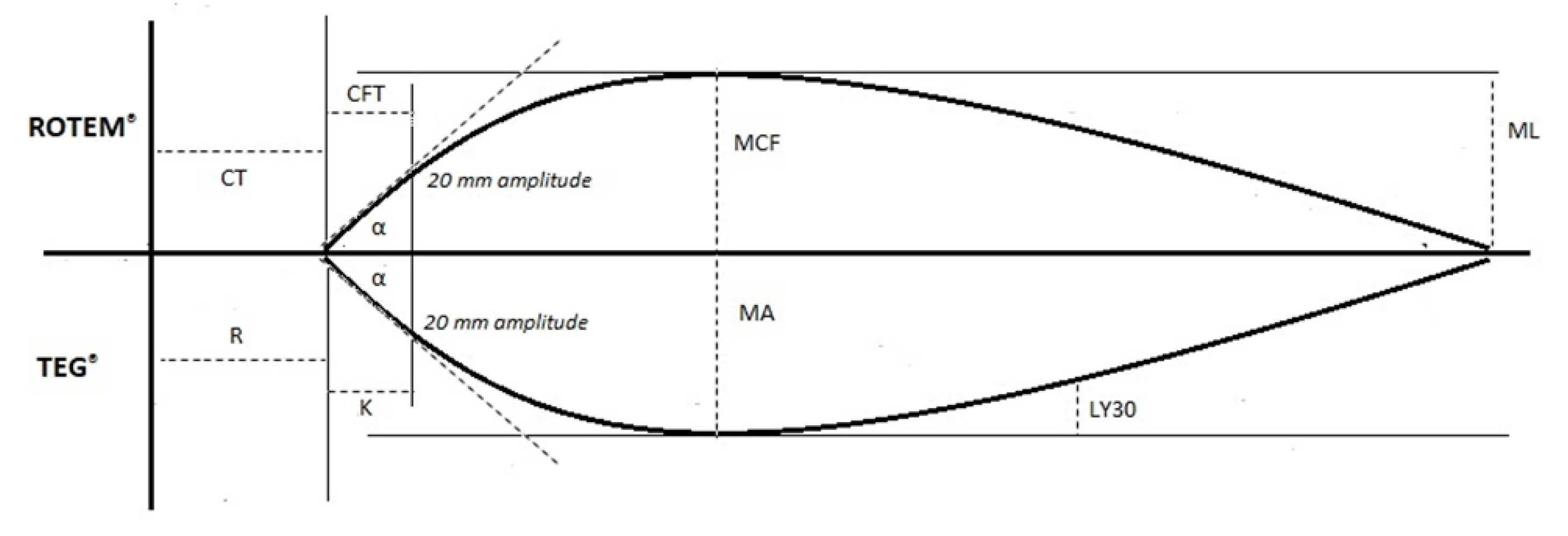
| TEG® Test Variable | Definition | ROTEM® Test Variable | Definition | Physiological Significance |
|---|---|---|---|---|
| Reaction time (R-time) | Time until clot amplitude of 2 mm is reached | Clotting time (CT) | Time until clot amplitude of 2 mm is reached | Initiation phase of enzymatic clotting factor activation. It is a measure of time taken to initiate coagulation |
| Kinetics time (K-time) | Time until clot amplitude of 20 mm is reached (from 2 mm amplitude) | Clot formation time (CFT) | Time until clot amplitude of 20 mm is reached (from 2 mm amplitude) | The amount of time it takes to reach a certain clot strength (amplitude of 20 mm) |
| Alpha angle (α) | Angle between central horizontal line and a tangent to the curve through the 20 mm amplitude point | Alpha angle (α) | Angle between central horizontal line and a tangent to the curve through the 20 mm amplitude point | Rate of clot formation and strengthening (kinetic measurement of fibrin–platelet interaction) |
| Amplitude 10 min after CT (A10) | Amplitude at 10 min after clotting time | Measure of clot strength (fibrin–platelet interaction) | ||
| Amplitude 30 min after CT (A30) | Amplitude at 30 min after clotting time | Measure of clot strength (fibrin–platelet interaction) | ||
| Maximum amplitude (MA) | Peak amplitude of clot | Maximum clot firmness (MCF) | Peak amplitude of clot | Measure of clot strength (fibrin–platelet interaction |
| Lysis at 30 min (LY30)Lysis at 60 min (LY60) | Percentage decrease in clot strength at 30 min after maximum amplitude (MA) Percentage decrease in clot strength at 60 min after maximum amplitude (MA) | Maximum lysis (ML) | Maximum percentage reduction in maximum clot firmness (MCF) | Measure of clot stability. Fibrinolytic-induced dissolution of the fibrin–platelet bond |
| TEG® Test | Activator | Rationale |
|---|---|---|
| Native TEG | None | Assessment of native blood coagulation |
| Kaolin TEG | Kaolin | Test of intrinsic pathway (Kaolin-mediated activation of factor XII). Faster results than the native test |
| Kaolin TEG | Kaolin and tissue factor | Test of both intrinsic and extrinsic pathways (Tissue factor-dependent activation of factor VII). Rapid assessment of blood coagulation |
| Kaolin TEG with heparinase | Kaolin and heparinase | Heparinase inactivates heparin. Assessment of heparin reversal on blood coagulation |
| TEG Functional Fibrinogen | Tissue factor and abciximab | Abciximab acts as a platelet GpIIb/IIIa inhibitor. Test of extrinsic pathway allowing for the quantification of fibrinogen contribution to clot strength after platelet inhibition |
| ROTEM® Test | Activator | Rationale |
|---|---|---|
| NATEM | None | Assessment of native blood coagulation |
| INTEM | Phospholipid and ellagic acid | Ellagic acid acts as intrinsic pathway activator. Test of intrinsic pathway, more sensitive to intrinsic pathway factor deficiencies |
| EXTEM | Tissue factor | Test of extrinsic pathway (tissue factor-dependent activation of extrinsic pathway). More sensitive to extrinsic pathway factor deficiencies. Fastest clot analysis |
| HEPTEM | Phospholipid, ellagic acid, and heparinase | Heparinase inactivates heparin. Assessment of heparin reversal on blood coagulation |
| APTEM | Tissue factor and aprotinin | Aprotinin inhibits fibrinolysis. Test of fibrinolysis |
| FIBTEM | Tissue factor and cytochalasin D | Cytochalasin D acts as a platelet inhibitor. Quantification of fibrinogen contribution to clot strength after platelet inhibition |
| Parameter | Neonatal Period (Mean Value) | Normalization |
|---|---|---|
| Platelets | Normal or increased | 1 year (after transient increases) |
| von Willebrand factor (vWF) | Increased (153%) | 3 months |
| FII | Decreased (40–66%) | 1 year |
| FVII | Decreased (40–66%) | 1 year (up to 16 years) |
| FIX | Decreased (40–66%) | 1 year |
| FX | Decreased (40–66%) | 1 year |
| FXI | Decreased (37–54%) | 1 year |
| FXII | Decreased (37–54%) | 1 year |
| FV | Normal or decreased (70%) | 1 year (up to 16 years) |
| FVIII | Normal or increased (100%) | 1 month |
| Prekallikrein (PK) | Decreased (37–54%) | 1 year |
| High-molecular-weight kininogen (HMWK) | Decreased (37–54%) | 1 year |
| Fibrinogen | Decreased or normal | 1 year |
| Antithrombin (AT) | Decreased (63%) | 3 months |
| Protein C (PC) | Decreased (35%) | 16 years |
| Protein S (PS) | Decreased (36%) | 3 months |
| Plasminogen | Decreased (36%) | 6 months |
| Alpha 2 antiplasmin | Normal or decreased (85%) | 6 months |
| Tissue plasminogen activator (tPA) | Increased | 1 week |
| D-dimer | Increased | 16 years |
| Author | Included Neonates (n) | Control Group | Type of blood Sample | Analyzing Method | Findings |
|---|---|---|---|---|---|
| Wiegele et al. [56] | 142 neonates. 55 preterm infants, 87 full-term infants | Cord blood | ROTEM | Significantly faster clot initiation and formation as well as higher clot strength in the term group | |
| Schott et al. [58] | 100 full-term neonates.50 delivered vaginally; 50 delivered by cesarean section | Cord blood | TEG | No differences between vaginal and cesarean delivery neonates in TEG measurements | |
| Mirabella et al. [57] | 85 full-term neonates | 40 adults | Cord blood | TEG | No between neonatal and adult TEG parameters No differences between neonatal and adult TEG value ranges |
| Sidlik et al. [53] | 101 neonates | Adults | Cord blood | ROTEM | Lower CT and CFT values and higher alpha angle in neonates (faster clot formation compared to adults). Accelerated fibrinolysis in the newborns compared to adults (shorter LI30) |
| Strauss et al. [54] | 231 (84 full-term and 47 preterm infants) | Institution’s reference ranges for adults and children | Cord blood | ROTEM | CT and CFT significantly shorter among preterm and term infants compared to adults (faster clot formation) Decreased MCF in preterm compared to term neonates and adults. Correlation between GA and CT and MCF |
| Edwards et al. [52] | 59 neonates (>34 weeks) | Institution’s reference ranges for adults and children | Cord blood | TEG | Accelerated initiation of coagulation and increased clot firmness and enhanced fibrinolytic activity compared to children (shorter R, higher angle, MA, CI, and G values) Accelerated initiation and propagation of coagulation compared to adults (shorter R, lower G values) |
| Cvirn et al. [55] | 20 full-term neonates | 20 adults | Cord blood | ROTEM | Lower MCF and α angle and longer CFT (FIBTEM) in neonates compared to adults |
| Author | Included Neonates (n) | Type of Blood Sample | Analyzing Method | Findings |
|---|---|---|---|---|
| Theodoraki et al. [65] | 215 full-term neonates | Whole blood | ROTEM | Positive correlation between LY30, LY45, and LY60 variables of EXTEM and INTEM assays and gestational age. ROTEM neonatal variables not influenced by maternal problems during pregnancy and delivery mode. Reduced A5 in INTEM and prolonged CT and CFT in INTEM and EXTEM assays were observed in neonates with higher hematocrit levels |
| Raffaeli et al. [94] | 60 neonates | Whole blood and cord blood | TEG | Placental blood leads to a procoagulant imbalance when testing is performed with TEG |
| Sokou et al. [63] | <37 weeks SGA: 22 <37 weeks AGA:25 ≥37 weeks SGA: 23 ≥37 weeks AGA: 23 | Whole blood | ROTEM | No statistically significant differences were noticed regarding all EXTEM parameters between AGA and SGA neonates |
| Raffaeli et al. [95] | 283 neonates VLBW: 201, ≥37 weeks: 72 | Whole blood | TEG | Healthy VLBWIs showed TEG profiles suggesting a relatively balanced hemostatic system, with slight hypocoagulability initially (compared with term neonates), gradually evolving to a somewhat more procoagulant phenotype over the first month |
| Liu et al. [96] | 371 full-term neonates | Whole blood | TEG | Negative correlation between age and K value. Positive correlation between age and α angle, MA, LY30. Positive correlation between MA and birthweight. R value of females was higher than that of males and higher in cesarean section than that of spontaneous delivery |
| Sokou et al. [62] | 282 neonates. 198 term and 84 preterm | Whole blood | ROTEM | Enhanced fibrinolytic activity in preterm neonates (LY60, significantly lower) |
| Sewell et al. [61] | 30 full-term neonates | Whole blood | TEG | Lower R and K values in neonates compared to older children. Higher fibrinolysis or rate of clot breakdown (LY30) and coagulation index (CI) in neonates compared to older children |
| Motta et al. [59] | 65 preterm neonates. <32 weeks: 32 32–37 weeks: 33 | Whole blood | TEG | Increased fibrinolysis (higher LY30) in early preterm neonates compared to moderate/late preterm neonates |
| Ravn et al. [64] | 149 children. Neonates: 30 1–18 months: 72 19–72 months: 47 | Whole blood | ROTEM | No sign of developmental changes in ROTEM assays, apart from CT in the EXTEM assay |
| Oswald et al. [97] | 51 infants (0–3 months) | Whole blood | TEM | Subjects aged 0–3 months exhibited accelerated initiation and propagation of coagulation and maximum clot firmness |
| Kettner et al. [98] | 40 neonates. 27–31 weeks: 13, 32–36 weeks: 9, 36–40 weeks: 7, 34–40 weeks corrected: 11 | Whole blood | TEG | When compared with the adult group, thromboelastography revealed no defects in coagulation from groups of clinically stable infants |
| Miller et al. [60] | 237 children Neonates: 37 | Whole blood | TEG | Neonatal values did not statistically differ from those of older patients (aged from 1 to 24 months) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannata, G.; Mariotti Zani, E.; Argentiero, A.; Caminiti, C.; Perrone, S.; Esposito, S. TEG® and ROTEM® Traces: Clinical Applications of Viscoelastic Coagulation Monitoring in Neonatal Intensive Care Unit. Diagnostics 2021, 11, 1642. https://doi.org/10.3390/diagnostics11091642
Cannata G, Mariotti Zani E, Argentiero A, Caminiti C, Perrone S, Esposito S. TEG® and ROTEM® Traces: Clinical Applications of Viscoelastic Coagulation Monitoring in Neonatal Intensive Care Unit. Diagnostics. 2021; 11(9):1642. https://doi.org/10.3390/diagnostics11091642
Chicago/Turabian StyleCannata, Giulia, Elena Mariotti Zani, Alberto Argentiero, Caterina Caminiti, Serafina Perrone, and Susanna Esposito. 2021. "TEG® and ROTEM® Traces: Clinical Applications of Viscoelastic Coagulation Monitoring in Neonatal Intensive Care Unit" Diagnostics 11, no. 9: 1642. https://doi.org/10.3390/diagnostics11091642
APA StyleCannata, G., Mariotti Zani, E., Argentiero, A., Caminiti, C., Perrone, S., & Esposito, S. (2021). TEG® and ROTEM® Traces: Clinical Applications of Viscoelastic Coagulation Monitoring in Neonatal Intensive Care Unit. Diagnostics, 11(9), 1642. https://doi.org/10.3390/diagnostics11091642







